Abstract
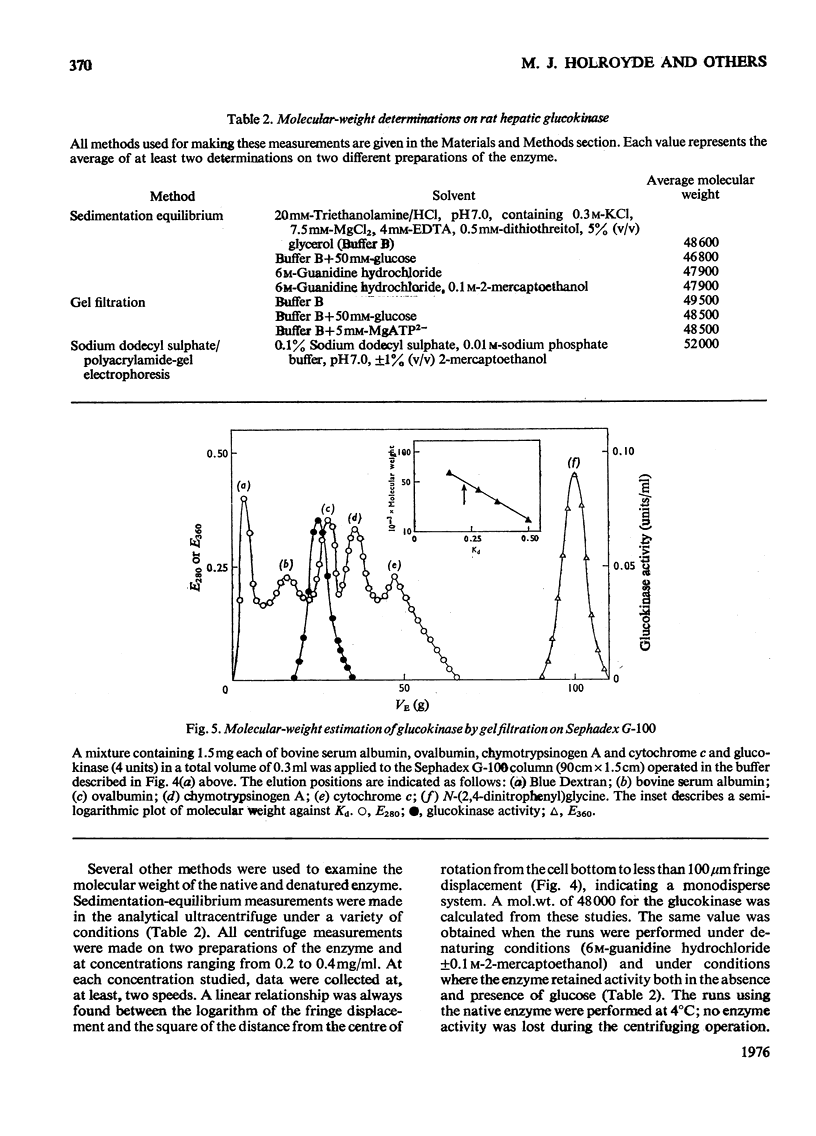
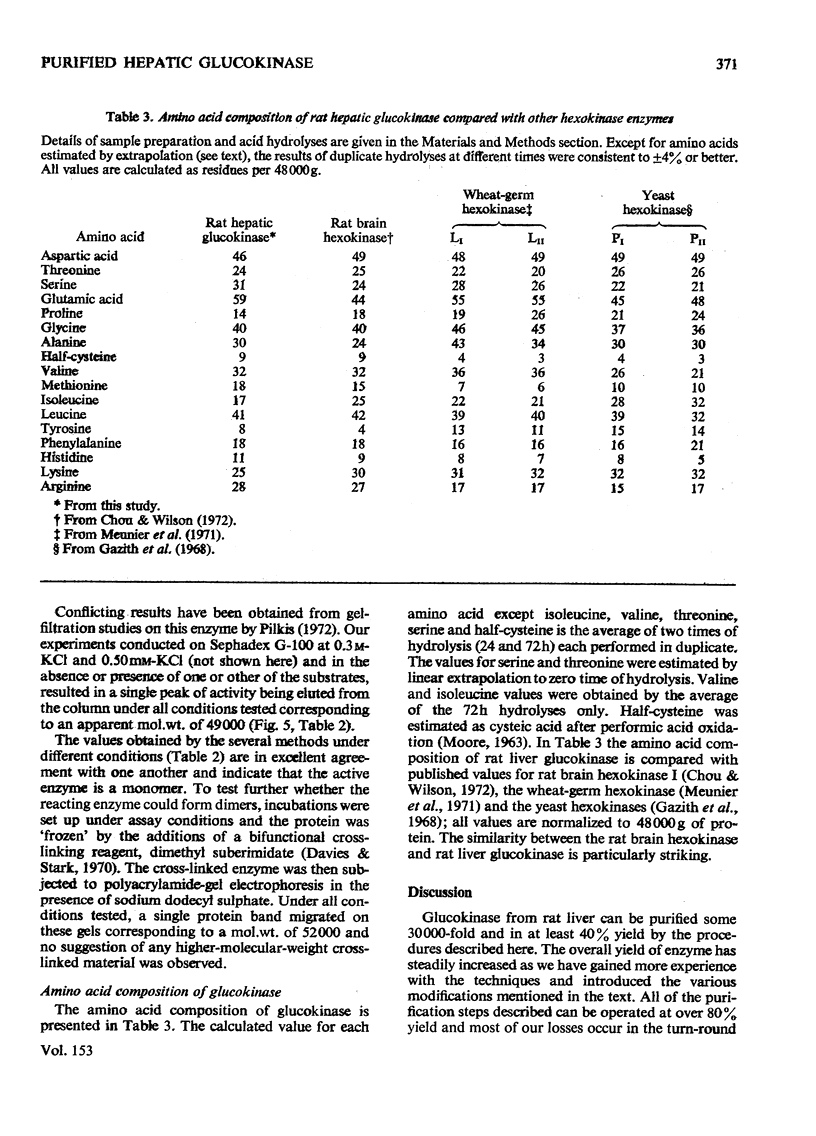
A new improved procedure for the purification of rat hepatic glucokinase (ATP-d-glucose 6-phosphotransferase, EC 2.7.1.2) is given. A key step is affinity chromatography on Sepharose-N-(6-aminohexanoyl)-2-amino-2-deoxy-d-glucopyranose. A homogeneous enzyme, specific activity 150 units/mg of protein, is obtained in about 40% yield. The molecular weight of the pure enzyme was determined by several procedures. In particular, sedimentation-equilibrium studies under a variety of conditions indicate a molecular weight of 48000 and no evidence for dimerization; reports in the literature of other values are discussed in the light of this evidence on the pure enzyme. The amino acid composition suggests that hepatic glucokinase is closely related to rat brain hexokinase and also the wheat "light" hexokinases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Berthillier G., Colobert L., Richard M., Got R. Glucokinases du foie de rat. Purification et propriétés des formes particulées. Biochim Biophys Acta. 1970 Apr 22;206(1):1–16. doi: 10.1016/0005-2744(70)90076-8. [DOI] [PubMed] [Google Scholar]
- Berthillier G., Got R. Effets de l'ATP et de la photo-oxydation sur l'agrégation de la glucokinase microsomique du foie de rat. Biochim Biophys Acta. 1972 Jan 20;258(1):88–98. doi: 10.1016/0005-2744(72)90968-0. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Wilson J. E. Purification and properties of rat brain hexokinase. Arch Biochem Biophys. 1972 Jul;151(1):48–55. doi: 10.1016/0003-9861(72)90471-7. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Gazith J., Schulze I. T., Gooding R. H., Womack F. C., Colowick S. P. Multiple forms and subunits of yeast hexokinase. Ann N Y Acad Sci. 1968 Jun 14;151(1):307–331. doi: 10.1111/j.1749-6632.1968.tb11898.x. [DOI] [PubMed] [Google Scholar]
- González C., Ureta T., Babul J., Rabajille E., Niemeyer H. Characterization of isoenzymes of adenosine triphosphate: D-hexose 6-phosphotransferase from rat liver. Biochemistry. 1967 Feb;6(2):460–468. doi: 10.1021/bi00854a014. [DOI] [PubMed] [Google Scholar]
