Abstracts
As the core of leaf functional traits, the trade-off relationship between the petiole and lamina expresses the plant's adaptability to the environment in terms of support structure and photosynthesis. We investigated the proportions of allometric growth in the relationship between the petiole and the lamina of broadleaf woody plants in temperate highland Tianshan Mountains montane forests through three dimensions (length, area, and mass), including the length of the lamina (LL) and the length of the petiole (PL), and the area of the lamina (LA) and petiole cross sectional area (PCA) versus the mass of the lamina (LM) and the mass of the petiole (PM), as well as exploring the characteristics of the variance in response to seasonal changes. We found that the functional traits in all three dimensions showed a clear convergent evolution as the seasons progressed, that is, a “seasonal effect” of increasing and then decreasing. The effect of the petiole–lamina relationship under spring was minimal in the area dimension; the effects of the three–dimensional relationships of the traits were all highest in summer, and the effect of the petiole–lamina relationship was lower in autumn. We also found that petiole traits are simultaneously and multiply affected by lamina traits, with LA and LM having additional effects on the length/mass and area dimensions, respectively. Compared to tree species, shrub species significantly require more light intensity and support capacity. Compound-leaved plants would invest more in photoluminescence, increasing leaf light capture efficiency and static load and dynamic resistance. Our results suggest that plants have rather complex trade-off mechanisms at the leaf level influencing their ability to adapt to the environment, emphasize the need for leaf-level studies on the relationships between functional traits in plants, and illustrate the importance of the season as a distinct time scale for plant trade-off mechanisms.
Keywords: Allometric relationship, Functional traits, Leaf, Season
Highlights
-
•
The relationship between laminae and petioles reflects the trade-off between photosynthesis and support functions in plants.
-
•
Mechanisms explaining the response of the petiole-lamina trade-off to the seasons from a three-dimensional perspective.
-
•
The functional traits in all three dimensions showed a clear convergent evolution as the seasons progressed, that is, a “seasonal effect” of increasing and then decreasing.
1. Introduction
There are often trade-offs between functional traits during plant growth and development, and these trade-offs are gradually formed through the continuous screening of nature and are the most common link between plant functional traits (Oktavia and Jin 2020). Trade-offs between plant functional traits have become one of the central issues in plant ecology (Forrestel et al., 2015). From the research in recent years, the study of plant functional traits has been derived from single or grouped traits to the relationship between functional traits (Brouat et al., 1998; Westoby and Wright 2003; Sun et al. 2006, 2017; Niklas et al., 2007; Filartiga et al., 2021).
As the main organ of plant energy acquisition, the functional traits of leaves reflect the allocation pattern of the plant's own biomass and its ability to adapt to the environment, and at the same time, the leaf functional traits contain some kind of intrinsic connection and allocation trade-offs. Usually, a complete broadleaf consists of two parts: the lamina and the petiole, which are closely related in structure and function (Li et al., 2021). In woody communities, overlapping leaves cause reduction in light capture efficiency, and this self-shading phenomenon can have serious effects on plants (Roig-Villanova and Martínez-García 2016; Perez et al., 2018). Allocating more biomass to petioles to make them elongate is one of the most effective strategies for plants to reduce self-shading because petioles can adjust the angle of growth of leaves to avoid overlapping with their own leaves and those of neighbouring plants (Falster and Westoby 2003; Niinemets et al., 2006; Sarlikioti et al., 2011; Zhong et al., 2019). Due to the light retention economics of the lamina and the mechanical biomechanical requirements of the petiole, leaves require more and larger laminae, and an increase in the plant's allocation of biomass to the leaf blade will inevitably lead to an increase in biomass investment in the petiole as well (Niinemets and Sack 2006). Patterns of biomass allocation between petiole and lamina as a basis for functional trade-offs, reflecting plant trade-offs between carbon benefits and support costs (Niinemets and Kull 1999; Niinemets et al., 2007a, Niinemets et al., 2007b). This allocation trade–off relationship at the leaf level is one of the key evolutionary mechanisms for improving energy transformation and material transport, and has significant implications on plant life history strategies.
Petiole-lamina trade-offs in plants are an idiosyncratic expression of their adaptation to their environment over long periods of evolutionary time (Niinemets 1998; Niinemets et al., 2007a, Niinemets et al., 2007b; Levionnois et al., 2020). The selective and filtering effects of the environment on plants dictate that the functional traits of the plant leaf change in response to the environment. Plants need to provide optimised leaves to resist external wind or other mechanical resistance and maintain the risk between maximum light capture and petiole breakage. Thin, long laminae transfer more bending stress to the petiole, while shorter, wider laminae adapt more readily to the effects of wind (Smith and Ennos 2003; Anten et al., 2009). Season is one of the important environmental factors that affect the growth and metabolism of plants. As different seasons contain changes in environmental factors such as light, temperature, humidity, etc. In order to adapt to the changes in the external environment, the physiological and ecological processes of the plants are also change. Accordingly, their biomass allocation strategies will also change (Zhang et al., 2022; Wang et al., 2023). Light-interception models predict that shade-tolerant species generally have large leaves because such leaves are more likely to capture light in low light. However, effective light capture by large leaves would appear to increase disproportionately with support requirements leading to petiole biomass (Pickup et al., 2005; Lusk et al., 2019; Zhu et al., 2019). As a result, shade-tolerant species require more petiole biomass than non-shade-tolerant species. High light intensity and windy environments have been shown to increase the proportion of plant biomass allocated to petioles, and low temperatures and drought often increase the risk of inefficient plant transport and petiole embolism, and may therefore require significant investment in petioles (Westoby and Wright 2003; Sun et al., 2006; Louf et al., 2018), where the petiole-lamina trade-off tends to support the “diminishing returns” hypothesis (Milla and Reich 2007; Niinemets et al., 2007a, Niinemets et al., 2007b; Sun et al., 2017). In addition to the economics of light interception, the requirement for adaptation to drought may have played a role in leaf evolution. There is an evidence that water and light availability are often negatively correlated during succession, resulting in compound-leaved plants being more likely to occur in arid environments than simple-leaved plants (Sack and Frole 2006; Poorter 2009; Fan et al., 2017). The process of leaf adaptation to environmental factors reflects the plant's own genetic characteristics (Tsukaya et al., 2002). As different seasons exhibit different environmental stresses, the ensuing trait variation in laminae will inevitably affect the plant's investment in biomass on the petiole (Gebauer et al., 2016; Roig-Villanova and Martínez-García 2016; Yoshinaka et al., 2018), and the petiole will then exhibit corresponding adaptive traits. However, there is still a gap in research on the relationships between petiole-lamina trade-offs in plants under different seasons.
