Abstract
With the availability of free antiretroviral therapy (ART) across India, HIV in adults has become a chronic disease with prolonged survival. The emergence of various non-communicable diseases in these prolonged survivors is a cause of concern. Metabolic dysfunction-associated steatotic liver disease (MASLD) in adults with HIV infection in India has not been explored to date. In this study, we attempted to assess the existence of MASLD in thirty adults registered at the Centre of Excellence in ART Care at a tertiary teaching hospital in New Delhi. This center provides free first-line, second-line, and third-line ART to patients as well as comprehensive HIV care including counseling, nutritional advice, and inpatient admissions for intercurrent illnesses. A total of 30 subjects were enrolled in the study to assess the occurrence of MASLD among people living with HIV (PLHIV) and its risk factors and to assess hepatic fibrosis in the subjects with MASLD using transient elastography and clinical fibrosis scores. The study population included 13 subjects on ART (43.3%) and 17 ART-naïve subjects (56.6%). All the study subjects underwent ultrasonography (USG) for the identification of the development of MASLD in them. Steatosis was identified as an increase in the echogenicity of the liver seen as an increase in the hepatorenal contrast and was further graded into the 3 grades of fatty liver. Out of the 30 subjects, 16.6% (5 out of 30) were found to have MASLD on USG, with grade 1 fatty changes seen in 4 (13.3%) and grade 2 fatty changes seen in 1 out of 30 subjects (3.3%). A majority (40%) of the subjects were underweight (body mass index [BMI] < 18.5). 22.7% of the male subjects included in the study had MASLD whereas none of the females had fatty changes in the liver on USG. Out of the study subjects, MASLD was detected in 17.6% of ART-naïve subjects while it was detected in 15.4% of subjects on ART. Although no statistically significant association was seen with any of these parameters, a few important trends were observed. These might be statistically significant in a higher power study with a larger sample size. Higher BMI (mean difference [MD] = 3.25, P = .09), waist circumference (MD = 3.84, P = .15), hip circumference (MD = 4.36, P = .14), and older age (MD = 6.56, P = .07) were observed to be associated with MASLD in our study, whereas the biochemical parameters and HIV-related factors were not seen to have any particular trend of association in our study. However, a higher median CD4 count was associated with MASLD as compared to the group without fatty changes on USG. On FibroScan, all 5 subjects with fatty changes in our study were found to have liver stiffness less than 7 kPa which corresponds to F0-F1 stage of fibrosis. Using the nonalcoholic fatty liver disease score, 2 subjects had scores corresponding to F0-F2 stage of fibrosis (as per METAVIR score) while the rest (3 out of 5) had indeterminate values. While on FIB4 scoring, 4 subjects had scores suggesting stage 0-1 fibrosis while 1 had a score suggestive of stage 4-6 fibrosis as per Ishak Fibrosis staging. As PLHIV with known diabetes mellitus, obesity, and hypothyroidism were excluded from our study, the prevalence of MASLD observed in our study underestimates the real prevalence of MASLD in this specific population. No significant association was observed between ART status or ART regimen and MASLD in our study subjects. However, in light of the existing evidence of association of dolutegravir (DTG) with significant weight gain, and the recent inclusion of DTG in the first-line ART regimen nationally in India, robust surveillance and large-scale studies are recommended to study the contribution of DTG to MASLD in PLHIV, if any.
Keywords: NAFLD, MASLD, PLHIV, antiretroviral therapy, India
Introduction
Globally, 38.0 million people were living with HIV at the end of 2023. The “treat all” policy of the World Health Organization (WHO) has helped increase antiretroviral therapy (ART) coverage and access and, greatly diminish mortality in people living with HIV (PLHIV). In 2019, 68% of adults and 53% of children living with HIV globally were receiving lifelong ART. By June 2020, 26 million people were accessing ART, marking a 2.4% increase from an estimate of 25.4 million at the end of 2019. By comparison, treatment coverage increased by an estimated 4.8% between January and June of 2019. Nearly 690 000 people died of HIV-related illnesses in 2019, 60% fewer than in 2004 and 40% fewer than in 2010. 1 HIV continues to be a major global public health issue, having claimed almost 33 million lives so far. However, with increasing access to effective HIV prevention, diagnosis, treatment, and care, including for opportunistic infections, HIV infection has become a manageable chronic health condition, enabling PLHIV to lead long and healthy lives. The increased life expectancy of PLHIV due to the early ART initiation has led to emergence of non-communicable diseases (NCDs) like hypertension, cardiovascular diseases, and liver disease as important contributors to morbidity and mortality in this population. The increased incidence of obesity, insulin resistance, dyslipidemias, and mitochondrial dysfunction are all attributable to both the HIV infection itself and combination antiretroviral therapy (cART) and are the main drivers behind the increased incidence of NCDs in PLHIV. The proportion of deaths caused by liver-related causes has increased between 8-fold and 10-fold in the post-antiretroviral treatment (ART) era whereas AIDS-related mortality has fallen more than 90-fold. 1 The pathogenesis of metabolic dysfunction-associated steatotic liver disease (MASLD) earlier called nonalcoholic fatty liver disease (NAFLD) in individuals with HIV infection includes metabolic syndrome, hyperuricemia, HIV-related lipodystrophy, genetic polymorphisms, medications, HIV itself, and the gut microbiome. The prevalence of MASLD in persons with HIV infection ranges from 30% to 65% depending on the modality of diagnosis. 