Abstract
Despite the evident demand and promising potential of disulfide-functionalized amino acids and peptides in linker chemistry and peptide drug discovery, those disulfurated specifically at the α-position constitute a unique yet rather highly underexplored chemical space. In this study, we have developed a method for preparing SS-linked amino acid/peptide derivatives through a base-catalyzed disulfuration reaction of azlactones, followed by the ring-opening functionalization. The disulfuration reaction proceeds under mild conditions, yielding disulfurated azlactones in excellent yields across a variety of N-dithiophthalimides and diverse azlactones derived from various amino acids and peptides. Leveraging the ready availability of N-dithiophthalimides from several bilateral disulfurating reagents, this method allows for the modular integration of functional molecules and azlactones into SS-linkage in two-step operations. Furthermore, due to the transformability of the azlactone moiety through ring-opening with various nucleophiles, our method provides a wide variety of functional molecule-tagged amino acids and oligopeptides bearing SS-linkages in a modular and time-efficient manner, serving as a valuable tool for linker chemistry and peptide chemistry.
An efficient method for preparing SS-linked amino acid/peptide derivatives has been developed via a base-catalyzed disulfuration reaction of azlactones, followed by ring-opening functionalization.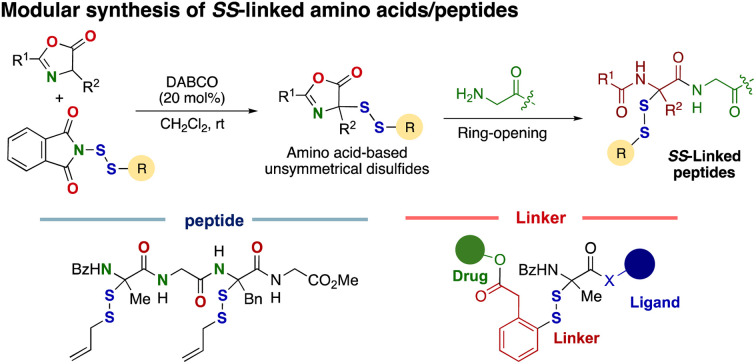
Introduction
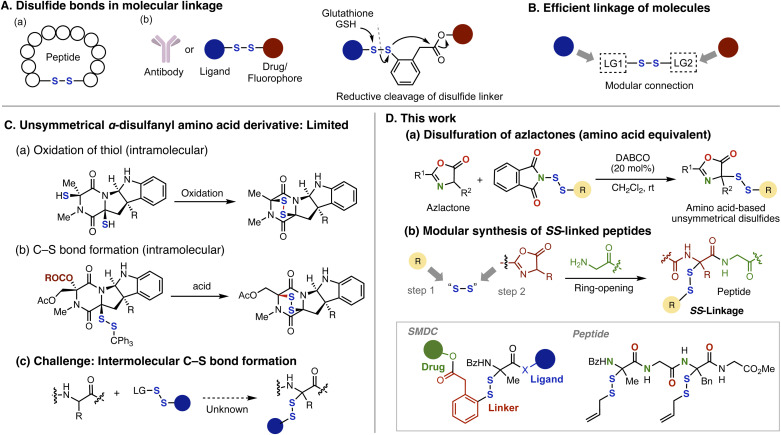
Disulfide bonds are prevalent in bioactive natural products1 and are essential for the proper folding of proteins.2 These linkages are also strategically used to advance drug discovery and development efforts. Within the realm of peptide-based drug development, utilizing disulfide bonds to create cyclic structures is a key strategy, which allows for generating constrained peptides that lead to improved potency, selectivity, membrane barrier permeability, and stability against proteases (Fig. 1A(a)).3 Moreover, the capacity of disulfide bonds to be cleaved by intracellular glutathione4 paves the way for their application as cleavable linkers5 in antibody–drug conjugates (ADCs),6 small molecule–drug conjugates (SMDCs),7 and prodrugs8 aimed at optimizing drug delivery (Fig. 1A(b)).9 This has led to an increasing need for synthetic methods that allow for the programmable introduction of biologically relevant entities on either side of a disulfide linkage (Fig. 1B).10,11 However, despite recent progress in bilateral disulfide reagents,10,11 achieving modular construction of unsymmetrical disulfides remains challenging due to the difficulty in controlling the two leaving groups.
Fig. 1. Preparation of amino acid/peptide-based disulfides. (A) Disulfide bonds in molecular linkage. (B) Ideal synthetic method for efficiently linking molecules. (C) Synthetic method for the preparation of α-disulfurated amino acid derivatives. (D) This work: modular preparation of SS-linked peptides through disulfuration reaction of azlactones. SMDC: small molecule–drug conjugates.
Despite the evident demand and promising potential of disulfide-functionalized amino acids and peptides,2,3,12 those disulfurated specifically at the α-position13 constitute a unique yet rather highly underexplored chemical space.14–16 The majority of known structures in this category belong to a wide-ranging family of natural products containing the venerable dithiodiketopiperazine nucleus (Fig. 1C(a and b)).15,16 Although the construction of this nucleus can be achieved intramolecularly by several techniques, including thiol oxidation (Fig. 1C(a))14,15 and nucleophilic disulfuration of iminium cation (Figure 1C(b)),16 translating these intramolecular processes to generate acyclic, unsymmetrical α-disulfurated amino acids and peptides is nontrivial due to selectivity issues (Fig. 1C(c)). In this respect, α-disulfuration of amino acid derivatives with a disulfide electrophile17,18via amino acid enolate intermediates is highly appealing yet remains unexplored, despite its great potential for accessing hitherto unexplored SS-linked amino acid/peptide chemical space in peptide chemistry and linker chemistry.
