Abstract
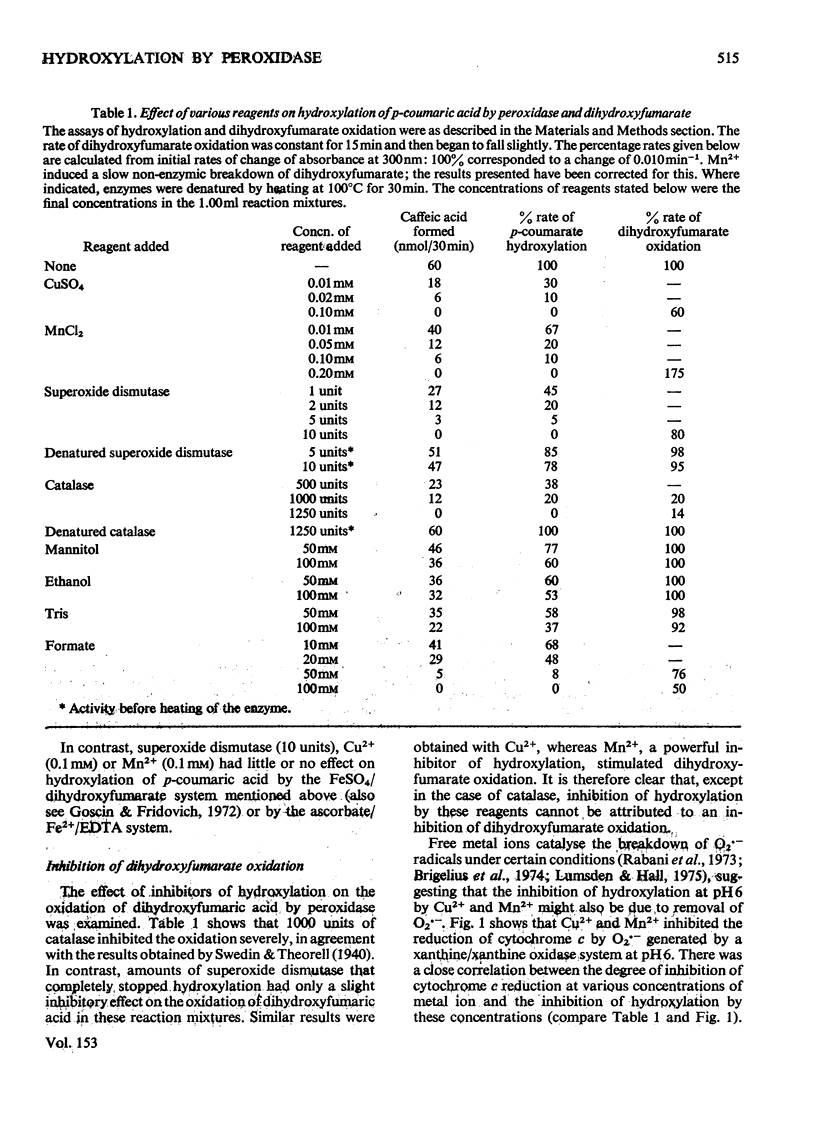
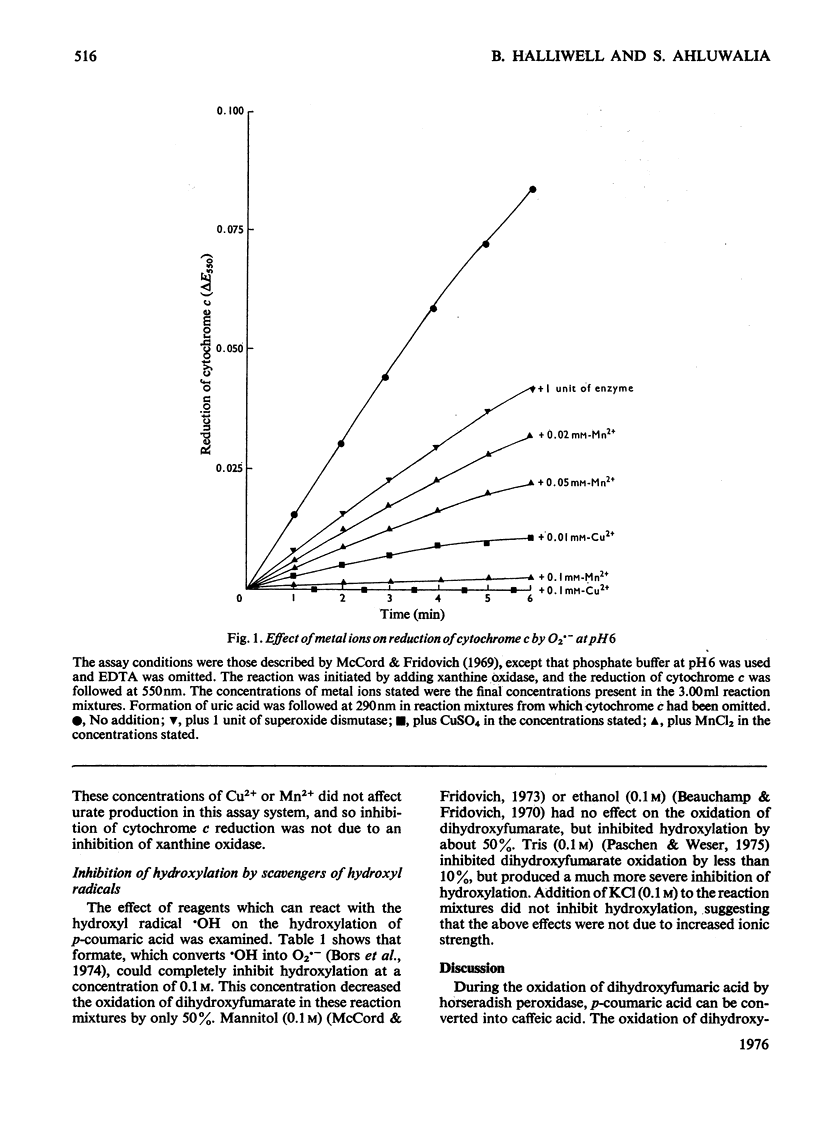
1. In the presence of dihydroxyfumarate, horseradish peroxidase catalyses the conversion of p-coumaric acid into caffeic acid at pH 6. This hydroxylation is completely inhibited by superoxide dismutase. 2. Dihydroxyfumarate cannot be replaced by ascorbate H2O2, NADH, cysteine or sulphite. Peroxidase can be replaced by high (10 mM) concentrations of FeSO4, but this reaction is almost unaffected by superoxide dismutase. 3. Hydroxylation by the peroxidase/dihydroxyfumarate system is completely inhibited by low concentrations of Mn2+ or Cu2+. It is proposed that this is due to the ability of these metal ions to react with the superoxide radical O2--. 4. Hydroxylation is partially inhibited by mannitol, Tris or ethanol and completely inhibited by formate. This seems to be due to the ability of these reagents to react with the hydroxyl radical -OH. 5. It is concluded that O2-- is generated during the oxidation of dihydroxyfumarate by peroxidase and reacts with H2O2 to produce hydroxyl radicals, which then convert p-coumaric acid into caffeic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Spöttl R., Michel C. The relevance of the superoxide anion radical in biological systems. Curr Top Radiat Res Q. 1974 May;9(3):247–309. [PubMed] [Google Scholar]
- Brigelius R., Spöttl R., Bors W., Lengfelder E., Saran M., Weser U. Superoxide dismutase activity of low molecular weight Cu2 plus-chelates studied by pulse radiolysis. FEBS Lett. 1974 Oct 1;47(1):72–75. doi: 10.1016/0014-5793(74)80428-x. [DOI] [PubMed] [Google Scholar]
