Abstract
Background:
Acute ischemic stroke (AIS) is a prevalent and challenging neurological condition associated with high mortality and morbidity rates. This study aimed to evaluate the therapeutic efficacy of repetitive transcranial magnetic stimulation (rTMS) combined with ginkgo diterpene lactone meglumine injection (GDLMI) on cognitive and neurological function recovery in patients with AIS.
Methods:
A total of 120 patients with AIS, admitted between January 2021 and January 2022, received rTMS combined with GDLMI after admission. Their cognitive and neurological functions were assessed using the Chinese version of the Montreal Cognitive Assessment (MoCA) and the National Institute of Health Stroke Scale (NIHSS) respectively before and after treatment. Additionally, serum levels of lipoprotein-associated phospholipase A2 (Lp-PLA2) and ischemia-modified albumin (IMA) were quantified. Statistical analyses were performed to elucidate potential correlations between Lp-PLA2 and IMA levels and clinical outcomes.
Results:
After treatment, patients with AIS exhibited significantly improved cognitive and neurological functions, increased MoCA score and decreased NIHSS score compared to those before treatment (p < 0.05). A linear correlation was observed between Lp-PLA2 and IMA levels and the recovery of cognitive function in AIS patients (r = –0.892/–0.764, p < 0.05). Before and after factor adjustment, Lp-PLA2 and IMA were identified as independent influencing factors for the efficiency in cognitive function recovery (p < 0.05). Similarly, Lp-PLA2 and IMA levels were linearly correlated with the recovery of neurological function in AIS patients (r = –0.887/–0.796, p < 0.05). Lp-PLA2 combined with IMA performed better than Lp-PLA2 or IMA alone in predicting the efficiency of rTMS plus GDLMI in promoting the cognitive and neurological function recovery (p < 0.05).
Conclusions:
rTMS combined with GDLMI can contribute to the cognitive and neurological function recovery in patients with AIS. Serum levels of Lp-PLA2 and IMA could serve as independent influencing factors for the efficiency in promoting cognitive and neurological function recovery.
Keywords: repetitive transcranial magnetic stimulation, ginkgo diterpene lactone meglumine injection, acute ischemic stroke, lipoprotein-associated phospholipase A2, ischemia-modified albumin
Introduction
Acute ischemic stroke (AIS) is a clinically common intractable disease with high mortality and morbidity rates. It has been found that AIS is often accompanied by varying degrees of cognitive and neurological impairment [1]. Consequently, early intervention is crucial for optimizing the recovery of cognitive and neurological functions. Ginkgo diterpene lactone meglumine injection (GDLMI) is a commonly used therapeutic agent in the treatment of AIS. It has been shown to facilitate the recovery of patients’ cognitive and neurological functions by attenuating the progression of brain injury [2]. However, clinical observations indicate that GDLMI alone demonstrates limited efficacy in clinical use. Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive, well-tolerated, and efficacious cortical stimulation therapy that has demonstrated therapeutic potential in the treatment of AIS [3]. Nevertheless, the synergistic effects of rTMS combined with GDLMI on the improvement of cognitive and neurological functions of AIS patients remain largely unexplored. Moreover, the prognostic trajectory of these patients is significantly affected by the initial severity of the disease.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) has been demonstrated to promote atherosclerosis, and its association with cognitive and neurological impairment in AIS has been verified [4]. rTMS has been shown to markedly modulate Lp-PLA2 activity [5]. Furthermore, Lp-PLA2 may serve as a biomarker for sub-acute stroke patients receiving treatment with GDLMI [6]. Elevated levels of ischemia-modified albumin (IMA), a kind of serum albumin, are frequently indicative of AIS progression [7]. However, the influence of rTMS or GDLMI on IMA expression remains to be elucidated.
This study aimed to elucidate the potential relationship between the combined application of rTMS and GDLMI and the levels of Lp-PLA2 and IMA in the context of cognitive and neurological function recovery in patients with AIS. The investigation sought to provide valuable clinical evidence for future treatment.