Lamina–petiole relationships of different dimensions (length, area and mass) exhibit different aspects of plant functional trade-offs or adaptations to environmental changes. However, current studies usually analyse their altitudinal gradients, hydraulic conditions, photosynthetic rates or mechanical stimulation by wind speed (Fan et al., 2017; Gleason et al., 2018; Louf et al., 2018; Song and Hong 2018; Lusk et al., 2019; Li et al., 2021), focusing mainly on the influence of spatial scales in this context. To the best of our knowledge, no study has so far explored petiole-lamina trade–off relationships in combination with seasonal factors. To enrich research in this field, we used typical broadleaf woody plants of the highland montane Tianshan Mountains forests in Xinjiang to explore the biomass allocation pattern among the petiole-lamina traits of broadleaf woody plants in this region, and investigated the mechanism of seasonal influence on the petiole-lamina trade-offs of woody plants from three dimensions. The main objectives of this study were (1) to determine the trade-off relationship between the petiole and lamina, (2) to assess the effect of season on the trade-off relationship between the petiole and the lamina, and (3) to validate the need for a multidimensional analysis of the relationship between the petiole and the lamina.
2. Materials and methods
2.1. Overview of the study area
The study area is located in the middle part of the northern slope of the Tianshan Mountains in Urumqi City, Ha Xionggou (84.63°–93.04°E, 43.60°–44.14°N) belongs to the Tianshan region within China, which has dry climate, low annual precipitation, high evapotranspiration, and fragile ecology, and vegetation types in descending order along the altitude are mountain grassland, mountain meadow, coniferous forest, alpine grassland, alpine cushion plants, and snow-covered glaciers. The region has a highland mountain climate, highlighting the process of biological evolution from a warm and humid flora gradually replaced by a modern dry Mediterranean flora, with average annual temperatures ranging from 5 °C to 14 °C and average annual precipitation mostly above 500 mm (Fig. 1).
Fig. 1.
The geographical range of our study is China Xinjiang Uygur Autonomous Region, Urumqi, Tianshan National Forest Park Haxiong Gou.
2.2. Sampling method
Field sampling was carried out in the study area in May (spring), July (summer), and September (autumn) of 2023, and the sampling site was set up in Ha Xionggou, Urumqi City, in the middle part of the northern slope of Tianshan Mountain, at an altitude of 1800m–1900m. Twelve species of woody plants, including six species of trees and six species of shrubs, belonging to eight families and ten genera, were selected in the study area (Table 1). For each sample, well-grown individuals with relatively uniform growth (i.e., mature and living in habitats with sufficiently viable and low disturbance) were
Table 1.
Statistical information on different functional species in this study.
| Latin name | Family | Genus | Vegetation type | Leaf shape |
|---|---|---|---|---|
| Betula pendula Roth. | Betulaceae | Betula | arbor | simple |
| Populus talassica Kom. | Salicaceae | Populus | arbor | simple |
| Ulmus pumila L. | Ulmaceae | Ulmus | arbor | simple |
| Populus alba var. pyramidalis Bge. | Salicaceae | Populus | arbor | simple |
| Fraxinus sogdiana Bunge | Oleaceae | Fraxinus | arbor | simple |
| Populus laurifolia Ledeb. | Salicaceae | Populus | arbor | simple |
| Cotoneaster melanocarpus Lodd. | Rosaceae | Cotoneaster | shrub | simple |
| Lonicera tatarica L. | Caprifoliaceae | Lonicera | shrub | simple |
| Berberis atrocarpa Schneid. | Berberidaceae | Berberis | shrub | simple |
| Spiraea hypericifolia L. | Rosaceae | Spiraea | shrub | simple |
| Rosa platyacantha Schrenk | Rosaceae | Rosa | shrub | compound |
| Caragana acanthophylla Kom. | Fabaceae | Caragana | shrub | compound |
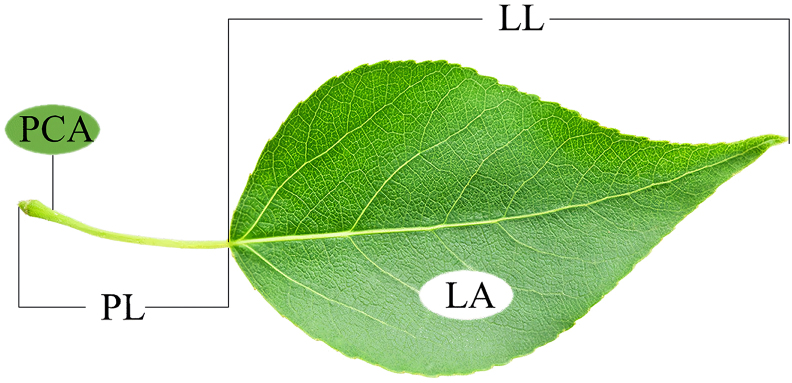
randomly selected and labeled, and the same individuals were collected once per season. Five current year branchlets (that is, the end of the branch to the first terminal node separated from the branch) in the same canopy were randomly selected for each individual, ensuring that all sample branches had intact foliage, with between 5 and 15 healthy, fully expanded and not insect bitten or damaged foliage on each branch to avoid significant differences in survival conditions, 4740 leaves in total (Table 2) They were then sealed in a zip-lock bag and brought back to the laboratory. For each sample, carefully separate the petiole from the lamina, and the following measurements of lamina traits versus petiole traits were taken for all leaves on the branchlet within 6 h of sampling (Fig. 2).
-
(1)
Lamina length (LL) and petiole length (PL): For each sample, leaves from different individuals were measured using Vernier calipers to measure LL and PL, respectively.
-
(2)
Lamina area (LA): For each sample, For each sample, laminae from different individuals were laid flat on white paper. LA was determined by scanning the laminae with a scanner three to four times in repetition and then analysing the pictures with image analysis software (Image J).
-
(3)
Petiole cross-sectional area (PCA): For each sample, at least 15 leaves from different individuals were selected to measure the diameter of the petiole (PD), that is, the diameter from the base of the leaf blade to the middle of the end of the petiole, and using a spiral micrometer (WD, Zhejiang, China), the PCA was calculated as follows: PCA = π ∗ (PD/2)2.
-
(4)
Lamina mass (LM) and petiole mass (PM): For each sample, the lamina and petioles were individually placed in ziplock bags, dried in an oven at 75 °C for 72 h to a constant mass and individually weighed to the nearest 0.0001 g. The dry mass obtained was then divided by the number of leaves to calculate the individual LM and PM.
Table 2.
The number of samples of each species collected in this study.
| Latin name | Twig number | Spring sample size | Summer sample size | Fall sample size |
|---|---|---|---|---|
| Betula pendula Roth. | 5 | 140 | 127 | 128 |
| Populus talassica Kom. | 5 | 132 | 133 | 140 |
| Ulmus pumila L. | 5 | 136 | 125 | 131 |
| Populus alba var. pyramidalis Bge. | 4 | 127 | 129 | 137 |
| Fraxinus sogdiana Bunge | 5 | 134 | 134 | 124 |
| Populus laurifolia Ledeb. | 4 | 134 | 123 | 130 |
| Cotoneaster melanocarpus Lodd. | 4 | 126 | 126 | 134 |
| Lonicera tatarica L. | 4 | 139 | 130 | 138 |
| Berberis atrocarpa Schneid. | 3 | 132 | 137 | 129 |
| Spiraea hypericifolia L. | 4 | 123 | 138 | 128 |
| Rosa platyacantha Schrenk | 3 | 136 | 135 | 121 |
| Caragana acanthophylla Kom. | 3 | 141 | 129 | 134 |
Fig. 2.