As an effective implementation of the disulfuration of amino acids and peptides, we report here the development of a disulfuration reaction of azlactones with N-(organodithio)phthalimides10,17 (Fig. 1D(a)). This class of disulfide electrophiles is notable for their ease of access and structural variability from various carbon10 or sulfur nucleophiles.17,19 In addition, the activated α-proton of azlactone allows for mild base-catalyzed conditions, circumventing the decomposition of base-sensitive α-disulfurated amino acid derivatives.20 Furthermore, the resulting disulfurated azlactones are amenable to ring-opening by a plethora of nucleophiles,21 including amino acids and peptides, thus affording an unprecedented class of disulfide-linked amino acid- and peptide-based compounds (Fig. 1D(b)). Indeed, the versatility and robustness of this method were showcased through its broad substrate scope, modular construction from various bilateral reagents, and further ring-opening diversification, successfully yielding densely functionalized amino acid/peptide derivatives, including drug-linker-ligand conjugates with a molecular weight over 1100, as well as oligopeptides with multiple disulfide moieties.
Results and discussion
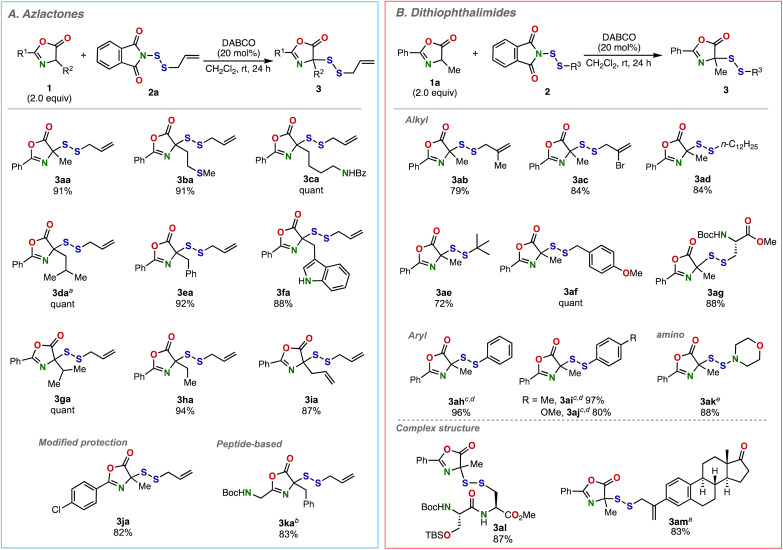
After conducting an extensive study, we have identified optimal reaction conditions for the allyldithiolation of azlactone 1a (R1 = Ph, R2 = Me) with N-(allyldithio)phthalimide (2a) (Fig. 2A). The reaction proceeded efficiently in the presence of a catalytic amount of DABCO (20 mol%) in CH2Cl2 at room temperature, producing the desired disulfurated azlactone 3aa in 91% isolated yield. This reaction system was successfully applied to a wide range of azlactones 1 and N-(organodithio)phthalimides 2 (Fig. 2A and B). A variety of azlactones 1 derived from proteinogenic amino acids such as Met, Lys, and Leu participated in the reaction with 2a, yielding the desired disulfides 3ba–3da in excellent yields (Fig. 2A).
Fig. 2. Reaction of various azlactones 1 with N-(organodithio)phthalimides 2. (A) Scope of azlactones 1. (B) Scope of N-(organodithio)phthalimides 2. Isolated yields are shown. aDABCO (1.0 equiv) was used. bAzlactone (6.0 equiv) and DIPEA (20 mol%) were used at −78 °C. cAzlactone (4.0 equiv) and DABCO (40 mol%) were used at 0 °C. dNMR yield. eK2CO3 (1.0 equiv) was used.
The use of azlactones prepared from bulkier proteinogenic amino acids (Phe, Trp, and Val) led to efficient allyldithiolation, producing 3ea–3ga in excellent yields. Ethyl- or allyl-substituted azlactones 1h and 1i efficiently reacted with 2a to afford the corresponding SS-linked azlactones 3ha and 3ia. Azlactone derived from 4-ClBz protected alanine also smoothly reacted to afford 3ja in good yield. Additionally, peptide-based azlactone 1k, prepared from Boc-Gly-Phe-OH, was dithiolated to afford 3ka in good yield under modified conditions. However, reaction of α-phenyl-substituted azlactone did not proceed.