2 In fact, due to the introduction of effective therapies against viral hepatitis and the increasing rates of obesity in many Asian as well as Western countries, it is likely that fatty liver disease will become the leading cause of liver cirrhosis in HIV-infected patients. A deeper understanding and identification of the associated modifiable risk factors, both traditional and HIV-specific, may be highly beneficial in reducing the incidence of this spectrum of liver disease and greatly improving the quality of life of PLHIV. The prevalence of MASLD worldwide in HIV-uninfected population has been estimated to be 25% (9.3%-31%), 3 while the prevalence in populations with HIV has been estimated to be 35%. 2 MASLD represents an important risk factor for the development and progression of liver disease, and with the availability of effective hepatitis B and C antiviral medications, it is conceivable that MASLD could become the most prominent liver disease affecting individuals with HIV in the future. It is foreseen that the burden of MASLD on morbidity and mortality is higher in developing countries like India due to the lack of economic resources for screening and diagnosis. Added to that, the prevalence of metabolic syndrome and obesity is rapidly rising in the Asian population. 3 However, there are limited studies in the Indian population assessing the prevalence and risk factors associated with MASLD in PLHIV. Hence, this study is of great importance to assess the burden of MASLD in PLHIV in the Indian population. This will help in planning and executing interventions designed for overall reduction in morbidity and mortality due to liver disease in PLHIV.
Methods
Study Design and Setting
This study was conducted in the Department of Medicine and ART center in a large teaching public hospital in New Delhi, India.
This study was done to assess the occurrence of MASLD among PLHIV. Assessment of hepatic fibrosis among PLHIV with MASLD using transient elastography and clinical fibrosis score was done. In addition, the risk factors associated with MASLD in these subjects were determined. These patients were enrolled at a Centre of Excellence in HIV Care affiliated with a tertiary-level teaching hospital in North India and were receiving free highly-active antiretroviral therapy. It was a cross-sectional study conducted for a period of 1 year. Since this was a pilot study, a convenience sample of 30 subjects was chosen. Subjects were screened for eligibility using the following criteria and consecutive subjects who consented to participate were enrolled.
Inclusion Criteria
Confirmed HIV infection.
Age: 18 years or more.
Exclusion Criteria
History of alcohol consumption >140 g/week for men and >70 g/week for women.
Subjects who were HBsAg-positive
Subjects who were anti-HCV antibody-positive
Any other known cause of liver disease, eg, autoimmune hepatitis
Obese subjects, those with known diabetes mellitus, hypothyroidism
Pregnancy and/or lactation.
Methodology
All enrolled study subjects were subjected to the following assessments:
Socio-Demographic Assessment: This included assessment of age/date of birth, gender, education, occupation, socio-economic status using the Modified Kuppuswamy scale, state of residence (urban or rural), marital status using a predetermined proforma.
Clinical Evaluation: Detailed clinical history was taken from all subjects. This included history of time of diagnosis of HIV, route of transmission, opportunistic infection-related history, ART details, known history of any liver disease or any systemic illness known to cause liver disease, history of intake of alcohol and other hepatotoxic drugs. A complete clinical assessment was performed, including measurements of blood pressure, weight, height, body mass index (BMI), and waist and hip circumference. A detailed general physical and systemic examination was done in all subjects.
Laboratory Investigations: All study subjects underwent the following investigations: hemogram and platelet count, liver function tests (alanine transaminase (ALT), aspartate transaminase (AST), bilirubin, alkaline phosphatase), serum albumin, fasting blood sugar, HbA1c, fasting lipid profile (total cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides [TGs]). CD4 count and viral load was also obtained in all patients.
Assessment for MASLD: All study subjects underwent the following assessment for MASLD. Ultrasonography (USG) (abdomen)—a curvilinear probe (3-5 MHz) or sector transducer was used to scan the liver. The liver was scanned to its entire extent from the dome of the diaphragm to the inferior margins and from lateral to the medial margins. Fatty liver is seen as an increased echogenicity of the liver parenchyma. Further grading of hepatic steatosis was done to study the extent of fatty changes.