Schematic diagram of leaf traits. LL: the length of the lamina; PL: the length of the petiole; LA: the area of the lamina; PCA: petiole cross sectional area.
2.3. Data processing and analysis
Standardised principal axis analysis and covariance analysis were used to compare the heterogeneity of slopes between lamina and petiole traits of different plant types and to compare differences in slopes with 1. If the slopes were homogeneous, common slopes were calculated between the species groups and differences in the intercepts under common slopes were compared. Data for leaf traits were logarithmically transformed before analyzes were performed to conform to a normal distribution. Petiole–lamina relationships were analysed using Meta-analysis, where the effect sizes of each group of petiole–lamina relationships were analysed by the “Metafor” software package and 95% confidence intervals (95% CI) were calculated, with petiole traits being considered as the dependent variable in all the dichotomous relationship analyses. To fully analyse the petiole-leaf trait correlations, linear mixed-effects models and partial correlation analyses were used to assess whether LA, LM, and LA would cause additional effects on the correlations of PL-LL, PCA-LA, and PM-LM. All analyses were performed in R 3.6.1.
3. Results
3.1. Variation in petiole-lamina trait
In all species, the regression slopes for the three dimensions of the petiole–lamina relationship were significantly positive throughout seasonal variation, with regression slopes significantly > 1 for the PL-LL relationship in summer and fall (Fig. 3a), < 1 for the PCA-LA and the PM-LM relationships in summer (Fig. 3c), and significantly > 1 in spring and fall (Fig. 3b).
Fig. 3.
Three-dimensional linear relationships (PL-LL, PCA-LA, PM-LM) for all woody plants under different seasons.
The regression slopes of the PL-LL relationship for tree species were < 1 in spring and significantly > 1 in both summer and fall, and the regression slopes of the PM-LM relationship were significantly < 1 in spring and summer and significantly > 1 in fall (Table 3).
Table 3.
Three-dimensional linear relationships (PL-LL, PCA-LA and PM-LM) were summarised separately for all species, tree species and shrub species under different seasons.
| Reason | Relationship | PL-LL |
PCA-LA |
PM-LM |
|||
|---|---|---|---|---|---|---|---|
| Slope(B) | Intercept(A) | Slope(B) | Intercept(A) | Slope(B) | Intercept(A) | ||
| Spring | All | 1.115 ± 0.166 | −0.829 ± 0.260 | 0.279 ± 0.025 | 0.304 ± 0.019 | 0.845 ± 0.098 | −1.457 ± 0.183 |
| Arbor | 0.866 ± 0.290 | −0.406 ± 0.466 | 0.230 ± 0.023 | 0.334 ± 0.024 | 0.886 ± 0.161 | −1.363 ± 0.256 | |
| Shrub | 1.516 ± 0.147 | −1.463 ± 0.226 | 0.454 ± 0.052 | 0.247 ± 0.025 | 0.622 ± 0.177 | −1.930 ± 0.358 | |
| Summer | All | 1.513 ± 0.165 | −1.612 ± 0.305 | 0.343 ± 0.031 | 0.160 ± 0.037 | 0.942 ± 0.108 | −1.248 ± 0.129 |
| Arbor | 1.911 ± 0.514 | −2.431 ± 0.985 | 0.288 ± 0.041 | 0.262 ± 0.059 | 0.976 ± 0.160 | −1.231 ± 0.146 | |
| Shrub | 1.529 ± 0.082 | −1.580 ± 0.146 | 0.268 ± 0.072 | 0.193 ± 0.060 | 0.936 ± 0.247 | −1.247 ± 0.352 | |
| Fall | All | 1.423 ± 0.180 | −1.405 ± 0.329 | 0.316 ± 0.016 | 0.220 ± 0.018 | 1.125 ± 0.102 | −1.036 ± 0.120 |
| Arbor | 1.684 ± 0.468 | −1.974 ± 0.905 | 0.317 ± 0.033 | 0.215 ± 0.050 | 1.400 ± 0.192 | −0.940 ± 0.151 | |
| Shrub | 1.584 ± 0.094 | −1.616 ± 0.162 | 0.341 ± 0.034 | 0.206 ± 0.027 | 0.951 ± 0.211 | −1.229 ± 0.311 | |
The mean ± 95% CI (confidence interval) of the slope (B) and intercept (A) of each linear relationship is shown.