- CHANCE B. Oxidase and peroxidase reactions in the presence of dihydroxymaleic acid. J Biol Chem. 1952 May;197(2):577–589. [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M. Aerobic aromatic hydroxylation catalyzed by horseradish peroxidase: absense of NIH shift. Biochim Biophys Acta. 1970 May 12;208(2):340–342. doi: 10.1016/0304-4165(70)90256-4. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Goscin S. A., Fridovich I. The role of superoxide radical in a nonenzymatic hydroxylation. Arch Biochem Biophys. 1972 Dec;153(2):778–783. doi: 10.1016/0003-9861(72)90398-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Hydroxylation of p-Coumaric acid by illuminated chloroplasts. The role of superoxide. Eur J Biochem. 1975 Jul 1;55(2):355–360. doi: 10.1111/j.1432-1033.1975.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide dismutase: a contaminant of bovine catalase. Biochem J. 1973 Oct;135(2):379–381. doi: 10.1042/bj1350379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. Z., Shen J. T., Ganong W. F. Evidence for the involvement of superoxide anion in dopamine-beta-hydroxylase system. Proc Soc Exp Biol Med. 1974 May;146(1):37–40. doi: 10.3181/00379727-146-38038. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Hall D. O. Chloroplast manganese and superoxide. Biochem Biophys Res Commun. 1975 May 19;64(2):595–602. doi: 10.1016/0006-291x(75)90363-0. [DOI] [PubMed] [Google Scholar]
- Matheson I. B., Etheridge R. D., Kratowich N. R., Lee J. The quenching of singlet oxygen by amino acids and proteins. Photochem Photobiol. 1975 Mar;21(3):165–171. doi: 10.1111/j.1751-1097.1975.tb06647.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Production of O2- in photolyzed water demonstrated through the use of superoxide dismutase. Photochem Photobiol. 1973 Feb;17(2):115–121. doi: 10.1111/j.1751-1097.1973.tb06340.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- NAIR P. M., VAIDYANATHAN C. S. A COLORIMETRIC METHOD FOR DETERMINATION OF PYROCATECHOL AND RELATED SUBSTANCES. Anal Biochem. 1964 Mar;7:315–321. [PubMed] [Google Scholar]