Grade 1: Diffusely increased echogenicity but periportal and diaphragmatic echogenicity is still appreciable.
Grade 2: Diffusely increased echogenicity obscuring periportal echogenicity but diaphragmatic echogenicity is still appreciable.
Grade 3: Diffusely increased echogenicity obscuring periportal as well as diaphragmatic echogenicity.
Assessment for Fibrosis: All study subjects found to have fatty liver on USG underwent the following assessment for fibrosis.
-
Transient Elastography:Using the EchoSens FibroScan 430 mini plus, the liver was assessed for severity of fibrosis. Liver stiffness is evaluated by measuring the velocity of a vibration wave (also called a shear wave) generated on the skin. Because fibrous tissue is harder than normal liver, the degree of hepatic fibrosis can be inferred from the liver hardness. A minimum of 10 valid readings, with at least 60% success rate and an interquartile range of less than or equal to 30% of the median value, are taken with the results expressed in kilopascals (kPa) and then the average is taken.
Interpretation: Liver Stiffness Fibrosis Severity (based on Brunt score)
<7 kPa F0-F1
7-8.7 kPa F2
>8.7-10.3 kPa F3
>10.3 kPa F4
-
Clinical Scores:
A) NAFLD score 4 —The score is calculated as NAFLD score = −1.675 + (0.037 × age [years]) + (0.094 × BMI [kg/m2]) + (1.13 × IFG/diabetes [yes = 1, no = 0]) + (0.99 × AST/ALT ratio) − (0.013 × platelet count [×109/L]) − (0.66 × albumin [g/dl]). It was done using the NAFLD score calculator (https://www.mdcalc.com/nafld-non-alcoholic-fatty-liver-disease-fibrosis-score).
Interpretation:
NAFLD Score Fibrosis Severity
<−1.455 F0-F2
−1.455 to 0.675 Indeterminate Score
>0.675 F3-F4
Fibrosis Severity Scale (meta-analysis of histological data in viral hepatitis [METAVIR] scoring)
F0 no fibrosis
F1 mild fibrosis
F2 moderate fibrosis
F3 severe fibrosis
F4 cirrhosis
B) FIB4 score 5 —The score is calculated as FIB-4 score = (age × AST)/(platelets × √(ALT)
It was done using the FIB4 score calculator (https://www.mdcalc.com/fibrosis-4-fib-4-index-liver-fibrosis).
Interpretation:
FIB4 score approximate fibrosis stage (based on Ishak fibrosis staging)
<1.45 0-1
1.45-3.25 2-3
>3.25 4-6
Statistical Analysis
The data was entered in MS Excel spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 25.0. For normally distributed continuous data, mean ± SD were calculated. For others that were not normally distributed, non-parametric tests were applied. The normality of data was tested using the Kolmogorov-Smirnov test. Qualitative variables were correlated using the chi-square test/Fisher's exact test. A P-value of <.05 was considered statistically significant.
Results
The results are presented in the following section.
All subjects included in the study were above 18 years of age. The mean age of the subjects was 33.36 ± 7.95 years (range 18-54 years). Maximum number of subjects were in the 31-40 years age group (46.7%). In the study population of 30 subjects, 22 (73.3%) were male and 8 (26.7%) were female. All subjects were literate, 85% had finished high school. The remaining 15% were graduates or with higher education. Forty percent were semi-skilled workers and 35% were skilled workers, the rest were unemployed. The majority of the subjects were residents of urban areas and belonged to the upper-lower socioeconomic class as per the modified Kuppuswamy scale.
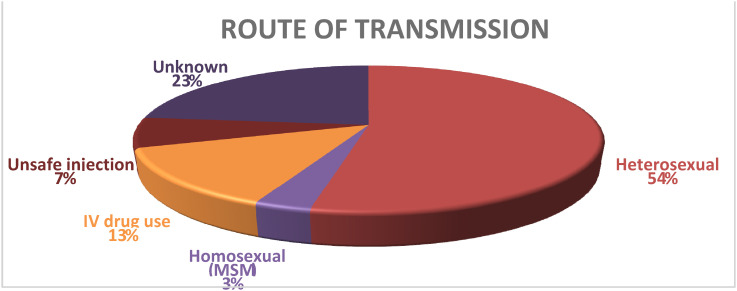
Route of Transmission
The self-perceived risk of HIV acquisition was enquired from all study subjects. The most common known route of HIV transmission was heterosexual route in 16 out of 30 (53.3%). The routes of transmission are shown in Figure 1.
Figure 1.
Routes of transmission of HIV in the subjects.
The study population included 13 subjects on ART (43.3%) and 17 ART-naïve subjects (56.6%). Out of the 13 study subjects, 5 (38.4%) were on tenofovir, lamivudine, and efavirenz (TLE) regimen and 4 (30.8%) were on tenofovir, lamivudine, and dolutegravir (TLD) regimen, while the other 4 patients were on second- or third-line regimens. The distribution of ART regimen in the study subjects has been summarized in Table 1.