Meanwhile, various N-(organodithio)phthalimides (2) were also demonstrated to participate in the reaction with alanine-derived azlactone 1a (Fig. 2B). A variety of alkyl-substituted N-(organodithio)phthalimides 2 successfully took part in this transformation to produce the desired products 3ab–3ag in high yields. Specifically, the reactions of 2-methylallyl- and 2-bromoallyl-substituted N-(organodithio)phthalimides 2b and 2c produced 3ab and 3ac without damaging the allyl moieties. n-Dodecyl, t-butyl, and p-methoxybenzyl substituted dithiophthalimides 2d–2f smoothly yielded the desired unsymmetrical disulfides 3ad–3af in high yields. The cysteine-based dithiophthalimide 2g could be successfully transformed into 3ag in high yield. The reactions of the aryl or amino-substituted dithiophthalimides 2h–2k took place efficiently to give 3ah–3ak in high yields under slightly modified conditions. Notably, the present reaction was also applicable to complex dithiophthalimides prepared from a peptide or estrone, affording the desired products 3al and 3am in high yields. Moreover, we found that the dithiophthalimide 2l is easily prepared by the elongation from cysteine-derived dithiophthalimide 2g (Scheme S1†), highlighting the flexible access to peptide-based dithiophthalimides, and thus to SS-peptide-tagged azlactones. Remarkably, the current method proved free from the formation of symmetrical disulfides as byproducts, which are often unavoidable with conventional disulfide synthesis.18a–c
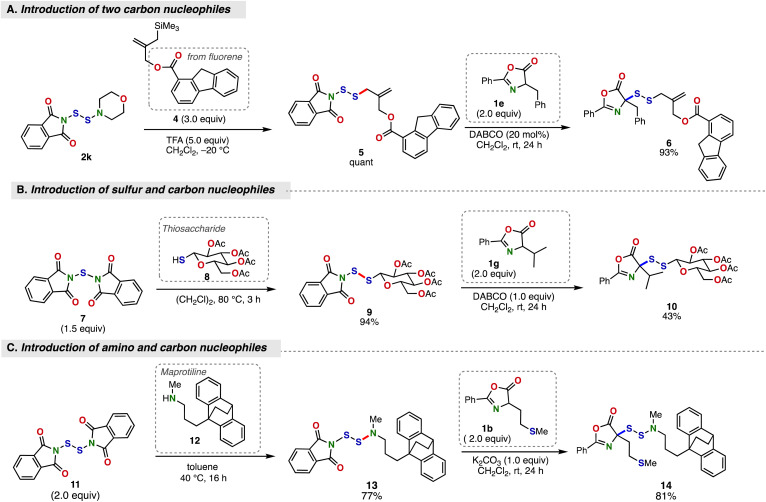
Encouraged by the successful execution of the disulfuration reaction of azlactones and the ready availability of N-dithiophthalimides,10,17,19 we further explored the scope of the present reaction in the context of modular synthesis of complex unsymmetrical disulfides starting from a series of sulfur electrophiles (Fig. 3). First, N-(morpholine-4-dithio)phthalimide (2k), a bilateral disulfide platform molecule we developed recently,10 was utilized to install fluorene-containing allylsilane nucleophile 4 and phenylalanine-derived azlactone 1e through sequential and chemoselective displacement events, providing the desired unsymmetrical disulfide 6 in high yield (Fig. 3A). Next, the construction of a disulfide bond from N,N′-thiobis(phthalimide) (7)17,19 and thiosaccharide 8 was followed by the introduction of valine-derived azlactone 1g to furnish the product 10 in moderate yield (Fig. 3B). Furthermore, we also accomplished the introduction of amine and azlactone nucleophiles into N,N′-dithiobis(phthalimide) (11)10 through the reaction with maprotiline (12; antidepressant drug) and methionine-derived azlactone 1b in high yield (Fig. 3C).
Fig. 3. Modular synthesis of SS-linked azlactones bearing various complex substituents. (A) Introduction of carbon and azlactone nucleophiles. (B) Introduction of sulfur and azlactone nucleophiles. (C) Introduction of amino and azlactone nucleophiles. Isolated yields are shown.
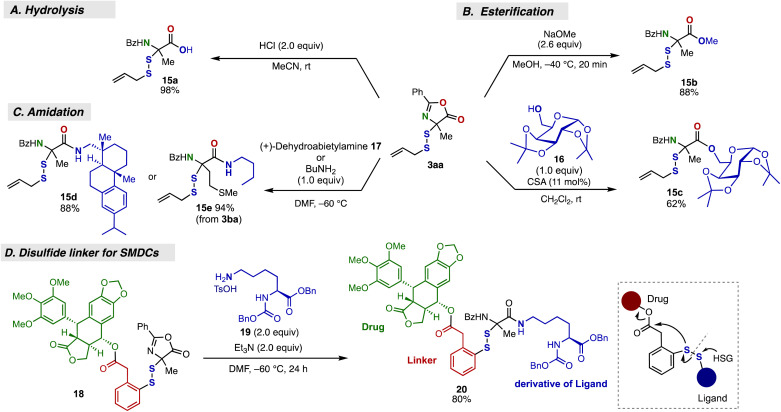
Besides the adaptability of the present reaction to modular disulfide synthesis, the disulfurated azlactone products proved to serve as viable precursors for diversification into disulfide-linked amino acid derivatives via ring-opening with various nucleophiles (Fig. 4).21 Thus, the disulfide-linked azlactone 3aa was quantitatively ring-opened by HCl to afford carboxylic acid 15a (Fig. 4A).21d The ring-opening esterification21c,d by NaOMe or saccharide 16 under basic or acid-mediated conditions afforded the α-disulfurated esters 15b and 15c in good yield (Fig. 4B). The amidation21a,b of 3aa was also achieved though ring-opening by dehydroabietylamine (17), thus producing the desired α-disulfurated amide 15d in good yield (Fig. 4C). The amidation process could also be applied to the disulfurated azlactone 3ba, derived from Met derivative, with butylamine to afford 15e in high yield (Fig. 4C). Furthermore, we successfully accomplished the preparation of a hybrid molecule that shows promise for use in SMDCs (small molecule–drug conjugates, Fig. 4D).7 The reaction of an azlactone 18 bearing drug and cleavable disulfide linker5a moieties with the lysine ε-amino moiety of a model molecule of a peptide-based targeting ligand (19)22 cleanly constructed the conjugate 20 with high molecular complexity in high yield. These results clearly demonstrate the utility of this method for linker chemistry.6,7
Fig. 4. Transformations of the azlactone moiety via the ring-opening by various nucleophiles. (A) Hydrolysis to carboxylic acid. (B) Esterification with alcohol. (C) Amidation with amine. (D) Preparation of an SMDC.