- Grossman S. H., Dorn C. G., Potter V. R. The preparation and characterization of pure rat liver glucokinase. J Biol Chem. 1974 May 25;249(10):3055–3060. [PubMed] [Google Scholar]
- Grossman S. H., Potter V. R. Identification of rat hepatic glucokinase after polyacrylamide disc electrophoresis. Anal Biochem. 1974 May;59(1):54–62. doi: 10.1016/0003-2697(74)90008-6. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., Easterby J. S. Wheatgerm hexokinase: physical and active-site properties. Eur J Biochem. 1974 Jun 1;45(1):147–160. doi: 10.1111/j.1432-1033.1974.tb03539.x. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Chesher J. M., Trayer I. P., Walker D. G. Studies on the use of sepharose-N-(6-aminohexanoyl)-2-amino-2-deoxy-D-glucopyranose for the large-scale purification of hepatic glucokinase. Biochem J. 1976 Feb 1;153(2):351–361. doi: 10.1042/bj1530351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Buc J., Navarro A., Ricard J. Regulatory behavior of monomeric enzymes. 2. A wheat-germ hexokinase as a mnemonical enzyme. Eur J Biochem. 1974 Nov 1;49(1):209–223. doi: 10.1111/j.1432-1033.1974.tb03826.x. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Buc J., Ricard J. Purification and characterization of wheat germ hexokinases. FEBS Lett. 1971 Apr 12;14(1):25–28. doi: 10.1016/0014-5793(71)80266-1. [DOI] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis S. J. Rat hepatic glucokinase: improved purification and some properties. Arch Biochem Biophys. 1972 Apr;149(2):349–360. doi: 10.1016/0003-9861(72)90333-5. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. An improved procedure for starch-gel electrophoresis: further variations in the serum proteins of normal individuals. Biochem J. 1959 Mar;71(3):585–587. doi: 10.1042/bj0710585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. The kinetics of coupled enzyme reactions. Applications to the assay of glucokinase, with glucose 6-phosphate dehydrogenase as coupling enzyme. Biochem J. 1974 Jul;141(1):205–209. doi: 10.1042/bj1410205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



