Abstract
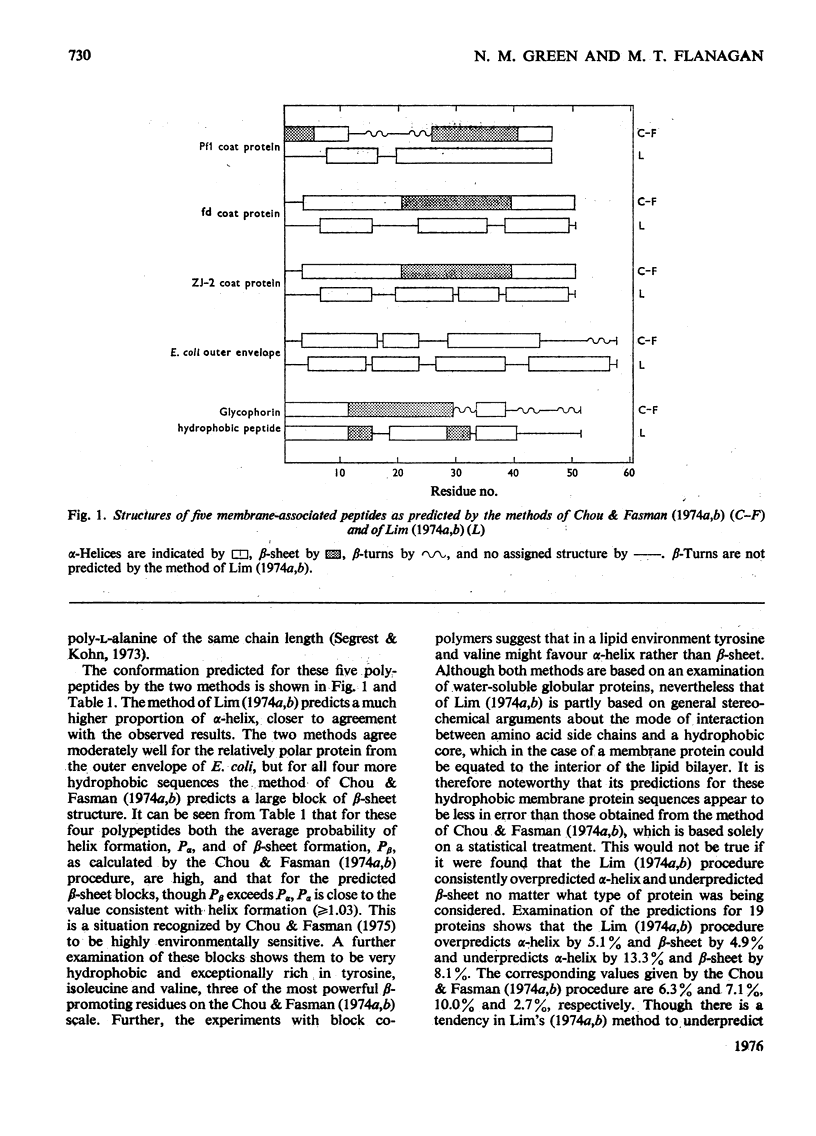
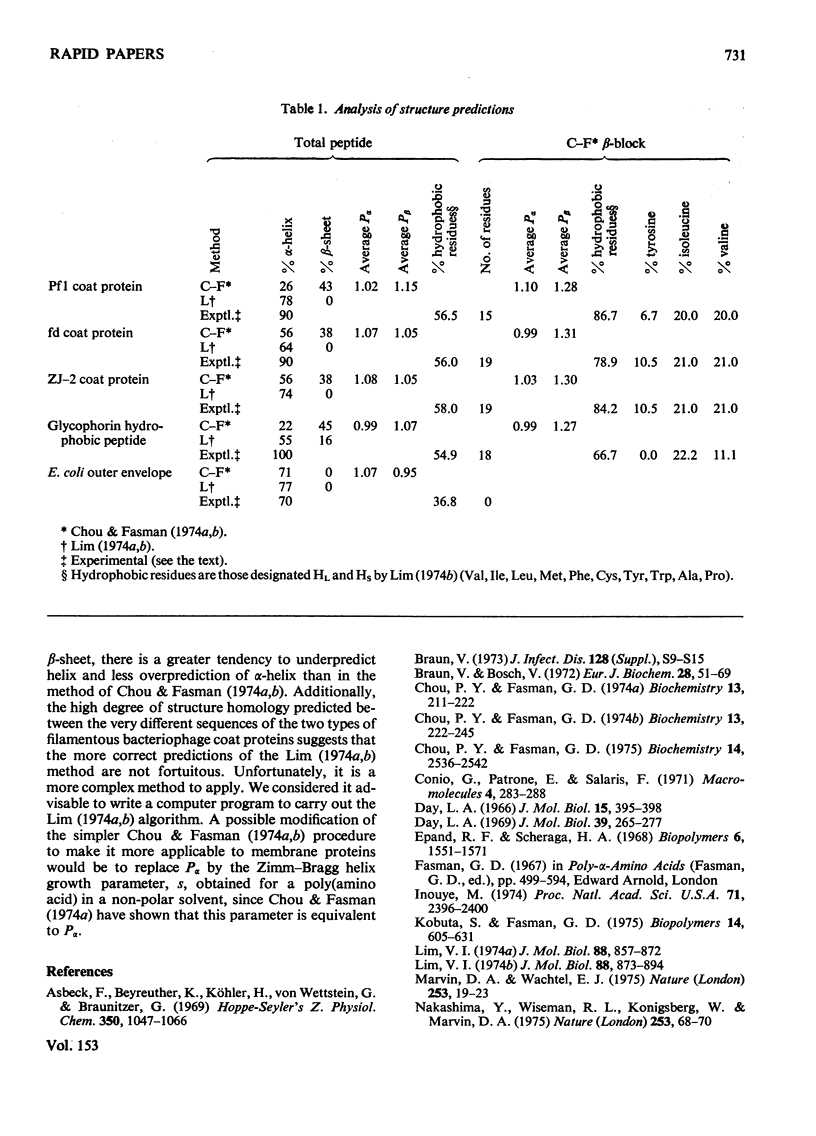
The methods of Chou & Fasman [Biochemistry (1974) 13, 211-222, 222-245] and of Lim [J. Mol. Biol. (1974)88, 857-872, 873-894] for predicting secondary structure from amino acid sequence have been applied to five predominantly helical membrane-associated peptides. The predictions from the method of Lim (1974a,b) are consistent with the experimental observations, whereas those from Chou & Fasman (1974a,b), although not inconsistent with alpha-helix, favour a beta-structure for several very hydrophobic regions. The results may be rationalized in terms of the effect of the solvent on the conformation of a polypeptide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. The conformation of glucagon: predictions and consequences. Biochemistry. 1975 Jun 3;14(11):2536–2541. doi: 10.1021/bi00682a037. [DOI] [PubMed] [Google Scholar]
- Day L. A. Conformations of single-stranded DNA and coat protein in fd bacteriophage as revealed by ultraviolet absorption spectroscopy. J Mol Biol. 1969 Jan;39(2):265–277. doi: 10.1016/0022-2836(69)90316-7. [DOI] [PubMed] [Google Scholar]
- Day L. A. Protein conformation in fd bacteriophage as investigated by optical rotatory dispersion. J Mol Biol. 1966 Jan;15(1):395–398. doi: 10.1016/s0022-2836(66)80239-5. [DOI] [PubMed] [Google Scholar]
- Epand R. F., Scheraga H. A. Conformations of poly-L-valine in solution. Biopolymers. 1968;6(11):1551–1571. doi: 10.1002/bip.1968.360061104. [DOI] [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S., Fasman G. D. The beta conformation of polypeptides of valine, isoleucine, and threonine in solution and solid-state: optical and infrared studies. Biopolymers. 1975 Mar;14(3):605–631. doi: 10.1002/bip.1975.360140314. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Wachtel E. J. Structure and assembly of filamentous bacterial viruses. Nature. 1975 Jan 3;253(5486):19–23. doi: 10.1038/253019a0. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Wiseman R. L., Konigsberg W., Marvin D. A. Primary structure and sidechain interactions of PFL filamentous bacterial virus coat protein. Nature. 1975 Jan 3;253(5486):68–71. doi: 10.1038/253068a0. [DOI] [PubMed] [Google Scholar]
- Noguchi H., Yang J. T. Volume and sound velocity changes accompanying the -helix to -form and coil to -helix transitions in aqueous solution. Biopolymers. 1971;10(12):2569–2579. doi: 10.1002/bip.360101216. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Barry C. D., Friedman J., Chou P. Y., Fasman G. D., Finkelstein A. V., Lim V. I., Pititsyn O. B., Kabat E. A., Wu T. T. Comparison of predicted and experimentally determined secondary structure of adenyl kinase. Nature. 1974 Jul 12;250(462):140–142. doi: 10.1038/250140a0. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Marchesi V. T., Guyer R. B., Terry W. Red cell membrane glycoprotein: amino acid sequence of an intramembranous region. Biochem Biophys Res Commun. 1972 Nov 15;49(4):964–969. doi: 10.1016/0006-291x(72)90306-3. [DOI] [PubMed] [Google Scholar]
- Snell C. R., Fasman G. D. Kinetics and thermodynamics of the helix leads to transconformation of poly(L-lysine) and L-leucine copolymers. A compensation phenomenon. Biochemistry. 1973 Mar 13;12(6):1017–1025. doi: 10.1021/bi00730a001. [DOI] [PubMed] [Google Scholar]
- Snell D. T., Offord R. E. The amino acid sequence of the B-protein of bacteriophage ZJ-2. Biochem J. 1972 Mar;127(1):167–178. doi: 10.1042/bj1270167. [DOI] [PMC free article] [PubMed] [Google Scholar]