- Paschen W., Weser U. Letter: Singlet oxygen decontaminating activity of erythrocuprein (superoxide dismutase). Biochim Biophys Acta. 1973 Nov 15;327(1):217–222. doi: 10.1016/0005-2744(73)90120-4. [DOI] [PubMed] [Google Scholar]
- Paschen W., Weser U. Problems concerning the biochemical action of superoxide dismutase (erythrocuprein). Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):727–737. doi: 10.1515/bchm2.1975.356.s1.727. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Falcioni G., Fioretti E., Brunoir M. Decay of oxyperoxidase and oxygen radicals; a possible role for myeloperoxidase. Biochem J. 1975 Feb;145(2):405–407. doi: 10.1042/bj1450405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligar S. G., Lipscomb J. D., Debrunner P. G., Gunsalus I. C. Superoxide anion production by the autoxidation of cytochrome P450cam. Biochem Biophys Res Commun. 1974 Nov 6;61(1):290–296. doi: 10.1016/0006-291x(74)90565-8. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., CLARK C. T., AXELROD J., BRODIE B. B. Ascorbic acid in aromatic hydroxylation. I. A model system for aromatic hydroxylation. J Biol Chem. 1954 Jun;208(2):731–739. [PubMed] [Google Scholar]
- YAMAZAKI I., PIETTE L. H. THE MECHANISM OF AEROBIC OXIDASE REACTION CATALYZED BY PEROXIDASE. Biochim Biophys Acta. 1963 Sep 3;77:47–64. doi: 10.1016/0006-3002(63)90468-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Yamazaki I. The reaction between indole 3-acetic acid and horseradish peroxidase. Arch Biochem Biophys. 1973 Jan;154(1):147–159. doi: 10.1016/0003-9861(73)90043-x. [DOI] [PubMed] [Google Scholar]