The regression slopes of the PL-LL relationship for shrub species were significantly > 1 in all three seasons, and the regression slopes of the PCA-LA relationship and the PM-LM relationship were significantly < 1. The regression slopes of the three dimensions of the petiole–lamina relationship varied seasonally, with the PCA-LA relationship showing the lowest slopes (all significantly < 1), but showing the highest intercepts (Table 3).
3.2. Petiole-lamina trade-offs across seasons and vegetation types
3.2.1. Petiole-lamina trait three–dimensional relationships
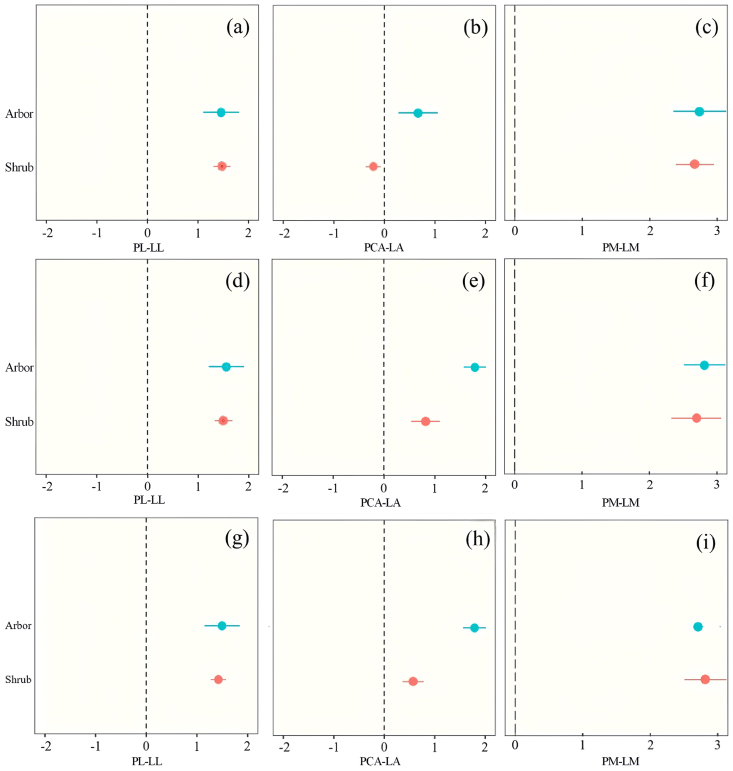
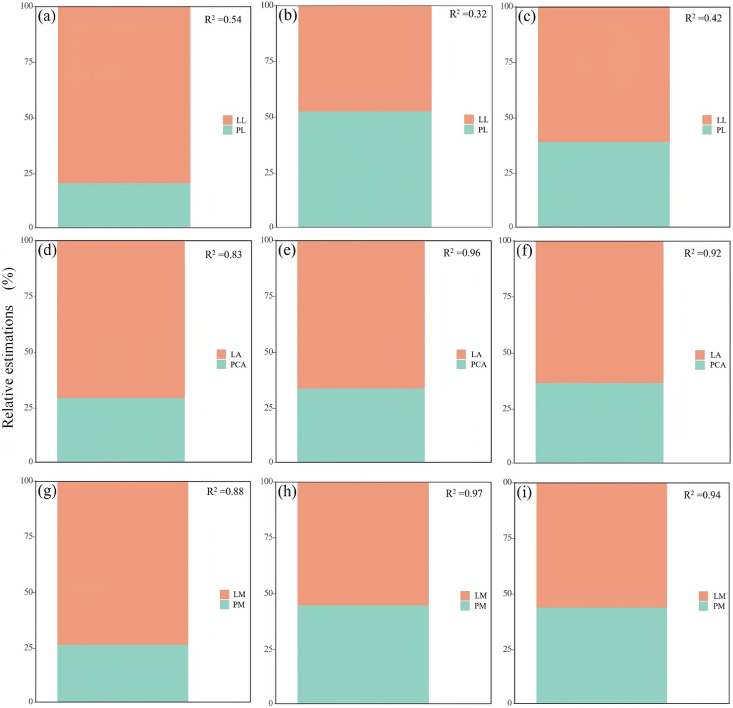
The mean effects of the three dimensions of the relationship of the petiole–lamina relationship changed roughly in the same way with seasons for almost all seasonal variations and vegetation types, showing a “seasonal effect” of increasing and then decreasing, the mean effect being greater in summer than in spring and fall, and the PL-LL relationship being significantly positive, with mean effect sizes of > 1. The effect size of the relationship was largest in summer and smallest in autumn. The effect sizes of the relationships were largest in summer and smallest in autumn (Fig. 5a). The PCA-LA relationship was significantly positive with a mean effect size > 1 for most seasonal variations and vegetation types, but it was significantly < 1 in spring (Fig. 5b). Shrub species showed a negative correlation in spring, followed by seasonal variations of < 1. The tree species had a mean effect of < 1 in spring and significantly > 1 in summer and fall (Fig. 5e) The mean effects of the PM-LM relationships were all highly significant > 1 (Fig. 5g ∼ i).
Fig. 5.
Three-dimensional Meta-analysis of different vegetation types under different seasons. Meta-analysis of different vegetation Types in Spring (a ∼ c), Summer (d ∼ f), Fall (g ∼ i).
3.2.2. LA-PL/LL, LM-PCA/LA, and LA-PM/LM relationships
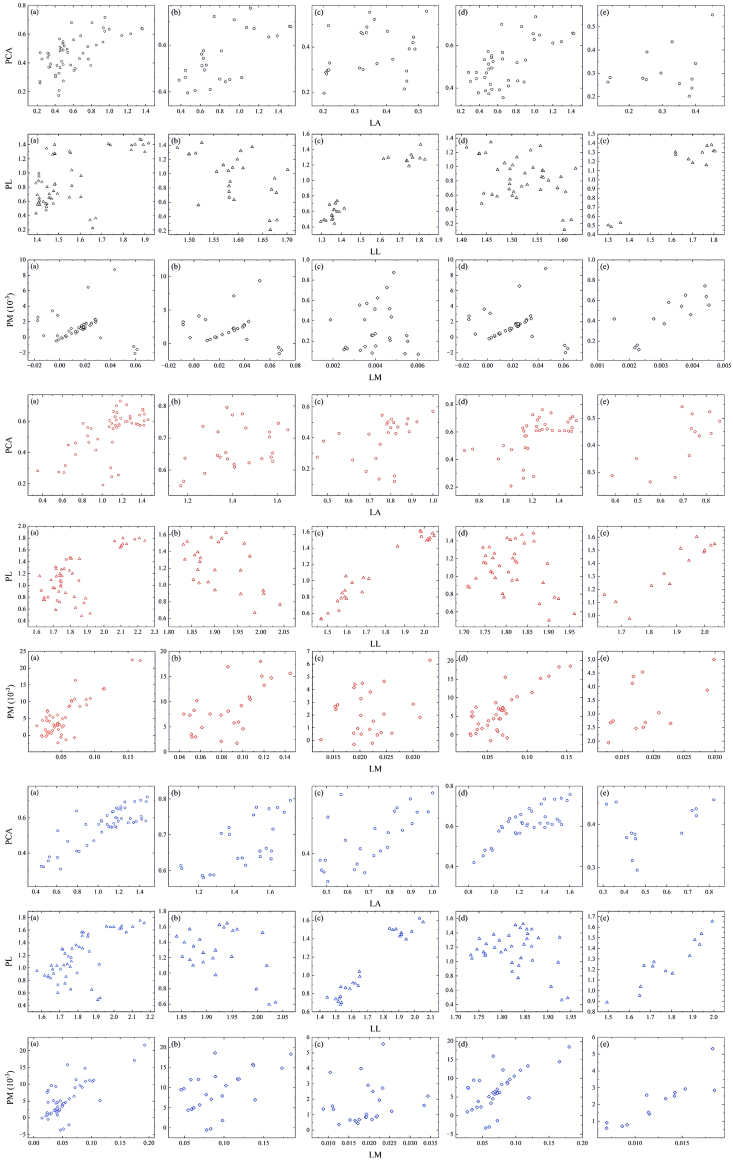
For the LA-PL/LL relationship between vegetation types, LA highly significantly affected LL in spring, with no significant difference for PL, and reached a maximum significant share of 31.02% for PL in summer, when tree species were negatively correlated (Fig. 6). Differences in leaf type altered the LA-PL/LL relationship, except in spring, when it showed significant differences in both summer and fall, with a negative correlation for monocarpic species in summer. The type of vegetation and the type of leaf did not have an additional effect on the seasonal pattern of the LM-PCA/LA relationship, with a significant percentage of PCA and LA of almost 50% in fall, LM showing a significant difference to LA and no significant difference for PCA.The LA-PM/LM relationship reached a highly significant level in spring, with LA to PM showing no significant difference in both summer and autumn, and with a negative correlation for woody species in summer, and a shift in the fall, shrub species shifted to positive correlations, and woody plants remained negatively correlated (Fig. 7). The pattern of petiole–lamina relationships was generally in line with the “seasonal effect” of increasing and then decreasing.
Fig. 6.
Modeling the mixed effects of different vegetation types across seasons. Mixed-effects model for leaf area and petiole area with fixed LA (PL-LL), fixed LM effects (PCA-LA) and fixed LA effects (PM-LM). (a ∼ c): Spring; (d ∼ f): Summer; (g ∼ i): Fall.
Fig. 7.
Modeling the mixed effects of different leaf types across seasons. Mixed-effects model for leaf area and petiole area with fixed LA (PL-LL), fixed LM effects (PCA-LA) and fixed LA effects (PM-LM). (a ∼ c): Spring; (d ∼ f): Summer; (g ∼ i): Fall.