Materials and Methods
Subjects
The study received ethical approval (No. JSXZH2021403) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants before enrollment. The study cohort comprised 120 patients with AIS who were treated at Xuzhou Central Hospital between January 2021 and January 2022. They included 68 males and 52 females, with a mean age of 56.84 5.19 years. Inclusion criteria were as follows: meeting the clinical diagnostic criteria for AIS [8]; experiencing first-onset AIS; voluntary participation and provision of written informed consent. Exclusion criteria encompassed: comorbid cerebral hemorrhage, massive cerebral infarction, transient ischemic attack, mental illness or benign/malignant tumors, and impaired communication abilities.
rTMS Combined with GDLMI
Patients received rTMS combined with GDLMI immediately after hospitalization. rTMS was administered using NS5000 transcranial magnetic stimulator (Wuhan Yiruide Medical Equipment New Technology Co., Ltd., Wuhan, China). The stimulation coil was placed over the dorsolateral region of the left frontal lobe, with the nasal-occipital line on the locating cap in the median head, and the CZ point at the midpoint of the connection between the posterior occipital tuberosity and the eyebrow center. The coil was tangent to the skull surface, with the stimulation focus centered at the intersection of the two circles. Stimulation parameters were set at 10 Hz, with 3 s/time with an interval of 30 s, for a total duration of 20 min. GDLMI treatment consisted of a daily intravenous injection of 5 mL GDLMI (Cat. No. Z20120024; Jiangsu Kanion Pharmaceutical Co., Ltd., Lianyungang, China) diluted with 250 mL of 0.9% sodium chloride. The combined treatment was administered for 14 consecutive days.
Assessment of Cognitive Function
Cognitive function assessment was conducted before and after treatment by two independent, qualified, and experienced evaluators using the validated Chinese version of the Montreal Cognitive Assessment (MoCA). The MoCA comprised 11 items across 8 domains, including executive function, attention and concentration, and language, among others [9]. The score ranges from 0 to 30 points, with a higher score indicating a superior cognitive function. A score 26 points is indicative of cognitive impairment. Following the administration of rTMS combined with GDLMI, participants were divided into two cohorts: a cognitive impairment group and a normal cognitive function group according to the presence or absence of cognitive impairment.
Assessment of Neurological Function
Neurological function was evaluated before and after treatment by two independent and experienced clinicians using the National Institute of Health Stroke Scale (NIHSS). This validated assessment tool comprised 11 items, including measures of consciousness and limb movement [10]. The NIHSS score ranges from 0 to 42 points, with higher scores indicating more severe neurological impairment. A score 1 is indicative of neurological impairment. Following rTMS combined with GDLMI, patients were divided into two cohorts: a neurological impairment group and a normal neurological function group according to the presence or absence of neurological impairment.
Detection of Serum Levels of Lp-PLA2 and IMA
Fasting venous blood samples (3 mL) were collected from each patient in the morning and centrifuged to obtain the serum. Serum levels of Lp-PLA2 and IMA were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen, Waltham, MA, USA, catalog number: EH304RB; MyBioSource, USA, catalog number: MBS263569). Absorbance measurements were performed using a multifunctional microplate reader (Mithras LB940, Berthold Technologies, Bad Wildbad, Germany).
Observation Indices
(1) Cognitive and neurological functions were assessed before and after treatment. (2) Demographic and clinical data were collected for four distinct groups: cognitive impairment group, normal cognitive function group, neurological impairment group, and normal neurological function group. Variables included age, sex, body mass index (BMI), medical history (hypertension, coronary heart disease, etc.), familial stroke history, smoking and alcohol consumption patterns, laboratory parameters [fasting blood glucose (FBG) and total cholesterol (TC)], duration from onset to admission and NIHSS score at admission. Comparative analyses were conducted to evaluate differences in Lp-PLA2 and IMA levels between the respective subgroups. (3) Logistic regression analysis was employed to evaluate the correlations between Lp-PLA2 and IMA levels and the efficiency of combined rTMS and GDLMI in promoting cognitive and neurological function recovery among patients with AIS. (4) The predictive efficacy of Lp-PLA2 and IMA for the efficiency of rTMS plus GDLMI in promoting cognitive and neurological function recovery in AIS patients was analyzed using receiver operating characteristic (ROC) curves.