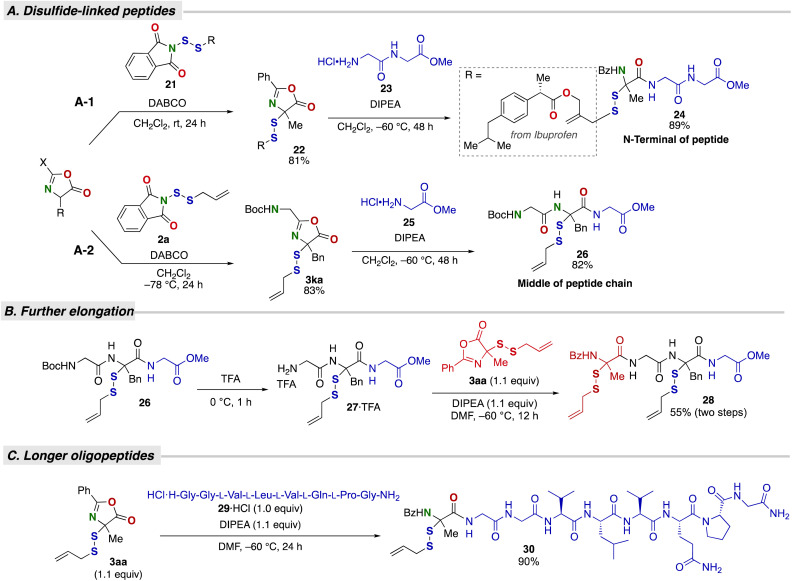
The present method is not only useful in linker chemistry but also in the preparation of α-disulfurated peptides. The reaction of alanine-derived azlactone 1a with ibuprofen-substituted dithiophthalimide 21, followed by ring-opening with HCl H-Gly-Gly-OMe (23), afforded a tripeptide 24 bearing a disulfide moiety at the N-terminus (Fig. 5A1).23 Meanwhile, the disulfuration reaction of azlactone 1k, derived from peptide (Boc-Gly-Phe-OH), and subsequent ring-opening with HCl H-Gly-OMe (25) resulted in the formation of a tripeptide 26 bearing an SS-linkage in the middle of the peptide chain (Fig. 5A2). The SS-linked peptide 26 was further elongated by TFA-mediated deprotection of the Boc group, followed by connection with another SS-linked azlactone 3aa, affording a tetrapeptide bearing two disulfide moieties (Fig. 5B). The synthesis of peptides α-(di)sulfide-substituted in the middle of the peptide chain has been challenging due to the instability of the N-deprotected (di)sulfide-substituted amino acids, even in the case of mono-sulfides.24 Our approach uniquely solves this problem by using dipeptide-derived azlactone, enabling peptide extension at both the C- and N-termini. Additionally, this method demonstrates the utility for the construction of the α-disulfurated variant of the α,α-disubstituted amino acid (dAA) moiety, which is a promising structure for enhancing potency and functioning as cell-penetrating peptides due to its restricted conformation and stabilized secondary structure when incorporated into the peptide.25
Fig. 5. Transformation into various peptide derivatives by ring-opening. (A) Construction of disulfide-linked peptides. (B) Further elongation. (C) Construction of disulfide-linked oligopeptides.
Furthermore, longer oligopeptides were also successfully introduced into a disulfide-substituted azlactone (Fig. 5C). The octapeptide HCl H-Gly-Gly-L-Val-L-Leu-L-Val-L-Gln-L-Pro-Gly-NH2 (29 HCl, Larazotide-NH2 HCl) gave the nonapeptide 30 in 90% yield. Importantly, these α-disulfurated peptides represent a largely uncharted chemical space in peptide chemistry, due to the lack of α-disulfuration reaction for amino acid/peptide derivatives, as well as the difficulty of the modular construction of unsymmetrical disulfides.10,11 Owing to its modular nature, simplicity, and wide substrate scope, including the oligopeptides, the present method offers an attractive route to unnatural disulfide-functionalized peptides, which may uniquely complement the chemistry of disulfide-linked peptides capitalizing on cysteine residues.
Conclusions
In conclusion, we have developed a method for the preparation of SS-linked amino acid/peptide derivatives through base-catalyzed disulfuration reaction of azlactones. The reaction is carried out under mild conditions and provides disulfurated azlactones in excellent yields across a variety of N-dithiophthalimides and diverse azlactones derived from various amino acids. Owing to the availability of N-dithiophthalimides from several bilateral disulfurating reagents, this method allows for the modular integration of functional molecules and azlactones into SS-linkage in two-step operations. Moreover, the introduced azlactone moiety can be ring-opened by various nucleophiles to yield a broad range of SS-linked amino acid derivatives, including complex molecules that show promise for use in SMDCs (small molecule–drug conjugates). The ring-opening with amino acids or peptides allows for the straightforward preparation of the hitherto unexplored α-disulfurated oligopeptides, especially bearing α,α-disubstituted moiety that is useful in peptide drug chemistry. Thus, the present method offers disulfide–peptide conjugation in a modular and time-efficient manner, serving as a valuable tool for linker chemistry and peptide drug discovery. Further investigations into the enantioselective reaction (Fig. S1†), the use of drug delivery vehicles, as well as their biological evaluations, are currently underway in our laboratory.