4. Discussion
Relationships between lamina length, area, and mass revealed inconsistent physiological relationships in global vegetation models in response to climate change, according to a survey of global economic profiles of lamina traits (Osnas et al., 2013). However, the study of functional trade-offs between petiole and laminae is still at a relatively basic stage, and its significance for plant evolutionary life history strategies has not yet been fully explored and understood. In the present study, we first attempt to show evidence of variation and correlation between different three-dimensional patterns of petiole–lamina relationships in broadleaved woody plants of montane forests on biological and temporal scales. Leaf form, vegetation type, and seasonal variation were also factors contributing to the variability of these relationships, suggesting that there is a clear anisotropic growth relationship between the petiole and the lamina. We did also find significant additional effects of a variety of leaf trait petiole trait relationships. These results may indicate that there are multiple mechanisms to simultaneously control petiole-lamina biomass allocation and its trade-off relationships in broad-leaved woody plants, and that these trait combinations are adaptively and evolutionarily important.
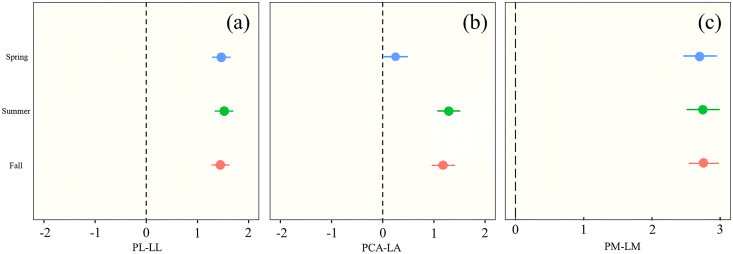
On the length scale, we found a positive correlation between PL and LL, and it was found that genetics determines that plants have a higher proportion of overlapping leaves that are longer in a given space (Tsukaya, 2005). This suggests that when species invest in biomass for petioles, they additionally increase the cost of investing in petiole length, taking into account the overlap between laminae that causes self-shading (Li et al., 2019). The regular leaf arrangement broadleaf effectively reduces porosity in the canopy, and self-shading by neighbouring leaves can be mitigated by changing the PL. The proportion of leaf biomass in the petiole is independent of leaf size, suggesting that biomass investment in the petiole is determined by spacing rather than support requirements (Perez et al., 2018; Wang et al., 2019). The PL-LL relationship was shown to vary proportionally during seasonal changes (Fig. 4). This demonstrates the generality of the overall balance between the cost of adaptation to light capture and support for petiole growth, and perhaps the role of convergent evolution, with plants conforming to this relationship in most environments. For most broad-leafed woody plants, this is a stable mechanism regardless of lamina size or mass. This study shows that the change in the effect size of seasonal advancement increases and then decreases. The equilibrium in spring (leaf growth period) is skewed toward increasing PL, implying that plants need to increase light capture efficiency by rapidly elongating petioles, while providing stronger support against wind (Vogel, 2009). The balance in summer (leaf stabilisation period) is biased towards increasing LL, where plants can better accumulate nutrients for their own growth and development. Shrubs are more biased towards decreasing PL than trees at this time, as a way of reducing overexposure to high UV light. Trees can allocate more biomass to lamina elongation due to self-shade. The equilibrium in fall (leaf decline period) was more biased towards increasing PL and decreasing biomass allocation to LL than in spring, and the increase in support cost would make the wind force more pronounced and accelerate leaf dieback. In addition in this study, the growth pattern of the LA-PL/LL relationship was found to be the same as that of PL-LL, which indicated that lamina growing parallel and perpendicularly to the petiole had the same effect on petiole growth, which could be the result of adaptation to the environment during the plant evolution, which could be the result of adaptation to the environment during convergent evolution in plants, leading to a reduction in the dependence between lamina and petiole traits. LA did not have an additional additive effect on PL, and for arboreal species, LA exerted a significant negative correlation on PL-LL (Fig. 6), an anisotropic growth relationship that demonstrated the inability of the plant's increased investment in laminae to keep up with the increased cost of petioles (Li et al., 2008), and an investment strategy that further increased this distributional discrepancy as the season progressed, leading to leaf abscission. This allocation pattern was also found in monocotyledonous species (Fig. 8). Compound-leaved species had the highest growth rates in the spring, probably because compound-leaved species are less efficiently supported, and petioles need more space to arrange leaflets along the axis and so have a greater mass fraction (Givnish, 1978).
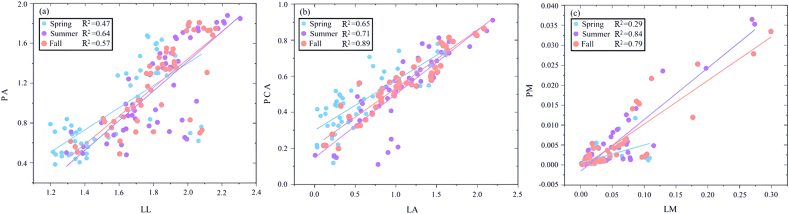
Fig. 4.
Three-dimensional Meta-analysis of woody plants types in different seasons. Length dimension (a), area dimension (b), and mass dimension (c).
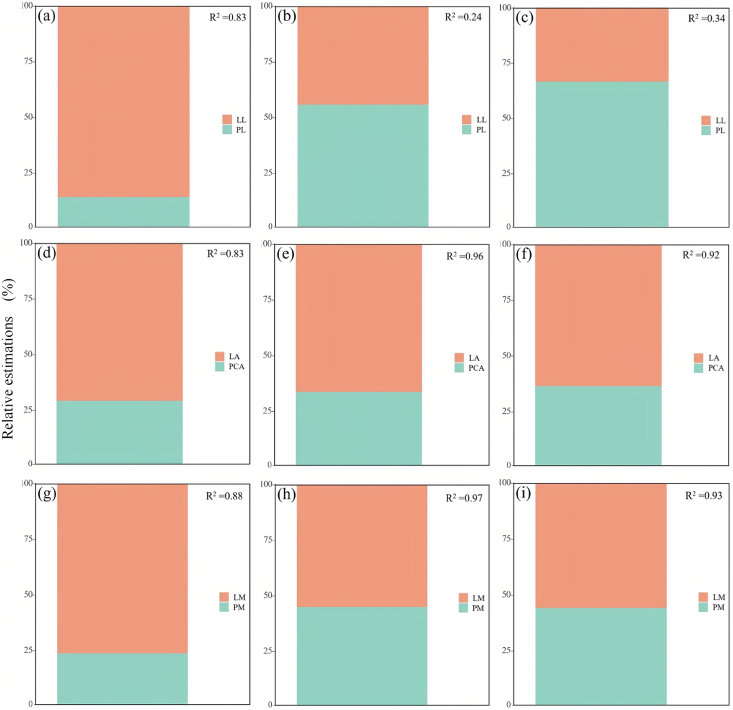
Fig. 8.