Statistical Analysis
Statistical analysis was conducted using SPSS version 26.0 software (IBM Inc., Armonk, NY, USA). The Kolmogorov-Smirnov test was employed to assess the normality of distribution, while Levene’s test was used to evaluate the homogeneity of variance. Continuous variables with normal distribution were expressed as mean standard deviation ( s) and analyzed using one-way F analysis or independent samples t-test, as appropriate. Non-normally distributed data underwent natural logarithmic transformation and were presented as a median and interquartile range [M(Qn)], with subsequent analysis using non-parametric tests. Categorical variables were described as [n (%)] and compared between two groups using the 2 test. The correlations between Lp-PLA2 and IMA levels and the efficiency of combined rTMS and GDLMI in promoting cognitive and neurological function recovery in AIS patients were subjected to logistic regression analysis. Pearson’s correlation analysis was conducted. The predictive efficacy of Lp-PLA2 and IMA for the efficiency of the rTMS plus GDLMI in promoting cognitive and neurological function recovery in AIS patients was analyzed using ROC curves. Statistical significance was set at p 0.05. All statistical tests were conducted as two-tailed analyses, with a significance level of = 0.05.
Results
Cognitive and Neurological Functions of AIS Patients before and after rTMS Combined with GDLMI
After treatment, patients with AIS exhibited significantly improved cognitive and neurological functions, increased MoCA score and decreased NIHSS score compared to those before treatment (p 0.05) (Table 1). Among the 120 patients, 18 and 25 patients still had mild cognitive impairment and mild neurological impairment, respectively.
Table 1.
Cognitive and neurological functions of 120 AIS patients before and after rTMS combined with GDLMI [( s), point].
| Before treatment | After treatment | t | p | |
| MoCA score | 18.32 1.29 | 27.93 4.31 | 23.400 | 0.001 |
| NIHSS score | 9.32 1.19 | 2.64 0.28 | 59.860 | 0.001 |
AIS, acute ischemic stroke; rTMS, repetitive transcranial magnetic stimulation; GDLMI, ginkgo diterpene lactone meglumine injection; MoCA, Montreal Cognitive Assessment; NIHSS, National Institute of Health Stroke Scale.
General Data, Lp-PLA2 and IMA of Cognitive Impairment and Normal Cognitive Function Groups
The proportions of patients with history of hypertension and coronary heart disease, family history of stroke, and smoking and drinking history, the levels of FBG, TC, Lp-PLA2 and IMA, and the NIHSS score at admission were lower, and the duration from onset to admission was shorter in the normal cognitive function group than those in the cognitive impairment group (p 0.05) (Table 2).
Table 2.
General data, Lp-PLA2 and IMA of cognitive impairment and normal cognitive function groups.
| Cognitive impairment group (n = 18) | Normal cognitive function group (n = 102) | t/2 | p | |
| Gender (male/female) | 9/9 | 59/43 | 0.383 | 0.536 |
| Age (year) | 56.78 6.43 | 56.99 6.52 | 0.126 | 0.900 |
| BMI (kg/m2) | 23.41 2.97 | 23.50 2.86 | 0.122 | 0.903 |
| Hypertension (Yes/No) | 11/7 | 30/72 | 6.835 | 0.009 |
| Coronary heart disease (Yes/No) | 13/5 | 28/74 | 13.635 | 0.001 |
| Family history of stroke (Yes/No) | 15/3 | 12/90 | 44.942 | 0.001 |
| Smoking and drinking history (Yes/No) | 16/2 | 31/67 | 20.685 | 0.001 |
| FBG (mmol/L) | 8.65 0.43 | 6.12 0.74 | 14.060 | 0.001 |
| TC (mmol/L) | 5.64 0.74 | 4.00 0.32 | 15.720 | 0.001 |
| Duration from onset to admission (h) | 6.34 0.53 | 4.24 0.65 | 12.950 | 0.001 |
| NIHSS at admission (point) | 11.97 1.08 | 8.01 0.78 | 18.660 | 0.001 |
| Lp-PLA2 (µg/L) | 261.96 25.89 | 68.77 7.92 | 61.650 | 0.001 |
| IMA (U/mL) | 123.54 13.67 | 78.65 9.31 | 17.460 | 0.001 |
Lp-PLA2, lipoprotein-associated phospholipase A2; IMA, ischemia-modified albumin; BMI, body mass index; FBG, fasting blood glucose; TC, total cholesterol.