Table 1.
ART regimen in recruited subjects.
| ART regimen | No. | % |
|---|---|---|
| TLD | 4 | 30.8 |
| TLE | 5 | 38.4 |
| RAL + LPV/r | 2 | 15.4 |
| T L + ATV/r | 1 | 7.7 |
| Z L + ATV/r | 1 | 7.7 |
| Total | 13 |
RAL, raltegravir; LPV/r, Lopinavir / ritonavir; TL, Tenofovir + Lamivudine; ATV/r, Atazanavir / ritonavir; ZL, Zidovudine + Lamivudine.
The mean BMI (kg/m2) of the study population was 20.77 ± 3.84 kg/m2. A majority (40%) of the subjects were underweight [BMI <18.5]. 30%of the study subjects were overweight/obese as per the Asian standards [BMI >22.9]. The BMI of the study subjects is summarized in Table 2.
Table 2.
BMI in the study subjects.
| BMI category | No. | % |
|---|---|---|
| <18.5 kg/m2 | 12 | 40.0 |
| 18.5-22.9 kg/m2 | 9 | 30.0 |
| 23.0-24.9 kg/m2 | 6 | 20.0 |
| 25.0-29.9 kg/m2 | 3 | 10.0 |
| Total | 30 |
Staging of the HIV infection was done in all the study subjects at study enrollment by using the WHO clinical staging system. The clinical stages identified are depicted in Table 3.
Table 3.
WHO clinical stage in the study subjects.
| WHO clinical stage | No. | % |
|---|---|---|
| Stage I | 5 | 16.6 |
| Stage II | 9 | 30.0 |
| Stage III | 9 | 30.0 |
| Stage IV | 7 | 23.3 |
| Total | 30 |
All patients underwent USG abdomen and fatty liver was detected in 5 out of 30 (17%) cases.22.7% of the male subjects included in the study had MASLD whereas none of the females had fatty changes in the liver on USG. Four out of 5 had Grade 1 fatty liver and one had Grade 2 fatty liver. No patient had Grade 3 fatty liver.
Out of the subjects who had MASFLD, 40% had stage I/II disease and 60% had stage III/IV disease. Out of the study subjects, MASLD was detected in 17.6% of ART-naïve subjects while it was detected in 15.4% of subjects on ART. The association of fasting blood glucose with MASLD was checked in the study population. Impaired fasting glucose (IFG) was defined as a fasting blood glucose of 100-125 mg/dL. The findings have been described in Table 4.
Table 4.
Association of IFG with MASLD.
| IFG | No MASLD | MASLD present | Total | P-value |
|---|---|---|---|---|
| No | 20 | 2 | 22 | .10 |
| Yes | 5 | 3 | 8 | |
| Total | 25 | 5 |
We analyzed 20 parameters for association with MASLD in the study subjects, including 13 biochemical parameters and some anthropometric measures. Although no statistically significant association was seen with any of these parameters, a few important trends were observed. These might be statistically significant in a higher power study with a larger sample size. Higher BMI (mean difference [MD] = 3.25, P = .09), waist circumference (MD = 3.84, P = .15), hip circumference (MD = 4.36, P = .14), and older age (MD = 6.56, P = .07) were observed to be associated with MASLD in our study, whereas the biochemical parameters and HIV-related factors were not seen to have any particular trend of association in our study. However, a higher median CD4 count was associated with MASLD as compared to the group without fatty changes on USG. This comparison is summarized in Table 5.
Table 5.
Associations of MASLD.