Three-dimensional partial correlation analysis of all species, vegetation types, and leaf types in different seasons. Partial correlation analysis of lamina area and petiole area under the influence of fixed LM (LA-PCA), fixed LA impact (LL-PL), fixed LA impact (LM-PM). Black, red and blue for spring, summer and fall respectively. (a): All species; (b): Arbor species; (c): Shrub species; (d): Monocotyledonous species; (e): Dipterocarp species.
On the area scale, according to the “pipeline model theory” (Shinozaki et al., 1964), the PCA increases proportionally with the increase of LA. However, the present study, contrary to the “pipeline model theory”, may be due to the fact that the petiole, in addition to supporting static loads and resisting dynamic loads from wind (Anten et al., 2010), is also used for protection and transport of nutrients, etc., so that the PCA actually represents the proportion of all the different types of tissue in the petiole. However, the proportions of these tissues in petioles are different and vary considerably between species or habitats (Givnish 2002; Taha and Malik 2012; Maiti et al., 2016). This may lead to an anisotropic growth relationship between PCA and LA, with greater changes occurring in PCA than in LA possibly due to a concomitant increase in the proportion of other tissues allocated to PCA as LA increases (Klepsch et al., 2016). Secondly, the xylem in the petiole consists of ducts of different functions and sizes. According to Hagen–Poiseuille's law (Niklas et al., 2009; Gebauer et al., 2019), small conduits of high density usually occupy more area than large conduits of low density, but large conduits of low density tend to transport more water than small conduits of high density due to lower resistance to water transport (Lintunen and Kalliokoski 2010). As a result, petioles of large leaves usually develop a small number of large ducts, which would be more in line with distributional requirements, which would weaken the dependence of PCA on LA. The balance is more biased toward increasing PCA in spring than in summer and autumn, probably because in spring the plant needs to ensure the survival of newborn laminae more than photosynthesis, and the large investment in petioles ensures that the laminae develop successfully under the stress of the external environment. Especially for shrub species, which are less able to cope with external environmental changes, more biomass needs to be allocated to PCA for subsequent life compared to tree species (Fig. 8). Recent studies have found that if the photosynthetic rate of laminae is independent of LA and the photosynthesis of petioles is negligible, then there should be a linear relationship between total carbon gain and LA. Therefore, it can be inferred that the relationship between LA and LM will be curvilinear (Lin et al., 2020).
On the quality scale, the usual reports found a positive correlation between PM and LM, suggesting that the proportion of petiole investment increases with LM (Li et al., 2008; Fan et al., 2017; Levionnois et al., 2020). The relationship between LM and PM may have achieved evolutionary stability, excessive biomass investment in petioles is destined to reduce the resources available for lamina development, and if too much investment is made in laminae, this will increase the risk of premature leaf drop due to the lack of adequate support structures (Yoshinaka et al., 2018). This is supported by the results of this study, where the relationship between LM and PM tends to stabilise under seasonal variation. However, this relationship was found to be positively heterogeneous in some studies, and as LM increases, PM will require more biomass increase than LM, and larger leaves must be invested more in the petiole than smaller leaves because larger leaves will suffer greater static loads on the petiole and greater dynamic loads on the surface resistance (Bal et al., 2011). Therefore, we can assume that the PM-LM anisotropic growth relationship is mainly caused by LA. The “diminishing returns” hypothesis suggests that the average value of the proportionality index controlling the relationship between LA and LM is less than 1 (Niklas and Enquist 2001), indicating that the increase in LA cannot keep up with the increase in LM. In the present study, it was found that the growth pattern of the plant under seasonal variation is in agreement with the hypothesis. This implies that plants have to provide different proportions of higher biomass investment to meet support requirements as LA increases, which explains the disproportionate increase in petiole mass relative to the lamina area and mass of the lamina. Particularly in the strong winds and freezing air of spring, plants need to make high investments in support structures such as petioles, which is consistent with the results of other studies (Anten et al., 2010; Louf et al., 2018). However, a negative correlation of LA-PM/LM was observed in summer and autumn, which implies that LA has a significantly lower impact on PM in summer and autumn, and this change is consistent with the growth strategy of the plant, where an increase in LM represents the maximum photosynthetic efficiency that the plant can carry out, storing the energy to get through the winter season while satisfying the plant's growth and development. In autumn, plants will accelerate leaf abscission by decreasing their investment in PM, which is consistent with the trade-offs that plants exhibit in different environments (Fig. 8).
In this study, most of the woody plants in spring will allocate biomass to the petiole, especially in the cross-sectional area. This is beneficial for the protection of newborn laminae; increased xylem will make leaves less susceptible to wind forces that can cause breakage, and smaller leaves reduce gnawing by phytophagous animals. During the summer months, plants will invest more in lamina length and area, but will prefer to increase area to improve light capture rather than elongate laminae. There is no point in producing robust, valuable, and well-protected leaves in fall for deciduous species that need to cope with harsh winter habitats (van Ommen Kloeke et al., 2012), when more biomass should be allocated to petiole elongation, while reducing xylem density, thus increasing the risk of leaf dieback. Tree species, with sturdy branchlets that can alter leaf spreading capacity and orientation, are less dependent on petioles in comparison and thus will increase the proportion of allocation to lamina. In terms of investment in petioles and laminae, the life history strategy of shrub species will more carefully trade off biophysical constraints and coupling functions between laminae and petioles, with their shorter petioles driving biomass allocation to laminae.
The PL-LL relationship was negatively correlated for single leaves and positively correlated for compound leaves. This may be due to the fact that compound leaves are composed of petiolules and leaflets. Petiolules extend the length and width of leaflets, helping to increase light interception by the lamina or leaflet while improving transport efficiency (Xu et al., 2009). However, due to the increase in petiole bending and torsional moments, the extension of the length and width of the compound lamina leads to a simultaneous increase in the static loads on the petiole and the dynamic loads on the lamina, which requires amount of leaf biomass to be allocated to the petiole. However, this allocation of greater investment in petioles found in compound leaves does not indicate that this strategy is detrimental to plant survival and reproduction, as compound-leaved plants have their own unique survival advantages. Monocotyledons have to invest more in twigs in order to meet their support requirements, but twigs are more costly because they are not only used for elongation, but additionally require diameter growth to support a wider canopy. Petioles in compound-leaved plants are a substitute for twigs to some extent, but the cost of petioles would be lower for twigs (Niklas, 1991). At the same time, we did not find variability in PCA-LA and PM-LM relationships between monocotyledon and dipterocotyledonous species, implying that changes in leaf trade-offs between plant protection and support costs and nutrient transport are independent of leaf form in leaf biomass allocation strategies.