Correlations of Lp-PLA2 and IMA with Cognitive Function Recovery in AIS Patients
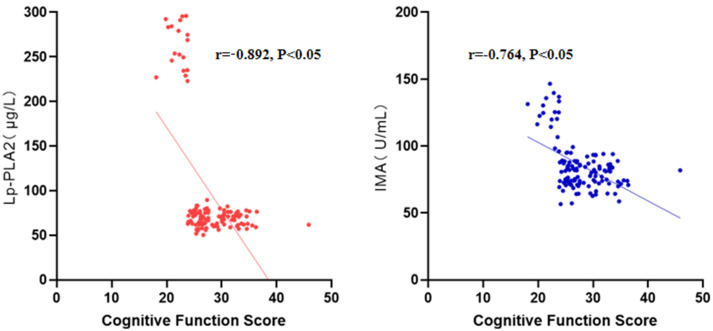
The correlation analysis revealed a statistically linear association between Lp-PLA2 and IMA levels and the recovery state of cognitive function in patients with AIS (r = –0.892/–0.764, p 0.05) (Fig. 1).
Fig. 1.
Correlations of Lp-PLA2 and IMA with cognitive function recovery in AIS patients.
Associations of Lp-PLA2 and IMA with the Efficiency of rTMS Combined with GDLMI in Promoting Cognitive Function Recovery in AIS Patients
Before and after factor adjustment, Lp-PLA2 and IMA were proven to be independent influencing factors for the efficiency of rTMS combined with GDLMI in promoting cognitive function recovery (p 0.05) (Table 3).
Table 3.
Associations of Lp-PLA2 and IMA with the efficiency of rTMS combined with GDLMI in promoting cognitive function recovery in AIS patients before and after factor adjustment.
| Before adjustment | After adjustment | |||||||||
| SE | Wald/ | OR (95% CI) | p | SE | Wald/ | OR (95% CI) | p | |||
| Lp-PLA2 | 1.083 | 0.323 | 11.242 | 2.954 (1.568–5.563) | 0.001 | 1.024 | 0.439 | 5.441 | 2.784 (1.178–6.583) | 0.020 |
| IMA | 1.437 | 0.401 | 12.842 | 4.208 (1.918–9.235) | 0.001 | 1.304 | 0.535 | 5.941 | 3.684 (1.291–10.513) | 0.015 |
The analysis was adjusted for several factors, including medical history of hypertension and coronary heart disease, family history of stroke, smoking and alcohol consumption habits, FBG, TC, duration from onset to admission, and NIHSS score at admission.
General Data, Lp-PLA2 and IMA of Neurological Impairment and Normal Neurological Function Groups
The proportions of patients with history of hypertension and coronary heart disease, family history of stroke, and smoking and drinking history, the levels of FBG, Lp-PLA2 and IMA, and the NIHSS score at admission were lower, and the duration from onset to admission was shorter in the normal neurological function group than those in the neurological impairment group (p 0.05) (Table 4).
Table 4.
General data, Lp-PLA2 and IMA of neurological impairment and normal neurological function groups.
| Neurological impairment group (n = 25) | Normal neurological function group (n = 95) | t/2 | p | |
| Gender (male/female) | 12/13 | 56/39 | 0.967 | 0.326 |
| Age (year) | 56.56 5.23 | 56.71 5.34 | 0.126 | 0.900 |
| BMI (kg/m2) | 23.14 2.51 | 23.16 2.56 | 0.034 | 0.972 |
| Hypertension (Yes/No) | 16/9 | 25/70 | 12.495 | 0.001 |
| Coronary heart disease (Yes/No) | 18/7 | 23/72 | 28.044 | 0.001 |
| Family history of stroke (Yes/No) | 10/15 | 17/78 | 5.546 | 0.019 |
| Smoking and drinking history (Yes/No) | 19/6 | 28/67 | 17.981 | 0.001 |
| FBG (mmol/L) | 8.92 1.02 | 6.43 0.52 | 16.950 | 0.001 |
| TC (mmol/L) | 4.72 0.36 | 4.77 0.45 | 0.514 | 0.609 |
| Duration from onset to admission (h) | 6.12 0.93 | 4.00 0.43 | 16.590 | 0.001 |
| NIHSS at admission (point) | 12.55 1.34 | 8.43 0.58 | 23.030 | 0.001 |
| Lp-PLA2 (µg/L) | 253.91 29.03 | 65.93 5.12 | 60.310 | 0.001 |
| IMA (U/mL) | 119.28 15.43 | 76.91 6.41 | 20.920 | 0.001 |
Correlations of Lp-PLA2 and IMA with Neurological Function Recovery in AIS Patients
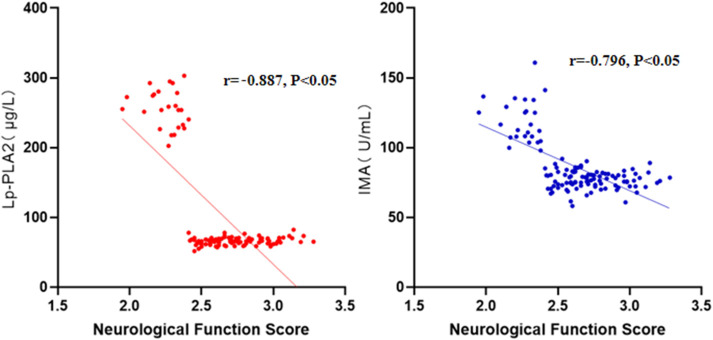
The results of correlation analysis showed that Lp-PLA2 and IMA levels were linearly correlated with the recovery state of neurological function in AIS patients (r = –0.887/–0.796, p 0.05) (Fig. 2).