| Variable/parameter | No MASLD (n = 25) | MASLD present (n = 5) | Total (n = 30) | P-value |
|---|---|---|---|---|
| Age (years) [mean ± SD] | 32.24 ± 7.75 | 38.80 ± 7.29 | 33.33 ± 7.95 | .07 |
| Weight (kg) [mean ± SD] | 51.56 ± 9.80 | 61.40 ± 13.41 | 53.20 ± 10.87 | .18 |
| BMI (kg/m2) [mean ± SD] | 20.23 ± 3.58 | 23.48 ± 4.38 | 20.77 ± 3.84 | .09 |
| Waist circumference (cm) [mean ± SD] | 72.40 ± 6.32 | 76.24 ± 6.42 | 73.04 ± 6.39 | .15 |
| Hip circumference (cm) [mean ± SD] | 98.24 ± 5.93 | 102.60 ± 5.73 | 98.97 ± 6.03 | .14 |
| Hemoglobin [mean ± SD] | 10.26 ± 3.22 | 11.82 ± 1.45 | 10.52 ± 3.04 | .23 |
| TLC [mean ± SD] | 6576.0 ± 2338.24 | 6088.0 ± 1255.36 | 6494.67 ± 2185.48 | .71 |
| Platelets (lakhs/mm3) [mean ± SD] | 2.27 ± 0.97 | 2.26 ± 0.87 | 2.27 ± 0.94 | .95 |
| ALT (U/L), median (IQR) | 44 (32-70) | 38 (23.5-116) | 40.5 (32-63) | .67 |
| AST (U/L), median (IQR) | 44 (33.5-66.0) | 32 (24.5-132.5) | 42 (32.75-60.0) | .27 |
| INR [mean ± SD] | 1.17 ± 0.19 | 1.07 ± 0.14 | 1.15 ± 0.18 | .35 |
| Total protein (g/dL) [mean ± SD] | 6.70 ± 1.01 | 6.64 ± 0.49 | 6.69 ± 0.93 | .97 |
| Albumin (g/dL) [mean ± SD] | 3.26 ± 0.56 | 3.44 ± 0.59 | 3.28 ± 0.56 | .57 |
| Total cholesterol (mg/dL) [mean ± SD] | 158.84 ± 23.22 | 167.20 ± 31.36 | 160.23 ± 24.33 | .24 |
| LDL cholesterol (mg/dL) [mean ± SD] | 92.40 ± 20.59 | 102.80 ± 25.90 | 94.13 ± 21.42 | .22 |
| HDL cholesterol (mg/dL) [mean ± SD] | 38.28 ± 6.01 | 39.0 ± 1.0 | 38.40 ± 5.48 | .55 |
| Triglycerides (mg/dL) [mean ± SD] | 135.28 ± 50.08 | 115.01 ± 9.27 | 131.90 ± 46.33 | .67 |
| CD4 count (cells/mm3), median (IQR) | 275 (133-433) | 489 (172-706) | 289 (133-446) | .22 |
| Duration of HIV diagnosis | 2 months (1 day-127.5 months) | 0 (0-43 months) | 1.5 months (1 day-82.8 months) | .15 |
| Duration of ART (months) | 54 (40-106) | 39.5 | 54 (32.5-93) |
Fibrosis in MASLD
The study subjects with MASLD on USG underwent assessment for hepatic fibrosis. Hepatic fibrosis was assessed using transient elastography and clinical fibrosis predictive scores. 2 scores were utilized in our study (FIB4 score and NAFLD score). The prevalence of fibrosis and its grading on each modality has been described in the following tables (Tables 6–8).
Table 6.
Fibrosis stage based on FIB4 score (ISHAK fibrosis staging) in MASLD subjects.
| Fibrosis stage | No. | % |
|---|---|---|
| Stage 0-1 | 4 | 80.0 |
| Stage 2-3 | 0 | 0.0 |
| Stage 4-6 | 1 | 20.0 |
| Total | 5 |
Table 7.
Fibrosis stage based on NAFLD score in MASLD patients.
| METAVIR fibrosis stage based on NAFLD score | No. | % |
|---|---|---|
| Stage F0-F2 | 2 | 40.0 |
| Indeterminate | 3 | 60.0 |
| Stage F3-F4 | 0 | 0.0 |
| Total | 5 |
Table 8.
Fibrosis stage based on FibroScan in MASLD patients.
| Fibrosis stage | Liver stiffness (kPa) | No. | % |
|---|---|---|---|
| F0-F1 | <7 | 5 | 100 |
| F2 | >7-8.7 | 0 | 0 |
| F3 | >8.7-10.3 | 0 | 0 |
| F4 | >10.3 | 0 | 0 |
Tables 9 and 10 depict the assessment of the subjects with MASLD and a comparison of the 2 scoring systems.
Table 9.
Assessment of subjects with MASLD for fibrosis using FIB4 score, NAFLD score, and FibroScan (N = 5).
| FIB4 score (Ishak score) | NAFLD score (METAVIR score) | FibroScan | |||
|---|---|---|---|---|---|
| Stage | No. (%) | Stage | No. (%) | Stage | No. (%) |
| 0-1 | 4 (80%) | F0-F2 | 2 (40%) | F0-F1 | 5 (100%) |
| 2-3 | 0 | Indeterminate | 3 (60%) | F2 | 0 |
| 4-6 | 1 (20%) | F3-F4 | 0 | F3 | 0 |
| F4 | 0 | ||||
Table 10.
