Abstract
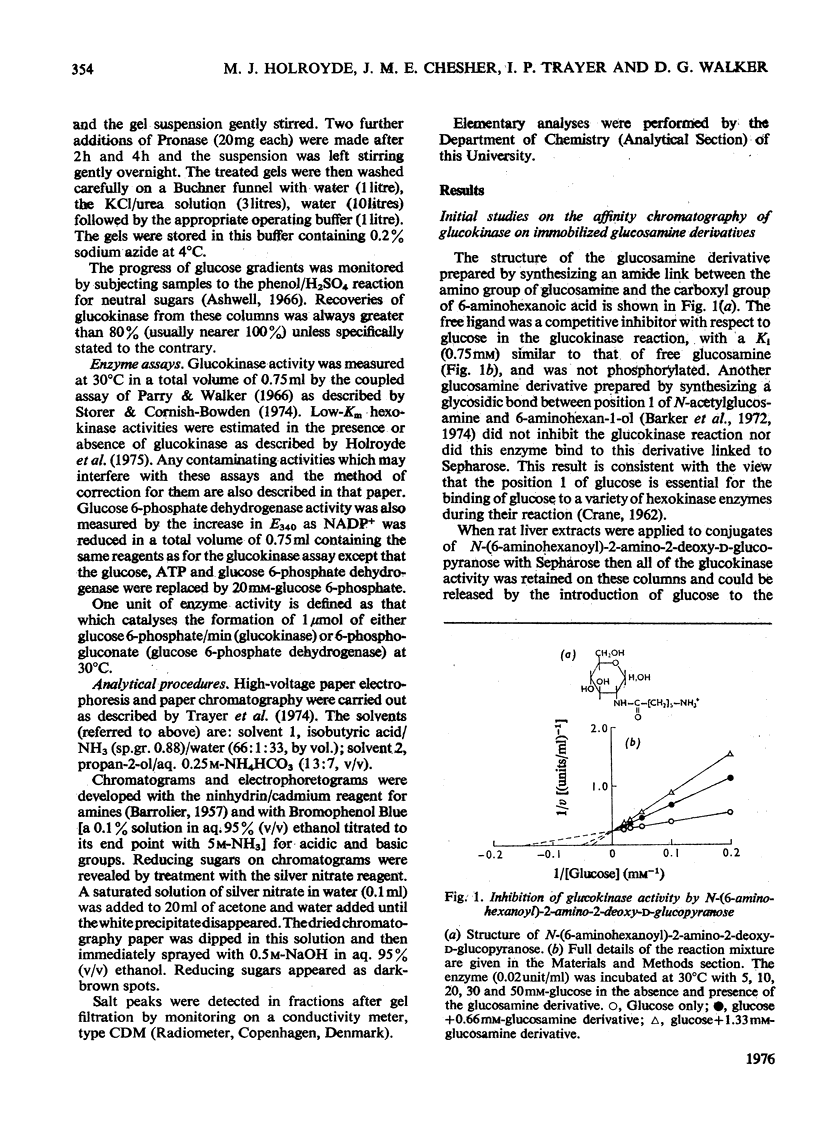
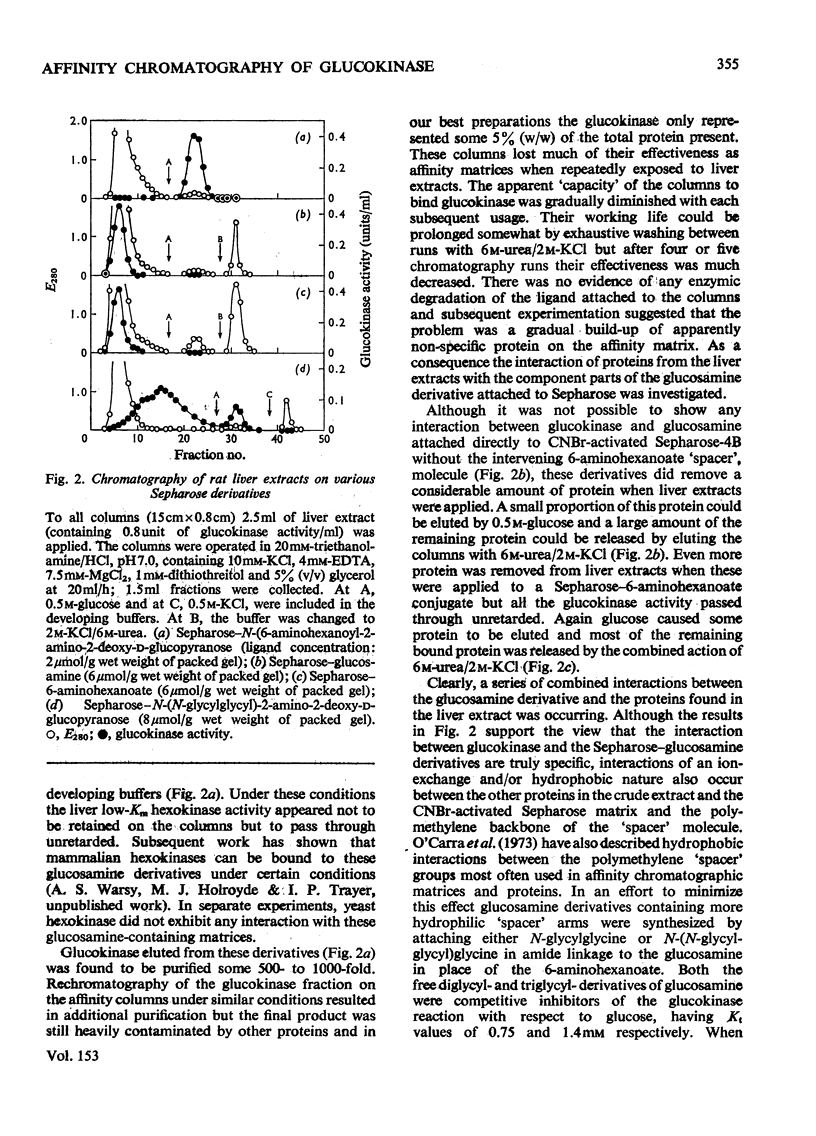
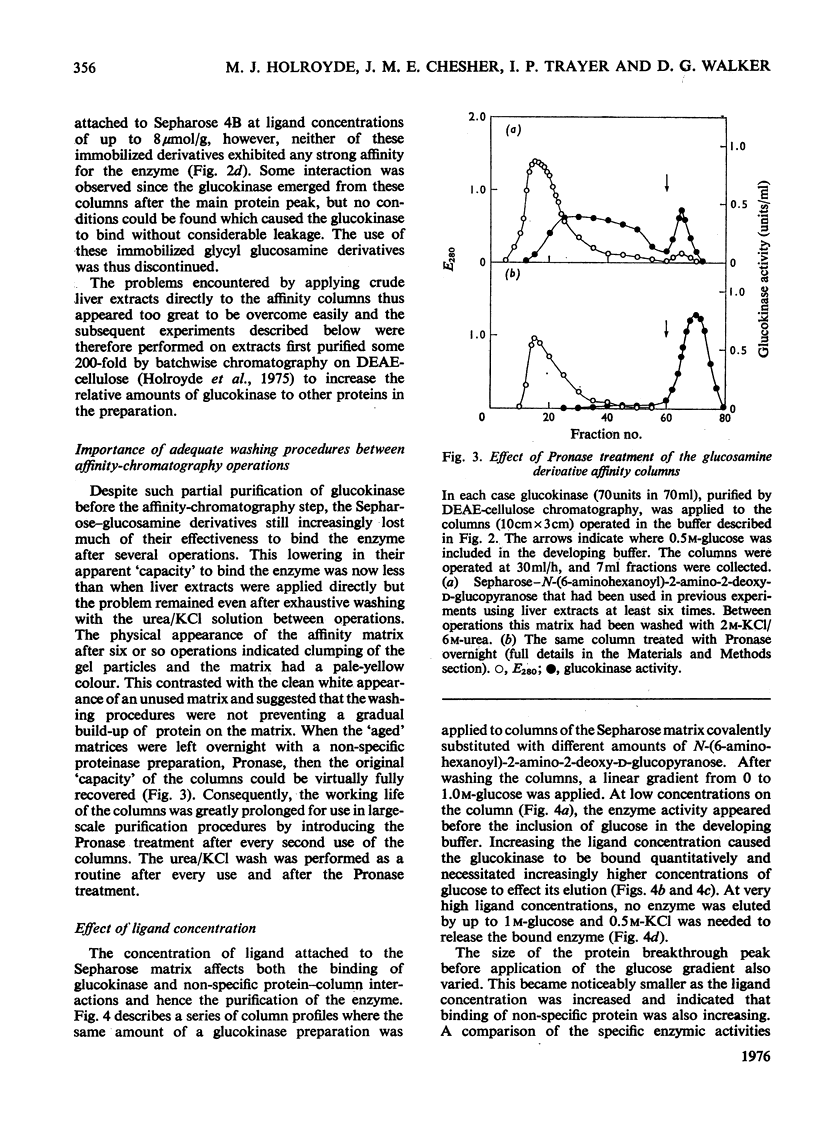
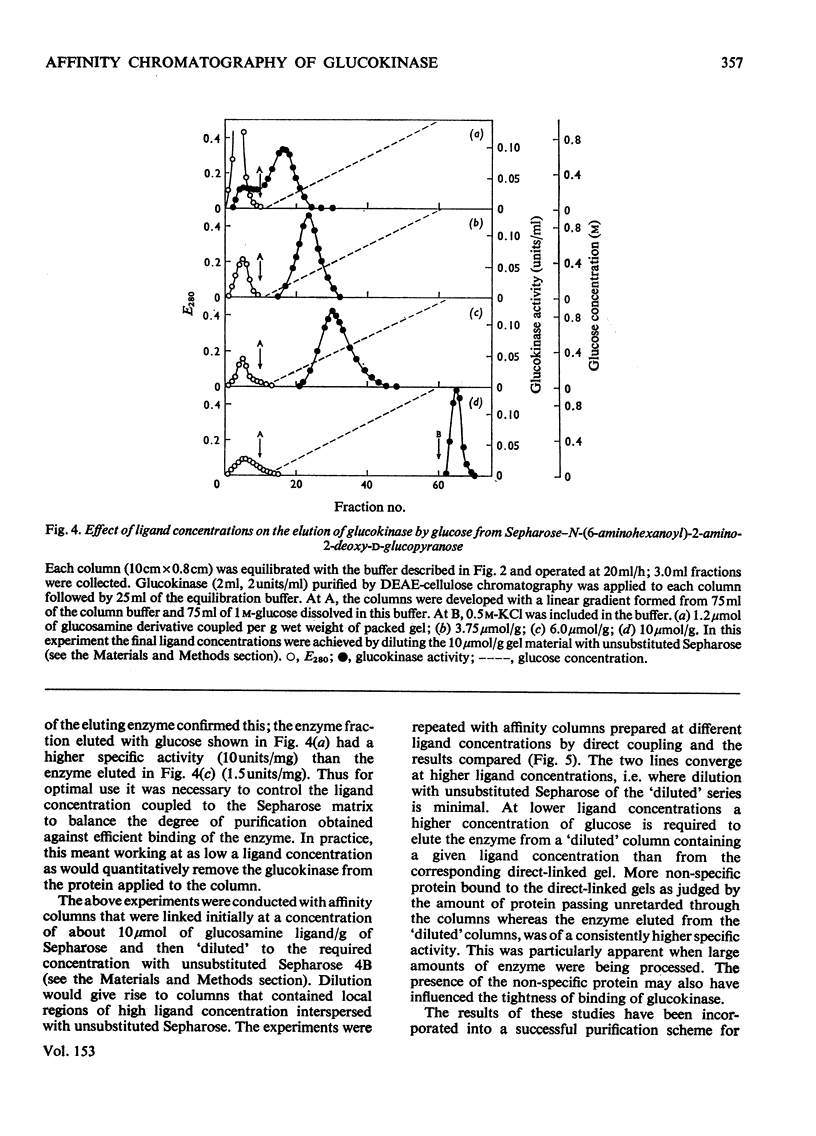
The synthesis of N-(6-aminohexanoyl)-2-amino-2-deoxy-D-glucose is described and it was shown to be a competitive inhibitor (Ki, 0.75 mM) with respect to glucose of rat hepatic glucokinase (EC 2.7.1.2). After attachment to CNBr-activated Sepharose 4B, this derivative was able to remove glucokinase quantitatively from crude liver extracts and release it when the columns were developed with glucose, glucosamine, N-acetyl-glucosamine or KC1. Repeated exposure of the columns to liver extracts led to rapid loss in their effectiveness as affinity matrices because proteins other than glucokinase are bound to the columns. The nature of such protein binding and methods for the rejuvenation of "used" columns are discussed along with the effect of the mode of preparation of the Sepharose-ligand conjugate and the concentration of bound ligand on the purification of glucokinase. Glucose 6-phosphate dehydrogenase is cited as an example of both non-specific protein binding to the affinity column and of the importance of the control of ligand concentration in removing such non-specifically bound proteins. Some guidelines emerged that should be generally applicable to other systems, particularly those which involve affinity chromatography of enzymes that are present in tissue extracts in very low amounts and possess only a relatively low association constant for the immobilized ligand.