Fig. 2.
Correlations of Lp-PLA2 and IMA with neurological function recovery in AIS patients.
Associations of Lp-PLA2 and IMA with the Efficiency of rTMS Combined with GDLMI in Promoting Neurological Function Recovery in AIS Patients
Before and after factor adjustment, Lp-PLA2 and IMA were demonstrated to be independent influencing factors for the efficiency of rTMS combined with GDLMI in promoting neurological function recovery (p 0.05) (Table 5).
Table 5.
Associations of Lp-PLA2 and IMA with the efficiency of rTMS combined with GDLMI in promoting neurological function recovery in AIS patients before and after factor adjustment.
| Before adjustment | After adjustment | |||||||||
| SE | Wald/2 | OR (95% CI) | p | SE | Wald/2 | OR (95% CI) | p | |||
| Lp-PLA2 | 1.145 | 0.451 | 6.446 | 3.142 (1.298–7.606) | 0.011 | 0.989 | 0.326 | 9.204 | 2.689 (1.419–5.093) | 0.002 |
| IMA | 1.443 | 0.563 | 6.569 | 4.233 (1.404–12.762) | 0.010 | 1.269 | 0.496 | 6.546 | 3.557 (1.346–9.404) | 0.011 |
The analysis was adjusted for different factors, including medical history of hypertension and coronary heart disease, family history of stroke, smoking and alcohol consumption history, FBG, duration from symptom onset to hospital admission, and NIHSS score on admission.
Predictive Efficacy of Lp-PLA2 and IMA for the Efficiency of rTMS Plus GDLMI in Promoting the Cognitive and Neurological Function Recovery in AIS Patients Using ROC Curves
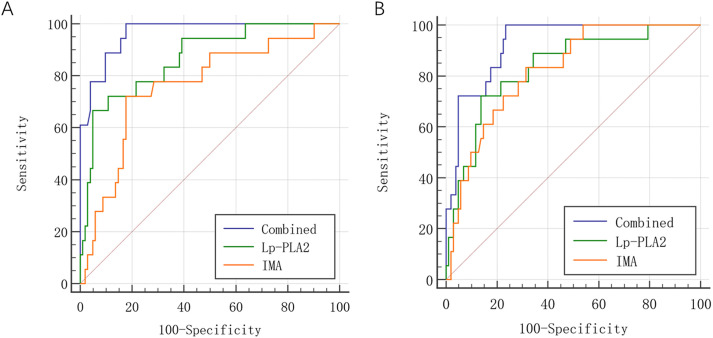
Based on ROC curve analysis, Lp-PLA2 combined with IMA performed better than Lp-PLA2 or IMA alone in predicting the efficiency of rTMS plus GDLMI in promoting the cognitive and neurological function recovery in AIS patients (p 0.05) (Table 6 and Fig. 3).
Table 6.