Proposed comparison between ISHAK staging and METAVIR scoring of hepatic fibrosis.
| ISHAK fibrosis stage | METAVIR score |
|---|---|
| 0-1 | F0-F1 |
| 2-3 | F2 |
| 4-5 | F3 |
| 6 | F4 |
Discussion
The present study assessed the prevalence of MASLD in 30 PLHIV attending a public ART center, the extent of hepatic fibrosis in those affected, and the risk factors associated with the development of MASLD. PLHIV with HBV/HCV coinfection, any known liver disease, a significant history of alcohol intake (defined as >140 g/week for men and >70 g/week for women), obesity, and known diabetes mellitus and hypothyroidism were excluded from the study as we wanted to study the prevalence of MASLD directly related to HIV infection only. In our study, the mean age of the subjects was 33.63 ± 7.95 years (range 18-54 years) and 73.3% were male. The majority of the subjects were residents of urban areas and belonged to the upper-lower socioeconomic class as per the modified Kuppuswamy scale. This is the usual profile of PLHIV accessing services through the ART centers in the national program—predominantly young, urban, upper lower socio-economic class.
The mean BMI of the study subjects was 20.77 ± 3.84 kg/m2 and 40% were underweight (BMI <18.5 kg/m2). Anand et al conducted a study to assess the anthropological and nutritional profile of PLHIV in India in 2014 and had similar observations, with a mean BMI of 19.73 ± 3.55 kg/m2 and 40% were underweight. 6 This is in stark contrast to the PLHIV population of the US where the data from NHANES 2003-2014 showed a mean BMI of 34.3 ± 2.3 kg/m2 among HIV-infected women and 26.2 ± 0.7 kg/m2 among HIV-infected men. 7 This reflects the prevalence of undernutrition among PLHIV in India. The reasons for this are manifold: lack of knowledge about a balanced diet, traditional low-protein diet, low socioeconomic status, food insecurity, psychosocial issues, social abuse or neglect by family, and the effect of HIV infection itself.
The study population comprised both ART naïve PLHIV and PLHIV receiving ART. Out of the subjects on ART, 69.2% were on first-line regimen and the rest were on other second-/third-line ART regimen. This high proportion of PLHIV on second and third-line ART regimen among the study subjects is possibly explained by the fact that our ART center is a designated Centre of Excellence in HIV Care where subjects are referred from all over North India for the initiation of second- and third-line ART regimen. Further, patients on second-line and third-line regimens would have been on ART for longer periods of time and hence an association between ART duration and MASLD could also be assessed.
All the study subjects underwent a USG for the identification of the development of MASLD in them. Steatosis was identified as an increase in the echogenicity of the liver seen as an increase in the hepatorenal contrast and was further graded into the 3 grades of fatty liver. Out of the 30 subjects, 16.6% (5 out of 30) were found to have MASLD on USG, with grade 1 fatty changes seen in 4 (13.3%) and grade 2 fatty changes seen in 1 out of 30 subjects (3.3%).
A meta-analysis of MASLD in PLHIV by Maurice et al described the prevalence of MASLD in PLHIV to be 35%. 8 Studies done in different parts of the world have described the prevalence of MASLD as varying from 28.8% to 48%. 8 A study performed in Canada by Vuille Lessard et al showed a 48% prevalence of MASLD in PLHIV on transient elastography, whereas studies done in Italy and Spain showed a prevalence of 36.9% and 40% respectively with computerised tomography (CT) used as the modality of diagnosis.9–11 A Chinese study using magnetic resonance spectrometry as the modality for diagnosis, described the prevalence of MASLD in PLHIV to be 28.8%, whereas a similar study in the Japanese PLHIV population used USG as the modality and observed the prevalence to be 31%.12,13
Multiple factors can explain the relatively lower prevalence of MASLD seen in our study as compared to the observations by others. Firstly, most of the studies included in the meta-analysis used CT, biopsy, or transient elastography as the diagnostic modality, which have much higher sensitivity as compared to USG for the detection of MASLD in the patients. 8 Another major factor was the exclusion of patients with traditional risk factors for MASLD such as obesity, diabetes mellitus, and hypothyroidism in this study which have well-known association with the development of MASLD and metabolic syndrome. In the present study, we wanted to estimate the prevalence of MASLD that can be directly attributed to HIV infection in the study subjects by excluding those with known risk factors. If we had recruited subjects without excluding these risk factors, the prevalence of MASLD in our population would also have been higher. To the best of our knowledge, there are no published studies assessing the prevalence of MASLD among PLHIV in India. However, there are studies describing the prevalence of MASLD in the Indian general population. Amarapurkar et al evaluated 1168 study subjects with an USG to study the prevalence of MASLD in the Indian population and found it to be 16.6%. 14 Similar studies conducted in the eastern coastal India and southern India found the prevalence of MASLD to be 24.5% and 32%, respectively.15,16