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Barker R., Chiang C. K., Trayer I. P., Hill R. L. Monosaccharides attached to agarose. Methods Enzymol. 1974;34:317–328. doi: 10.1016/s0076-6879(74)34032-3. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Barry S., O'Carra P. Affinity chromatography of nicotinamide-adenine dinucleotide-linked dehydrogenases on immobilized derivatives of the dinucleotide. Biochem J. 1973 Dec;135(4):595–607. doi: 10.1042/bj1350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau B., Malek G. A new convenient reagent for peptide syntheses. J Am Chem Soc. 1968 Mar 13;90(6):1651–1652. doi: 10.1021/ja01008a045. [DOI] [PubMed] [Google Scholar]
- Brodelius P., Mosbach K. Separation of the isoenzymes of lactate dehydrogenase by affinity chromatography using an immobilized AMP-analogue. FEBS Lett. 1973 Sep 15;35(2):223–226. doi: 10.1016/0014-5793(73)80290-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography of macromolecules. Adv Enzymol Relat Areas Mol Biol. 1972;36:29–89. doi: 10.1002/9780470122815.ch2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Er-el Z., Zaidenzaig Y., Shaltiel S. Hydrocarbon-coated sepharoses. Use in the purification of glycogen phosphorylase. Biochem Biophys Res Commun. 