Predictive efficacy of Lp-PLA2 and IMA for efficiency of rTMS plus GDLMI in promoting the cognitive and neurological function recovery in AIS patients.
| Predictive efficacy for cognitive function recovery | Predictive efficacy for neurological function recovery | |||||||||
| Sensitivity (%) | Specificity (%) | Youden index | AUC | 95% CI | Sensitivity (%) | Specificity (%) | Youden index | AUC | 95% CI | |
| Lp-PLA2 | 85.50 | 81.23 | 0.67 | 0.867 | 0.735–0.998 | 83.24 | 81.27 | 0.64 | 0.825 | 0.781–0.948 |
| IMA | 79.07 | 80.12 | 0.59 | 0.776 | 0.597–0.954 | 81.19 | 80.33 | 0.62 | 0.814 | 0.762–0.927 |
| Combination | 90.90 | 83.03 | 0.74 | 0.964 | 0.900–1.020 | 86.71 | 80.11 | 0.67 | 0.921 | 0.876–1.000 |
AUC, area under the curve.
Fig. 3.
Receiver operating characteristic (ROC) curves for predictive efficacy of Lp-PLA2 and IMA for efficiency of rTMS plus GDLMI in promoting (A) the cognitive and (B) neurological function recovery in AIS patients.
Discussion
Ginkgolides are the major component of GDLMI, which can effectively inhibit platelet activating factor-induced thrombosis and platelet aggregation, alleviate ischemic brain damage through the signal transducer and activator of transcription 3 pathway, and reduce the level of oxidative stress, thereby maintaining the cognitive and neurological function recovery in AIS patients [11]. rTMS is a non-invasive and effective therapy that induces electrical currents in cerebral tissue, promoting dopamine release and enhancing cortical excitability, thereby modulating cerebral blood flow and metabolism [12, 13]. In this study, the cognitive and neurological functions of AIS patients recovered to a certain extent after treatment using rTMS combined with GDLMI, but cognitive and neurological impairment was still present in some cases. Possibly, some related indices affected the efficiency of the recovery process.
Lp-PLA2, also known as platelet-activating factor acetylhydrolase, is secreted by macrophages, lymphocytes, mast cells, and platelets, which can bind various lipoproteins dominated by low-density lipoprotein through interacting with apolipoprotein B in the blood circulation [14, 15]. Lp-PLA2 can produce lysophosphatidylcholine via hydrolyzing oxidized phospholipids, produce oxidatively modified low-density lipoproteins via oxidizing non-esterified fatty acids, and release pro-inflammatory and pro-atherosclerotic metabolites to the circulation [16]. In the pathological state, Lp-PLA2 upregulates cytokine and adhesion factor levels, thereby disrupting vascular endothelial homeostasis [17]. As a sensitive index for assessing acute ischemia time, IMA has N-terminal binding sites altered under the action of acid and reactive oxygen species, which weakens its ability to bind metal ions [18, 19, 20]. When brain tissue is under hypoxic and ischemic states, excessive free radicals are generated, followed by a cascade reaction that extends over time [20, 21, 22]. Consequently, IMA is excessively generated during the progression of AIS.
In this study, the levels of Lp-PLA2 and IMA were significantly higher in patients with neurological impairment than those in patients with normal neurological function after rTMS combined with GDLMI, and Lp-PLA2 and IMA levels were linearly correlated with the efficiency of the combined therapy in promoting neurological function recovery. These findings suggest that Lp-PLA2 and IMA are associated with the process of neurological function recovery [23]. However, the correlation between IMA and cognitive impairment in AIS remains largely unexplored. In this investigation, the potential association between Lp-PLA2 and IMA levels and cognitive function recovery was further analyzed by grouping the patients based on the improvement degree in cognitive function. The results revealed that elevated levels of Lp-PLA2 and IMA were observed in patients exhibiting cognitive impairment. Consequently, these findings suggest that Lp-PLA2 and IMA levels are correlated with cognitive function recovery in patients with AIS [24].
Furthermore, our findings indicated that Lp-PLA2 and IMA were associated with treatment efficiency. The combination of rTMS and GDLMI demonstrated superior predictive efficacy compared to rTMS or GDLMI alone. Therefore, the efficiency of rTMS combined with GDLMI in promoting cognitive and neurological function recovery in AIS patients can be predicted based on the levels of Lp-PLA2 and IMA [7]. This prognostic information enables the early implementation of targeted interventions to optimize cognitive and neurological function recovery in these patients.
Nevertheless, this study has several limitations. First, the data were derived from a single medical center, potentially limiting the generalizability of the findings. Second, the sample size (n = 120) is relatively small. Additionally, our methodology was confined to comparing the scores of cognitive and neurological impairments before and after treatment. Without a negative control group for comparison, the efficacy assessment of rTMS plus GDLMI on AIS may be affected by the natural history of the disease itself, the placebo effect, and other treatments that may affect the development and prognosis of the disease. Consequently, the results may have bias. Further multicenter studies with larger sample sizes and a negative control group are needed to confirm our findings.