In conclusion, our study illustrates the importance of interpreting plant functional trade-offs from a leaf perspective and provides a new perspective for explaining global patterns of leaf change. Petiole–lamina relationships may be significantly influenced by environmental change, vegetation type, and leaf form. We identified evidence for trade-offs between heterochronic growth among leaf functional traits in temperate woody plants along the seasons, with the effect of the petiole–lamina relationship being the smallest in the area dimension under spring, suggesting that the plant prefers to enhance lamina support and protection. The effect of all three dimensions of the relationship being the highest under summer, suggesting the importance of the plant's increased efficiency of light trapping and nutrient transport under suitable environmental conditions. Under autumn, the effects of petiole–lamina relationships decreased, reflecting the tendency of plants to selfwither while satisfying the basic energy supply of leaves. We also found that leaf traits cause multiple effects on petiole traits, suggesting a complex mechanism between functional traits. We understand the unique seasonal adaptive strategies of temperate forest woody plants during evolution in terms of allometric growth ratios between lamina and petiole traits, and we need closer studies to determine the extent to which this convergent evolution among plants is universal.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
CRediT authorship contribution statement
Wenjie Guo: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Lu Gong: Writing – review & editing, Software, Project administration, Funding acquisition, Conceptualization. Yan Luo: Writing – review & editing, Visualization, Project administration. Qian Guo: Validation, Software.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by The Third Comprehensive Scientific Investigation Project in Xinjiang (2021XJKK0900). The authors would like to thank all those who participated in the field collection of samples for their contribution to this study.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Contributor Information
Wenjie Guo, Email: 986135346@qq.com.
Lu Gong, Email: gonglu721@163.com.
References
- Anten N.P., Alcala H.R., Schieving F., et al. Wind and mechanical stimuli differentially affect leaf traits in plantago major. New Phytol. 2010;188:554–564. doi: 10.1111/j.1469-8137.2010.03379.x. [DOI] [PubMed] [Google Scholar]
- Anten N.P., Von W.E.J., Pawlowski M., et al. Interactive effects of spectral shading and mechanical stress on the expression and costs of shade avoidance. Am. Nat. 2009;173:241–255. doi: 10.1086/595761. [DOI] [PubMed] [Google Scholar]
- Bal K.D., Bouma T.J., Buis K., et al. Trade-off between drag reduction and light interception of macrophytes: comparing five aquatic plants with contrasting morphology. Funct. Ecol. 2011;25:1197–1205. [Google Scholar]
- Brouat C., Gibernau M., Amsellem L., et al. Corner's rules revisited: ontogenetic and interspecific patterns in leaf–stem allometry. New Phytol. 1998;139:459–470. [Google Scholar]
- Falster D.S., Westoby M. Leaf size and angle vary widely across species: what consequences for light interception? New Phytol. 2003;158:509–525. doi: 10.1046/j.1469-8137.2003.00765.x. [DOI] [PubMed] [Google Scholar]
- Fan Z.X., Sterck F., Zhang S.B., et al. Tradeoff between stem hydraulic efficiency and mechanical strength affects leaf–stem allometry in 28 ficus tree species. Front. Plant Sci. 2017;8:1619. doi: 10.3389/fpls.2017.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filartiga A.L., Klimes A., Altman J., et al. Comparative anatomy of leaf petioles in temperate trees and shrubs. Ann. Bot. 2021;129:567–582. doi: 10.1093/aob/mcac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrestel E.J., Ackerly D.D., Emery N.C. The joint evolution of traits and habitat: ontogenetic shifts in leaf morphology and wetland specialization in lasthenia. New Phytol. 2015;208:949–959. doi: 10.1111/nph.13478. [DOI] [PubMed] [Google Scholar]
- Gebauer R., Albrechtova P., Plichta R., et al. The comparative xylem structure and function of petioles and twigs of mistletoe Loranthus europaeus and its host Quercus pubescence. Trees. 2019;33:933–942. [Google Scholar]
- Gebauer R., Vanbeveren S.P., Volarik D., et al. Petiole and leaf traits of poplar in relation to parentage and biomass yield. For. Ecol. Manag. 2016;362:1–9. [Google Scholar]
- Givnish T. Tropical Trees as Living Systems. Cambridge University Press; Cambridge: 1978. On the adaptive significance of compound leaves, with special reference to tropical trees. [Google Scholar]
- Givnish T.J. Ecological constraints on the evolution of plasticity in plants. Evol. Ecol. 2002;16:213–242. [Google Scholar]
- Gleason S.M., Blackman C.J., Gleason S.T., et al. Vessel scaling in evergreen angiosperm leaves conforms with murray's law and area-filling assumptions: implications for plant size, leaf size and cold tolerance. New Phytol. 2018;218:1360–1370. doi: 10.1111/nph.15116. [DOI] [PubMed] [Google Scholar]
- Klepsch M., Lange A., Angeles G., et al. The hydraulic architecture of petioles and leaves in tropical fern species under different levels of canopy openness. Int. J. Plant Sci. 2016;177:209–216. [Google Scholar]
- Levionnois S., Coste S., Nicolini E., et al. Scaling of petiole anatomies, mechanics and vasculatures with leaf size in the widespread Neotropical pioneer tree species Cecropia obtusa Trécul (Urticaceae) Tree Physiol. 2020;40:245–258. doi: 10.1093/treephys/tpz136. [DOI] [PubMed] [Google Scholar]
- Li G., Yang D., Sun S. Allometric relationships between lamina area, lamina mass and petiole mass of 93 temperate woody species vary with leaf habit, leaf form and altitude. Funct. Ecol. 2008;22:557–564. [Google Scholar]
- Li L., Zhang T., Zhao C., et al. Leaf and stem traits variation of stellera chamaejasme linn. with slope aspect in alpine steppe. Ecol. Res. 2019;34:119–126. [Google Scholar]
- Li Y., Kang X., Zhou J., et al. Geographic variation in the petiole–lamina relationship of 325 eastern Qinghai–Tibetan woody species: analysis in three dimensions. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.748125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Niklas K.J., Wan Y., et al. Leaf shape influences the scaling of leaf dry mass vs. area: a test case using bamboos. Ann. For. Sci. 2020;77:1–15. [Google Scholar]
- Lintunen A., Kalliokoski T. The effect of tree architecture on conduit diameter and frequency from small distal roots to branch tips in Betula pendula, Picea abies and Pinus sylvestris. Tree Physiol. 2010;30:1433–1447. doi: 10.1093/treephys/tpq085. [DOI] [PubMed] [Google Scholar]
- Louf J.F., Nelson L., Kang H., et al. How wind drives the correlation between leaf shape and mechanical properties. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-34588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk C.H., Grierson E.R., Laughlin D.C. Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency. New Phytol. 2019;223:1319–1327. doi: 10.1111/nph.15849. [DOI] [PubMed] [Google Scholar]
- Maiti R., Rodriguez H.G., Manue J.J., et al. Comparative petiole anatomy of 36 woody plant species in northeastern Mexico and its significance in taxonomy and adaptation. Int. J. Bio-resour. Stress Manage. 2016;7:350–360. [Google Scholar]