Data availability
The data supporting this article have been reported as part of the ESI.†
Author contributions
K. K. conceptualized and directed the project. K. K. designed the experiments. K. K., M. I., Y. T., H. A., K. H., H. O. performed and analyzed the experiments. K. K. wrote the original draft. N. Y. and K. K. reviewed and edited the manuscript. All authors contributed to discussions.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank Prof. Shin-ichi Fukuzawa (Chuo University) for fruitful discussions. This study was supported by the Kato Memorial Bioscience Foundation (K. K.), the Tokyo Biochemical Research Foundation (K. K.), the Takahashi Industrial and Economic Research Foundation (K. K.), a Meiji Seika Award in Synthetic Organic Chemistry, Japan (K. K.), The Uehara Memorial Foundation (K. K.), the NOVARTIS Foundation (Japan) for the Promotion of Science (K. K.), the Research Foundation for Pharmaceutical Sciences (K. K.), Kobayashi Foundation (K. K.), The Noguchi Institute, the Shitagau Noguchi Research Grant (Grant number, NJ202312 (K. K.)), Tohoku University-AIST Matching Support Program (K. K.), JSPS KAKENHI (Grant numbers, JP20K15288 and JP22K14687: Young Scientists; K. K.), JSPS Research Fellows (Grant Number, JP23KJ0191; H. A.), and Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED (Grant no. JP23ama121040 (N. Y.)).
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07187e
Notes and references
- For selected reviews: ; (a) Jiang C.-S. Müller W. E. G. Schröder H. C. Guo Y.-W. Chem. Rev. 2012;112:2179–2207. doi: 10.1021/cr200173z. [DOI] [PubMed] [Google Scholar]; (b) Li B. Wever W. J. Walsh C. T. Bowers A. A. Nat. Prod. Rep. 2014;31:905–923. doi: 10.1039/c3np70106a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jiang X., Topics in Current Chemistry, Sulfur Chemistry, Springer, Berlin, 2018 [Google Scholar]
- For selected reviews: ; (a) Hogg P. J. Trends Biochem. Sci. 2003;28:210–214. doi: 10.1016/S0968-0004(03)00057-4. [DOI] [PubMed] [Google Scholar]; (b) Lu S. Fan S.-B. Yang B. Li Y.-X. Meng J.-M. Wu L. Li P. Zhang K. Zhang M.-J. Fu Y. Luo J. Sun R.-X. He S.-M. Dong M.-Q. Nat. Methods. 2015;12:329–331. doi: 10.1038/nmeth.3283. [DOI] [PubMed] [Google Scholar]
- For selected reviews for stapled peptides: ; (a) Verdine G. L. Hilinski G. J. Methods Enzymol. 2012;503:3–33. doi: 10.1016/B978-0-12-396962-0.00001-X. [DOI] [PubMed] [Google Scholar]; (b) Góngora-Benítez M. Tulla- Puche J. Albericio F. Chem. Rev. 2014;114:901–926. doi: 10.1021/cr400031z. [DOI] [PubMed] [Google Scholar]; (c) Lau Y. H. de Andrade P. Wu Y. Spring D. R. Chem. Soc. Rev. 2015;44:91–102. doi: 10.1039/c4cs00246f. [DOI] [PubMed] [Google Scholar]
- (a) Saito G. Swanson J. A. Lee K.-D. Adv. Drug Delivery Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]; (b) Cheng R. Feng F. Meng F. Deng C. Feijen J. Zhong Z. J. Controlled Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- For selected reviews for disulfide linkers: ; (a) Ojima I. Acc. Chem. Res. 2008;41:108–119. doi: 10.1021/ar700093f. [DOI] [PubMed] [Google Scholar]; (b1) Tsuchikama K. An Z. Protein Cell. 2018;9:33–46. doi: 10.1007/s13238-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; . For selected examples: ; (c) Zhao R. Y. Wilhelm S. D. Audette C. Jones G. Leece B. A. Lazar A. C. Goldmacher V. S. Singh R. Kovtun Y. Widdison W. C. Lambert J. M. Chari R. V. J. J. Med. Chem. 2011;54:3606–3623. doi: 10.1021/jm2002958. [DOI] [PubMed] [Google Scholar]; (d) Sadowsky J. D. Pillow T. H. Chen J. Fan F. He C. Wang Y. Yan G. Yao H. Xu Z. Martin S. Zhang D. Chu P. dela Cruz-Chuh J. O'Donohue A. Li G. Rosario G. D. He J. Liu L. Ng C. Su D. Phillips G. D. L. Kozak K. R. Yu S.