Conclusions
In conclusion, rTMS combined with GDLMI can contribute to the cognitive and neurological function recovery in AIS patients. Serum levels of Lp-PLA2 and IMA may serve as independent predictive factors for the efficiency in promoting the cognitive and neurological function recovery. Therefore, the levels of Lp-PLA2 and IMA should be early monitored to predict the efficacy in cognitive and neurological function recovery and to improve the clinical efficacy.
Availability of Data and Materials
The data and materials are available from the corresponding author upon reasonable request.
Acknowledgment
Not applicable.
Author Contributions
MH, XW and CS designed this study. MH, XW and TW performed this study. TW analyzed the data. MH, XW and TW drafted this paper. CS significantly revised this paper. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
The study has received ethical approval by Xuzhou Central Hospital (No. JSXZH2021403). Informed consent was obtained for the study. This study was performed in accordance with the Declaration of Helsinki.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Rabinstein AA. Update on Treatment of Acute Ischemic Stroke. Continuum (Minneapolis, Minn.) . 2020;26:268–286. doi: 10.1212/CON.0000000000000840. [DOI] [PubMed] [Google Scholar]
- [2].Chen C, Lv H, Shan L, Long X, Guo C, Huo Y, et al. Antiplatelet effect of ginkgo diterpene lactone meglumine injection in acute ischemic stroke: A randomized, double-blind, placebo-controlled clinical trial. Phytotherapy Research: PTR . 2023;37:1986–1996. doi: 10.1002/ptr.7720. [DOI] [PubMed] [Google Scholar]
- [3].Dionísio A, Duarte IC, Patrício M, Castelo-Branco M. The Use of Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation: A Systematic Review. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association . 2018;27:1–31. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.008. [DOI] [PubMed] [Google Scholar]
- [4].Li X, Xu L, Xu Z. The diagnostic and prognostic performance of Lp-PLA2 in acute ischemic stroke. Medicina Clinica . 2021;156:437–443. doi: 10.1016/j.medcli.2020.11.034. [DOI] [PubMed] [Google Scholar]
- [5].Zhu S, Wei X, Yang X, Huang Z, Chang Z, Xie F, et al. Plasma Lipoprotein-associated Phospholipase A2 and Superoxide Dismutase are Independent Predicators of Cognitive Impairment in Cerebral Small Vessel Disease Patients: Diagnosis and Assessment. Aging and Disease . 2019;10:834–846. doi: 10.14336/AD.2019.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fan Q, Zhou J, Wang Y, Xi T, Ma H, Wang Z, et al. Chip-based serum proteomics approach to reveal the potential protein markers in the sub-acute stroke patients receiving the treatment of Ginkgo Diterpene Lactone Meglumine Injection. Journal of Ethnopharmacology . 2020;260:112964. doi: 10.1016/j.jep.2020.112964. [DOI] [PubMed] [Google Scholar]
- [7].Ma J, Shen L, Bao L, Yuan H, Wang Y, Liu H, et al. A novel prognosis prediction model, including cytotoxic T lymphocyte-associated antigen-4, ischemia-modified albumin, lipoprotein-associated phospholipase A2, glial fibrillary acidic protein, and homocysteine, for ischemic stroke in the Chinese hypertensive population. Journal of Clinical Laboratory Analysis . 2021;35:e23756. doi: 10.1002/jcla.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke . 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- [9].Chen KL, Xu Y, Chu AQ, Ding D, Liang XN, Nasreddine ZS, et al. Validation of the Chinese Version of Montreal Cognitive Assessment Basic for Screening Mild Cognitive Impairment. Journal of the American Geriatrics Society . 2016;64:e285–e290. doi: 10.1111/jgs.14530. [DOI] [PubMed] [Google Scholar]
- [10].Zeitlberger AM, Flynn MC, Hollenstein M, Hundsberger T. Assessment of neurological function using the National Institute of Health Stroke Scale in patients with gliomas. Neuro-oncology Practice . 2021;8:699–705. doi: 10.1093/nop/npab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dong Y, Li H, Dong Q. The effect of intravenous ginkgolide on clinical improvement of patients with acute ischemic stroke. Neurological Research . 2020;42:260–266. doi: 10.1080/01616412.2020.1724462. [DOI] [PubMed] [Google Scholar]