The socio-demographic, anthropological, clinical, and immunological parameters that predicted MASLD in PLHIV were assessed in our study. As the study subjects were only 30, the study may not be powered enough to obtain meaningful statistical associations. Still, an attempt was made to assess the risk factors associated with MASLD. All 5 subjects, who were detected to have MASLD on USG, were male. As per existing data, the overall prevalence of MASLD appears to be higher in men, however, the prevalence of NASH with more advanced stages of fibrosis appears to be somewhat higher in women. 17 Differential rates of disease progression resulting in an increased risk of severe fibrosis in post-menopausal women compared to men are possibly attributed to loss of the protective effects of estrogen against fibrogenesis. 18
The mean BMI of subjects with MASLD was higher (23.48 ± 4.38 kg/m2) compared to those without MASLD (20.23 ± 3.58 kg/m2). The same was seen for the mean weight (61.40 kg vs 51.56 kg), waist circumference (76.24 cm vs 72.40 cm), and hip circumference (102.60 cm vs 98.24 cm). Though these differences were large, however, none of these parameters achieved statistical significance, owing probably to the small sample size. Maurice et al described a significant association of MASLD with high BMI (MD = 2.92), waist circumference (MD = 8.05), and type 2 diabetes mellitus (OR = 1.61) in their meta-analysis of MASLD in PLHIV. 8
This observation reflects the important role of obesity, insulin resistance, and adipose tissue-mediated cytokines in the pathogenesis and development of MASLD. The proposed mechanisms are the imbalance of adipose-derived signals secondary to increased adipose tissue, especially decreased adiponectin levels, leading to increased free fatty acids (FFA) supply due to increased lipolysis from both visceral/subcutaneous adipose tissue and/or increased intake of dietary fat, decreased FFA oxidation, increased de novo hepatic lipogenesis and decreased hepatic very low-density lipoprotein-TG secretion. These mechanisms lead to the accumulation of various lipids (predominantly TGs) in the liver resulting in hepatic steatosis. This association is very crucial, especially in light of the increased incidence of weight gain and obesity observed with the newer ART regimen, especially with dolutegravir (DTG) and tenofovir alafenamide (TAF). Only recently our national program has transitioned to a DTG-based first-line ART for all PLHIV. We need robust surveillance systems to monitor weight gain on DTG (if any) in our population. No significant association was seen between ART status and MASLD in our study subjects. 15.4% of the subjects on ART had MASLD while 17.6% of the ART-naïve study subjects had MASLD (P = 1.0). Duration of ART, duration since HIV diagnosis, and WHO clinical stage of the disease also did not have any association with MASLD in the study subjects. Similar results were obtained in the meta-analysis by Maurice et al 8 In the study conducted by Nishijima et al in Japan, 435 Asians with HIV infection were analyzed for the association of MASLD with both traditional and HIV-related risk factors. Multivariate analysis showed that no HIV-specific variables, including treatment with dideoxynucleoside analogs (didanosine, stavudine, and zalcitabine) and cumulative duration of ART, were associated with MASLD. 12
The association of CD4 count with MASLD was also assessed in our study. The median CD4 in the subjects with MASLD was 489 cells/mm3 whereas the subjects without MASLD on USG had a median CD4 count of 275 cells/mm3. This observation, although statistically insignificant (P = .22), is consistent with other published studies assessing the parameter. In a study conducted by Guaraldi et al, the mean CD4 count in the subjects with MASLD was 585.9 cells/mm3 as compared to 509.1 cells/mm3 in the subjects without MASLD. 11 Similarly, higher CD4 counts were observed in PLHIV with MASLD as compared to those without MASLD in studies conducted by Lui et al (551 vs 483 cells/mm3) and Nishijima et al (421 vs 343 cells/mm3). 13 The role of ART in the development of MASLD is still controversial given the contrasting results of various studies. Several antiretroviral drugs cause unfavorable metabolic changes such as dyslipidemia and insulin resistance. Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTIs) cause metabolic disturbances by inhibiting the replication of the mitochondrial DNA by binding to intramitochondrial polymerase gamma, leading to impairment of oxidative phosphorylation promoting the formation of reactive oxygen species and subsequently, mitochondrial injury. 19 Another class of ARTs, protease inhibitors (PIs), especially the early generation PIs, also display an unfavorable metabolic profile with an increased risk for insulin resistance and dyslipidemia.
The recent inclusion of DTG in the first-line ART regimen worldwide merits special attention in this context as DTG is well known to cause significant weight gain. In the ADVANCE trial, mean weight gain in the DTG-containing regimen was 7.1 kg when combined with TAF and 4.3 kg when combined with TDF, as compared to 2.3 kg in the group on TLE regimen, and was greater among women than men. 20 The viral suppression achieved with DTG, the high genetic barrier to resistance, and its side effect profile are remarkable upgrades over efavirenz and other non nucleoside reverse transcriptase inhibitors as the incidence of drug-induced liver disease and neuropsychiatric events are much lower with DTG. However, the increased weight gain and the higher association with obesity and insulin resistance leading to the development of T2DM can indirectly lead to increased development of MASLD and increased cardiovascular mortality. To gauge this effect, more large-scale cohort studies need to be conducted.