-F. Xu K. Leipold D. Wai J. Bioconjugate Chem. 2017;28:2086–2098. doi: 10.1021/acs.bioconjchem.7b00258. [DOI] [PubMed] [Google Scholar]; (e) Wang X. Borges C. A. Ning X. Rafi M. Zhang J. Park B. Takemiya K. Sterzo C. L. Taylor W. R. Riley L. Murthy N. Bioconjugate Chem. 2018;29:1729–1735. doi: 10.1021/acs.bioconjchem.8b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Pei Z. Chen C. Chen J. dela Cruz-Chuh J. Delarosa R. Deng Y. Fourie-O’Donohue A. Figueroa I. Guo J. Jin W. Khojasteh S. C. Kozak K. R. Latifi B. Lee J. Li G. Lin E. Liu L. Lu J. Martin S. Ng C. Nguyen T. Ohri R. Phillips G. L. Pillow T. H. Rowntree R. K. Stagg N. J. Stokoe D. Ulufatu S. Verma V. A. Wai J. Wang J. Xu K. Xu Z. Yao H. Yu S.-F. Zhang D. Dragovich P. S. Mol. Pharmaceutics. 2018;15:3979–3996. doi: 10.1021/acs.molpharmaceut.8b00431. [DOI] [PubMed] [Google Scholar]; (g) Hebels E. R. Dietl S. Timmers M. Hak J. van den Dikkenberg A. Rijcken C. J. F. Hennink W. E. Liskamp R. M. J. Vermonden T. Bioconjugate Chem. 2023;34:2375–2386. doi: 10.1021/acs.bioconjchem.3c00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews for ADCs: ; (a) Chari R. V. J. Miller M. L. Widdison W. C. Angew. Chem., Int. Ed. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]; (b) Beck A. Goetsch L. Dumontet C. Corvaïa N. Nat. Rev. Drug Discovery. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou K. C. Rigol S. Angew. Chem., Int. Ed. 2019;58:11206–11241. doi: 10.1002/anie.201903498. [DOI] [PubMed] [Google Scholar]
- For selected reviews for SMDCs: ; (a) Krall N. Scheuermann J. Neri D. Angew. Chem., Int. Ed. 2013;52:1384–1402. doi: 10.1002/anie.201204631. [DOI] [PubMed] [Google Scholar]; (b1) Razzak M. Nat. Rev. Clin. Oncol. 2013;10:668. doi: 10.1038/nrclinonc.2013.201. [DOI] [PubMed] [Google Scholar]; . For selected examples; ; (c) Krall N. Pretto F. Neri D. Chem. Sci. 2014;5:3640–3644. [Google Scholar]; (d) Krall N. Pretto F. Decurtins W. Bernardes G. J. L. Supuran C. T. Neri D. Angew. Chem., Int. Ed. 2014;53:4231–4235. doi: 10.1002/anie.201310709. [DOI] [PubMed] [Google Scholar]; (e) Cazzamalli S. Corso A. D. Widmayer F. Neri D. J. Am. Chem. Soc. 2018;140:1617–1621. doi: 10.1021/jacs.7b13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected examples of prodrugs: ; (a) Vrudhula V. M. MacMaster J. F. Li Z. Kerr D. E. Senter P. D. Bioorg. Med. Chem. Lett. 2002;12:3591–3594. doi: 10.1016/s0960-894x(02)00784-9. [DOI] [PubMed] [Google Scholar]; (b) Santra S. Kaittanis C. Santiesteban O. J. Perez J. M. J. Am. Chem. Soc. 2011;133:16680–16688. doi: 10.1021/ja207463b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a selected review: ; Ulrich S. Acc. Chem. Res. 2019;52:510–519. doi: 10.1021/acs.accounts.8b00591. [DOI] [PubMed] [Google Scholar]
- Asanuma H. Kanemoto K. Watanabe T. Fukuzawa S.-i. Angew. Chem., Int. Ed. 2023;62:e202219156. doi: 10.1002/anie.202219156. [DOI] [PubMed] [Google Scholar]
- Examples of bilateral disulfurating reagents: ; (a) Xue J. Jiang X. Nat. Commun. 2020;11:4170. doi: 10.1038/s41467-020-18029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tian Q. Li Y. Angew. Chem., Int. Ed. 2023;62:e202302861. doi: 10.1002/anie.202302861. [DOI] [PubMed] [Google Scholar]
- For selected reviews of disulfide-containing amino acids and peptides: ; (a) Zhao J. Jiang X. Chin. Chem. Lett. 2018;29:1079–1087. [Google Scholar]; (b) Wang N. Saidhareddy P. Jiang X. Nat. Prod. Rep. 2020;37:246–275. doi: 10.1039/c8np00093j. [DOI] [PubMed] [Google Scholar]
- Importance of α-sulfurated peptides: ; (a) Chen Y. Wang J. Li G. Yang Y. Ding W. Front. Chem. 2021;9:595991. doi: 10.3389/fchem.2021.595991. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mo T. Ji X. Yuan W. Mandalapu D. Wang F. Zhong Y. Li F. Chen Q. Ding W. Deng Z. Yu S. Zhang Q. Angew. Chem., Int. Ed. 2019;58:18793–18797. doi: 10.1002/anie.201908490. [DOI] [PubMed] [Google Scholar]; (c) Hudson G. A. Burkhart B. J. DiCaprio A. J. Schwalen C. J. Kille B. Pogorelov T. V. Mitchell D. A. J. Am. Chem. Soc. 2019;141:8228–8238. doi: 10.1021/jacs.9b01519. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Flühe L. Knappe T. A. Gattner M. J. Schäfer A. Burghaus O. Linne U. Marahiel M. A. Nat. Chem. Biol. 2012;8:350–357. doi: 10.1038/nchembio.798. [DOI] [PubMed] [Google Scholar]; (e) Flühe L. Marahiel M. A. Curr. Opin. Chem. Biol. 2013;17:605–612. doi: 10.1016/j.cbpa.2013.06.031. [DOI] [PubMed] [Google Scholar]