1972 Oct 17;49(2):383–390. doi: 10.1016/0006-291x(72)90422-6. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Craven D. B., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 2. Some parameters relating to the selection and concentration of the immobilised ligand. Eur J Biochem. 1974 Jan 16;41(2):335–340. doi: 10.1111/j.1432-1033.1974.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Allen M. B., Storer A. C., Warsy A. S., Chesher J. M., Trayer I. P., Cornish-Bowden A., Walker D. G. The purification in high yield and characterization of rat hepatic glucokinase. Biochem J. 1976 Feb 1;153(2):363–373. doi: 10.1042/bj1530363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 3. The binding of glycerokinase and lactate dehydrogenase in relation to column geometry and dynamics. Eur J Biochem. 1974 Jan 16;41(2):341–345. doi: 10.1111/j.1432-1033.1974.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 4. Variation of the binding of dehydrogenases and kinases with pH. Eur J Biochem. 1974 Jan 16;41(2):347–351. doi: 10.1111/j.1432-1033.1974.tb03276.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Mosbach K. Biospecific affinity chromatography in aqueous-organic cosolvent mixtures. The effect of ethylene glycol on the binding of lactate dehydrogenase to an immobilised-AMP analogue. Eur J Biochem. 1975 Mar 3;52(1):99–105. doi: 10.1111/j.1432-1033.1975.tb03977.x. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Pons M., Descomps B., Crastes de Paulet A. Affinity chromatography: large-scale purification of the soluble oestradiol-17- dehydrogenase of human placenta. FEBS Lett. 1972 Jun 15;23(2):175–179. doi: 10.1016/0014-5793(72)80334-x. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S., Griffin T. Spacer arms in affinity chromatography: use of hydrophilic arms to control or eliminate nonbiospecific adsorption effects. FEBS Lett. 1974 Jul 15;43(2):169–175. doi: 10.1016/0014-5793(74)80993-2. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. The kinetics of coupled enzyme reactions. Applications to the assay of glucokinase, with glucose 6-phosphate dehydrogenase as coupling enzyme. Biochem J. 1974 Jul;141(1):205–209. doi: 10.1042/bj1410205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Hill R. L. The purification and properties of the A protein of lactose synthetase. J Biol Chem. 1971 Nov;246(21):6666–6675. [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Small D. P., Bottomley R. C. Preparation of adenosine nucleotide derivatives suitable for affinity chromatography. Biochem J. 1974 Jun;139(3):609–623. doi: 10.1042/bj1390609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon R. J. Chromatography of lipophilic proteins on adsorbents containing mixed hydrophobic and ionic groups. Biochem J. 1972 Feb;126(3):765–767. doi: 10.1042/bj1260765. [DOI] [PMC free article] [PubMed] [Google Scholar]