- Milla R., Reich P.B. The scaling of leaf area and mass: the cost of light interception increases with leaf size. Proc. Biol. Sci. 2007;74:2109–2115. doi: 10.1098/rspb.2007.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U. Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs. Plant Ecol. 1998;134:1–11. [Google Scholar]
- Niinemets U., Kull O. Biomass investment in leaf lamina versus lamina support in relation to growth irradiance and leaf size in temperate deciduous trees. Tree Physiol. 1999;19:349–358. doi: 10.1093/treephys/19.6.349. [DOI] [PubMed] [Google Scholar]
- Niinemets U., Portsmuth A., Tena D., et al. Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann. Bot. 2007;100:283–303. doi: 10.1093/aob/mcm107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U., Portsmuth A., Tobias M. Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol. 2006;171:91–104. doi: 10.1111/j.1469-8137.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- Niinemets U., Portsmuth A., Tobias M. Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: a neglected source of leaf physiological differentiation? Funct. Ecol. 2007;21:28–40. [Google Scholar]
- Niinemets U., Sack L. Structural determinants of leaf light-harvesting capacity and photosynthetic potentials. Prog. Bot. 2006;67:385–419. [Google Scholar]
- Niklas K.J. Flexural stiffness allometries of angiosperm and fern petioles and rachises: evidence for biomechanical convergence. Evolution. 1991;45:734–750. doi: 10.1111/j.1558-5646.1991.tb04342.x. [DOI] [PubMed] [Google Scholar]
- Niklas K.J., Cobb E.D., Niinemets U., et al. “Diminishing returns” in the scaling of functional leaf traits across and within species groups. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8891–8896. doi: 10.1073/pnas.0701135104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas K.J., Cobb E.D., Spatz H.C. Predicting the allometry of leaf surface area and dry mass. Am. J. Bot. 2009;96:531–536. doi: 10.3732/ajb.0800250. [DOI] [PubMed] [Google Scholar]
- Niklas K.J., Enquist B.J. Invariant scaling relationships for interspecific plant biomass production rates and body size. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2922–2927. doi: 10.1073/pnas.041590298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktavia D., Jin G. Variations in leaf morphological and chemical traits in response to life stages, plant functional types, and habitat types in an old-growth temperate forest. Basic Appl. Ecol. 2020;49:22–33. [Google Scholar]
- Osnas J.L.D., Lichstein J.W., Reich P.B., et al. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science. 2013;340:741–744. doi: 10.1126/science.1231574. [DOI] [PubMed] [Google Scholar]
- Perez R.P., Dauzat J., Pallas B., et al. Designing oil palm architectural ideotypes for optimal light interception and carbon assimilation through a sensitivity analysis of leaf traits. Ann. Bot. 2018;121:909–926. doi: 10.1093/aob/mcx161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup M., Westoby M., Basden A. Dry mass costs of deploying leaf area in relation to leaf size. Funct. Ecol. 2005;19:88–97. [Google Scholar]
- Poorter L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol. 2009;181:890–900. doi: 10.1111/j.1469-8137.2008.02715.x. [DOI] [PubMed] [Google Scholar]
- Roig V.I., Martinez G.J.F. Plant responses to vegetation proximity: a whole life avoiding shade. Front. Plant Sci. 2016;7:236. doi: 10.3389/fpls.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L., Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. [DOI] [PubMed] [Google Scholar]
- Sarlikioti V., De V.P., Marcelis L. Exploring the spatial distribution of light interception and photosynthesis of canopies by means of a functional–structural plant model. Ann. Bot. 2011;107:875–883. doi: 10.1093/aob/mcr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Yoda K., Hozumi K., et al. A quantitative analysis of plant form-the pipe model theory: I. Basic analyses. Jpn. J. Ecol. 1964;14:97–105. [Google Scholar]
- Smith V., Ennos A. The effects of air flow and stem flexure on the mechanical and hydraulic properties of the stems of sunflowers Helianthus annuus L. J. Exp. Bot. 2003;54:845–849. doi: 10.1093/jxb/erg068. [DOI] [PubMed] [Google Scholar]
- Song J.H., Hong S.P. Comparative petiole anatomy of the tribe Sorbarieae (Rosaceae) provide new taxonomically informative characters. Nord. J. Bot. 2018;36 [Google Scholar]
- Sun J., Fan R., Niklas K.J., et al. “Diminishing returns” in the scaling of leaf area vs. dry mass in Wuyi mountain bamboos, southeast China. Am. J. Bot. 2017;104:993–998. doi: 10.3732/ajb.1700068. [DOI] [PubMed] [Google Scholar]
- Sun S., Jin D., Shi P. The leaf size–twig size spectrum of temperate woody species along an altitudinal gradient: an invariant allometric scaling relationship. Ann. Bot. 2006;97:97–107. doi: 10.1093/aob/mcj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha Y.A.E., Malik S.A. Taxonomic significance of anatomical characters in some species of the family Myrtaceae. Am. J. Plant Sci. 2012;5:572–581. [Google Scholar]
- Tsukaya H. Leaf shape: genetic controls and environmental factors. Int. J. Dev. Biol. 2005;49:547–555. doi: 10.1387/ijdb.041921ht. [DOI] [PubMed] [Google Scholar]
- Tsukaya H., Kozuka T., Kim G.T. Genetic control of petiole length in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:1221–1228. doi: 10.1093/pcp/pcf147. [DOI] [PubMed] [Google Scholar]
- van Ommen Kloeke, A.E.E., Douma J.C., Ordonez J.C., et al. Global quantification of contrasting leaf life span strategies for deciduous and evergreen species in response to environmental conditions. Global Ecol. Biogeogr. 2012;21:224–235. [Google Scholar]
- Vogel S. Leaves in the lowest and highest winds: temperature, force and shape. New Phytol. 2009;183:13–26. doi: 10.1111/j.1469-8137.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- Wang M., Jin G., Liu Z. Variation and relationships between twig and leaf traits of species across successional status in temperate forests. Scand. J. For. Res. 2019;34:647–655. [Google Scholar]
- Wang Z., White J., Hockaday W. The molecular composition of leaf lipids changes with seasonal gradients in temperature and light among deciduous and evergreen trees in a sub-humid ecosystem. Org. Geochem. 2023;187 [Google Scholar]
- Westoby M., Wright I.J. The leaf size–twig size spectrum and its relationship to other important spectra of variation among species. Oecologia. 2003;135:621–628. doi: 10.1007/s00442-003-1231-6. [DOI] [PubMed] [Google Scholar]
- Xu F., Guo W., Xu W., et al. Leaf morphology correlates with water and light availability: what consequences for simple and compound leaves? Prog. Nat. Sci. 2009;19:1789–1798. [Google Scholar]
- Yoshinaka K., Nagashima H., Yanagita Y., et al. The role of biomass allocation between lamina and petioles in a game of light competition in a dense stand of an annual plant. Ann. Bot. 2018;121:1055–1064. doi: 10.1093/aob/mcy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bi G., Li T., et al. Color shade nets affect plant growth and seasonal leaf quality of Camellia sinensis grown in Mississippi, the United States. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.786421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M., Castro D.P., Puyravaud J.P., et al. Convergent xylem widening among organs across diverse woody seedlings. New Phytol. 2019;222:1873–1882. doi: 10.1111/nph.15734. [DOI] [PubMed] [Google Scholar]
- Zhu G., Niklas K.J., Li M., et al. “Diminishing returns” in the scaling between leaf area and twig size in three forest communities along an elevation gradient of Wuyi mountain, China. Forests. 2019;10:1138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.