- For intermolecular oxidation for symmetrical disulfides: ; (a) Joshaghani M. Rafiee E. Shahbazi F. Jafari H. Amiri S. Omidi M. Arkivoc. 2007;1:164–172. [Google Scholar]; (b) Grace C. R. R. Erchegyi J. Samant M. Cescato R. Piccand V. Riek R. Reubi J. C. Rivier J. E. J. Med. Chem. 2008;51:2676–2681. doi: 10.1021/jm701445q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Golovanov I. S. Leonov A. V. Lesnikov V. K. Pospelov E. V. Frolov K. V. Korlyukov A. A. Nelyubina Y. V. Novikov V. V. Sukhorukov A. Y. Dalton Trans. 2022;51:4284–4296. doi: 10.1039/d1dt04104e. [DOI] [PubMed] [Google Scholar]
- Selected examples: ; (a) Overman L. E. Sato T. Org. Lett. 2007;9:5267–5270. doi: 10.1021/ol702518t. [DOI] [PubMed] [Google Scholar]; (b) Kim J. Ashenhurst J. A. Movassaghi M. Science. 2009;324:238–241. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Iwasa E. Hamashima Y. Fujishiro S. Higuchi E. Ito A. Yoshida M. Sodeoka M. J. Am. Chem. Soc. 2010;132:4078–4079. doi: 10.1021/ja101280p. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou K. C. Totokotsopoulos S. Giguère D. Sun Y.-P. Sarlah D. J. Am. Chem. Soc. 2011;133:8150–8153. doi: 10.1021/ja2032635. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Codelli J. A. Puchlopek A. L. A. Reisman S. E. J. Am. Chem. Soc. 2012;134:1930–1933. doi: 10.1021/ja209354e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Kurogi T. Okaya S. Fujiwara H. Okano K. Tokuyama H. Angew. Chem., Int. Ed. 2016;55:283–287. doi: 10.1002/anie.201507830. [DOI] [PubMed] [Google Scholar]; (g) Sato S. Hirayama A. Ueda H. Tokuyama H. Asian J. Org. Chem. 2017;6:54–58. [Google Scholar]
- (a) Kim J. Movassaghi M. J. Am. Chem. Soc. 2010;132:14376–14378. doi: 10.1021/ja106869s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Boyer N. Movassaghi M. Chem. Sci. 2012;3:1798–1803. doi: 10.1039/C2SC20270K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Takeuchi R. Shimokawa J. Fukuyama T. Chem. Sci. 2014;5:2003–2006. [Google Scholar]; (d) Adams T. C. Payette J. N. Cheah J. H. Movassaghi M. Org. Lett. 2015;17:4268–4271. doi: 10.1021/acs.orglett.5b02059. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Snaddon T. N. Scaggs T. D. Pearson C. M. Fyfe J. W. B. Org. Lett. 2019;21:4873–4877. doi: 10.1021/acs.orglett.9b01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transformation of N-(organodithio)phthalimides: ; (a) Zou J. Chen J. Shi T. Hou Y. Cao F. Wang Y. Wang X. Jia Z. Zhao Q. Wang Z. ACS Catal. 2019;9:11426–11430. [Google Scholar]; (b) Gao W.-C. Tian J. Shang Y.-Z. Jiang X. Chem. Sci. 2020;11:3903–3908. doi: 10.1039/d0sc01060j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gao W.-C. Liu J. Jiang X. Org. Chem. Front. 2021;8:1275–1279. [Google Scholar]; (d) Wang D. Li W. Shi K. Pan Y. J. Org. Chem. 2023;88:2550–2556. doi: 10.1021/acs.joc.1c02556. [DOI] [PubMed] [Google Scholar]; (e) Asanuma H. Kanemoto K. Org. Lett. 2024;26:438–443. doi: 10.1021/acs.orglett.3c03419. [DOI] [PubMed] [Google Scholar]; (f) Li B. Liu B.-X. Rao W. Shen S.-S. Sheng D. Wang S.-Y. Org. Lett. 2024;26:3634–3639. doi: 10.1021/acs.orglett.4c01109. [DOI] [PubMed] [Google Scholar]