- [12].Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Therapeutic Advances in Neurological Disorders . 2019;12:1756286419878317. doi: 10.1177/1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Du J, Yang F, Hu J, Hu J, Xu Q, Cong N, et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. NeuroImage. Clinical . 2019;21:101620. doi: 10.1016/j.nicl.2018.101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qiao J, Zhou K, Huang C, Fu S, Xing Y, Zhang B. Comparison of serum Lp-PLA2 levels in ischemic stroke patients with H-type hypertension or non-H-type hypertension. Journal of Clinical Laboratory Analysis . 2020;34:e23068. doi: 10.1002/jcla.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chong Y, Wen M, Feng F. Lp-PLA2 Predicts the Risk of Ischemic Stroke: A Prospective Cohort Study in the Chinese Population. The Neurologist . 2021;26:284–285. doi: 10.1097/NRL.0000000000000351. [DOI] [PubMed] [Google Scholar]
- [16].Wang Y, Hu S, Ren L, Lei Z, Lan T, Cai J, et al. Lp-PLA2 as a risk factor of early neurological deterioration in acute ischemic stroke with TOAST type of large arterial atherosclerosis. Neurological Research . 2019;41:1–8. doi: 10.1080/01616412.2018.1493850. [DOI] [PubMed] [Google Scholar]
- [17].Zhong C, Chen T, Shen Y, Zhang Y, Liu Y, Ning L. The effects of serum ischemia modified albumin on diagnosis of cerebral infarction and vertebral basilar artery stenosis. Brain Injury . 2021;35:1457–1461. doi: 10.1080/02699052.2021.1972145. [DOI] [PubMed] [Google Scholar]
- [18].Shevtsova A, Gordiienko I, Tkachenko V, Ushakova G. Ischemia-Modified Albumin: Origins and Clinical Implications. Disease Markers . 2021;2021:9945424. doi: 10.1155/2021/9945424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aslan Koşar P, Tepebaşı MY, Şengeze N, İlhan İ, Büyükbayram Hİ, Kutluhan S. Effect of methylenetetrahydrofolate reductase gene polymorphisms and oxidative stress in silent brain infarction. Molecular Biology Reports . 2021;48:3955–3962. doi: 10.1007/s11033-021-06395-w. [DOI] [PubMed] [Google Scholar]
- [20].Lidong D, Zhanghong X, Huawu M, Xiaofang H, Junhua G, Kaifu K, et al. Ischemia Modified Albumin and miR-126 Play Important Role in Diagnosis of Posterior Circulation Transient Ischemic Attack and Prediction of Secondary Cerebral Infarction. Neurology India . 2021;69:75–80. doi: 10.4103/0028-3886.310100. [DOI] [PubMed] [Google Scholar]
- [21].Elshony HS, Okda MA, El-Kabany RA. Ischemia-modified albumin and fibulin-5 as diagnostic and prognostic markers for acute cerebrovascular disease. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery . 2021;57:12. [Google Scholar]
- [22].Tayman C, Öztekin O, Serkant U, Yakut I, Aydemir S, Kosus A. Ischemia-Modified Albumin May Be a Novel Marker for Predicting Neonatal Neurologic Injury in Small-For-Gestational-Age Infants in Addition to Neuron-Specific Enolase. American Journal of Perinatology . 2017;34:349–358. doi: 10.1055/s-0036-1588026. [DOI] [PubMed] [Google Scholar]
- [23].Gamboa NT, Kalani MY. Computational Materials, Chemistry, and Biochemistry: From Bold Initiatives to the Last Mile: In Honor of William A. Goddard’s Contributions to Science and Engineering . Springer: Cham; 2021. Development of Biomarkers and Point-of-Care Tests for Cerebrovascular Pathology: A Marriage of Chemistry, Biology, and Medicine; pp. 817–853. [Google Scholar]
- [24].Ma Y, Chen Y, Yang T, He X, Yang Y, Chen J, et al. Blood biomarkers for post-stroke cognitive impairment: A systematic review and meta-analysis. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association . 2024;33:107632. doi: 10.1016/j.jstrokecerebrovasdis.2024.107632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available from the corresponding author upon reasonable request.