- For selected reviews of disulfide synthesis: ; (a) Witt D. Synthesis. 2008;16:2491–2509. [Google Scholar]; (b) Ong C. L. Titinchi S. Juan J. C. Khaligh N. G. Helv. Chim. Acta. 2021;104:e2100053. [Google Scholar]; (c1) Sun Q. Xu Y. Yang L. Zheng C.-L. Wang G. Wang H.-B. Fang Z. Wang C.-S. Guo K. Chem.–Asian J. 2024;19:e202400124. doi: 10.1002/asia.202400124. [DOI] [PubMed] [Google Scholar]; . Selected examples of other electrophilic disulfurations: ; (d) Xiao X. Xue J. Jiang X. Nat. Commun. 2018;9:2191. doi: 10.1038/s41467-018-04306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang W. Lin Y. Ma Y. Tung C.-H. Xu Z. Org. Lett. 2018;20:3829–3832. doi: 10.1021/acs.orglett.8b01418. [DOI] [PubMed] [Google Scholar]; (f) Zhang Q. Li Y. Zhang L. Luo S. Angew. Chem., Int. Ed. 2021;60:10971–10976. doi: 10.1002/anie.202101569. [DOI] [PubMed] [Google Scholar]; (g) Chen W. Liu X.-y. Sheng D. Jiang Y.-F. Rao W. Shen S.-S. Yang Z.-Y. Wang S.-Y. Org. Chem. Front. 2024;11:830–835. [Google Scholar]; (h) Zhang C.-P. Wang T.-Z. Wu K. Liang Y.-F. ChemCatChem. 2024;16:e202400128. [Google Scholar]
- (a) Harpp D. N. Steliou K. Chan T. H. J. Am. Chem. Soc. 1978;100:1222–1228. [Google Scholar]; (b) Abe Y. Horii T. Kawamura S.-i. Nakabayashi T. Bull. Chem. Soc. Jpn. 1979;52:3461–3462. [Google Scholar]; (c1) Haseltine J. N. Danishefsky S. J. J. Org. Chem. 1990;55:2576–2578. [Google Scholar]; . Other examples: ; (d) Nicolaou K. C. Li R. Lu Z. Pitsinos E. N. Alemany L. B. Aujay M. Lee C. Sandoval J. Gavrilyuk J. J. Am. Chem. Soc. 2018;140:12120–12136. doi: 10.1021/jacs.8b06955. [DOI] [PubMed] [Google Scholar]
- (a) Fisk J. S. Mosey R. A. Tepe J. J. Chem. Soc. Rev. 2007;36:1432–1440. doi: 10.1039/b511113g. [DOI] [PubMed] [Google Scholar]; (b) Alba A.-N. R. Rios R. Chem.–Asian J. 2011;6:720–734. doi: 10.1002/asia.201000636. [DOI] [PubMed] [Google Scholar]; (c) Shirakawa S. Maruoka K. Angew. Chem., Int. Ed. 2013;52:4312–4348. doi: 10.1002/anie.201206835. [DOI] [PubMed] [Google Scholar]; (d) Metz A. E. Kozlowski M. C. J. Org. Chem. 2015;80:1–7. doi: 10.1021/jo502408z. [DOI] [PubMed] [Google Scholar]; (e) Liu S. Kumatabara Y. Shirakawa S. Green Chem. 2016;18:331–341. [Google Scholar]
- (a) Barnes D. K. Campaigne E. Shriner R. L. J. Am. Chem. Soc. 1948;70:1769–1772. doi: 10.1021/ja01185a030. [DOI] [PubMed] [Google Scholar]; (b) Liu B. Zhang Y. Huang G. Zhang X. Niu P. Wu J. Yu W. Chang J. Org. Biomol. Chem. 2014;12:3912–3923. doi: 10.1039/c4ob00309h. [DOI] [PubMed] [Google Scholar]; (c) Pereira A. A. de Castro P. P. de Mello A. C. Ferreira B. R. V. Eberlin M. N. Amarante G. W. Tetrahedron. 2014;70:3271–3275. [Google Scholar]; (d) Yang J. Sun W. He Z. Yu C. Bao G. Li Y. Liu Y. Hong L. Wang R. Org. Lett. 2018;20:7080–7084. doi: 10.1021/acs.orglett.8b03020. [DOI] [PubMed] [Google Scholar]
- (a) Lütje S. Slavik R. Fendler W. Herrmann K. Eiber M. Methods. 2017;130:42–50. doi: 10.1016/j.ymeth.2017.06.026. [DOI] [PubMed] [Google Scholar]; (b) Murce E. Spaan E. Beekman S. van den Brink L. Handula M. Stuurman D. de Ridder C. Dalm S. U. Seimbille Y. Pharmaceuticals. 2023;16:1072. doi: 10.3390/ph16081072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples: ; (a) Uraguchi D. Asai Y. Ooi T. Angew. Chem., Int. Ed. 2009;48:733–737. doi: 10.1002/anie.200803661. [DOI] [PubMed] [Google Scholar]; (b) Le D. N. Hansen E. Khan H. A. Kim B. Wiest O. Dong V. M. Nat. Chem. 2018;10:968–973. doi: 10.1038/s41557-018-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Milewska K. D. Malins L. R. Org. Lett. 2022;24:3680–3685. doi: 10.1021/acs.orglett.2c01297. [DOI] [PubMed] [Google Scholar]; (b) Hayashi J. Kobayashi D. Denda M. Otaka A. Org. Lett. 2024;26:5167–5171. doi: 10.1021/acs.orglett.4c01685. [DOI] [PubMed] [Google Scholar]
- For selected reviews of dAAs: ; (a) Tanaka M. Chem. Pharm. Bull. 2007;55:349–358. doi: 10.1248/cpb.55.349. [DOI] [PubMed] [Google Scholar]; (b) Grauer A. König B. Eur. J. Org Chem. 2009:5099–5111. [Google Scholar]; (c) Oba M. ChemBioChem. 2019;20:2041–2045. doi: 10.1002/cbic.201900204. [DOI] [PubMed] [Google Scholar]; (d) Tsuchiya K. Kurohara T. Fukuhara K. Misawa T. Demizu Y. Processes. 2022;10:924. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been reported as part of the ESI.†