Several biochemical parameters including hemoglobin, TLC, platelet count, ALT, AST, serum protein, serum albumin, international normalised ratio, total cholesterol, LDL, high-density lipoprotein (HDL), and serum TG levels were analyzed for association with MASLD in the study subjects. None of them had a statistically significant association with the development of MASLD. IFG was also analyzed for association with the development of MASLD. 37.5% of the subjects with IFG had MASLD whereas only 9% of the subjects without IFG had fatty changes. This is a significant observation though this difference was not statistically significant, probably due to the small size of the group with IFG (P = .10). IFG represents a component of metabolic syndrome and is often an early sign of insulin resistance. High fasting glucose level was seen to be an independent risk factor with a statistically significant association with MASLD in PLHIV by Maurice et al. 8 A study by Jimba et al studied the association of MASLD with fasting plasma glucose (FPG) levels in the Japanese population. The prevalence of MASLD increased with increasing FPG levels: 27% in the subgroup with normal fasting glucose, 43% in IFG, and 62% in newly diagnosed diabetes. 21
Fibrosis
In our study, the subjects who were detected to have fatty changes on USG were then assessed for fibrosis. Identification and quantification of fibrosis are clinically important because fibrosis correlates with clinical outcomes. The individuals with MASLD were assessed for hepatic fibrosis using both clinical scores and transient elastography.
There are multiple clinical scoring systems to predict hepatic fibrosis. We have included NAFLD score and FIB4 score in our study to assess the subjects for fibrosis based on clinical and laboratory parameters. Both scoring systems have shown promise in identifying advanced fibrosis in MASLD patients with fairly high accuracy.22,23 Cut-offs established based on results of previous studies comparing the scoring systems with liver biopsy findings have been used to maximize the positive and negative predictive values.
Transient elastography is one of the best radiological modalities to detect advanced hepatic fibrosis. Liver stiffness is evaluated by measuring the velocity of a vibration wave (also called a shear wave) generated on the skin. Because fibrous tissue is harder than normal liver, the degree of hepatic fibrosis can be inferred from the liver hardness. The cut-offs corresponding to different grades of hepatic fibrosis on liver biopsy have been established for specific liver diseases. The cut-off values for liver stiffness on FibroScan in MASLD have been accepted from the study by Wong et al in 2010 which corresponds to the Brunt score of hepatic fibrosis on biopsies. 24
On FibroScan, all 5 subjects with fatty changes in our study were found to have liver stiffness less than 7 kPa which corresponds to F0-F1 stage of fibrosis.
Using the NAFLD score, 2 subjects had scores corresponding to F0-F2 stage of fibrosis (as per METAVIR score) while the rest (3 out of 5) had indeterminate values. While on FIB4 scoring, 4 subjects had scores suggesting stage 0-1 fibrosis while 1 had a score suggestive of stage 4-6 fibrosis as per Ishak Fibrosis staging (Table 9). The proposed comparison between Ishak fibrosis stages and METAVIR scoring has been shown in Table 10.
To summarize, MASLD is an important disorder and is part of the spectrum of metabolic diseases and NCDs that are on the rise in PLHIV. As the burden of Opportunistic infections is reducing among PLHIV with earlier initiation of ART, NCDs are emerging as significant contributors to the overall morbidity and mortality in these patients. With increasing life span due to effective ART, the burden of NCDs is escalating. Even among developing and resource-constrained nations, the most important cause of mortality among PLHIV is now NCDs. The unique metabolic milieu of the PLHIV—chronic inflammation, insulin resistance, metabolic abnormalities, and long-term ART—all play a part in the development and progression of NCDs.
MASLD is an important metabolic disease among these. In light of the existing literature and the above observations, we emphasize that MASLD in PLHIV is an understudied clinical entity that greatly contributes to the overall mortality and morbidity in the HIV-infected population.
MASLD contributes directly to mortality secondary to liver disease through progression to cirrhosis and end-stage liver disease, as well as increases cardiovascular mortality due to the associated features of insulin resistance and metabolic syndrome. A noteworthy point to understand is that if detected early, timely interventions in the diet and lifestyle along with the available pharmacological therapeutic options can drastically help in decelerating the progression of liver disease and the onset of fibrosis leading to a cascading decrease in mortality.
Studies on MASLD are yet lacking in our country. Ours is among the first Indian studies to systematically study MASLD among PLHIV. Hence, there is a dearth of data on the exact burden and impact of the disease in the Indian PLHIV. It is imperative that larger, multicenter studies across ART centers in India should be done to understand the extent of the problem. The national program must now focus its attention on ameliorating NCDs among PLHIV and develop strategies for the screening, diagnosis, and management of these conditions among them.
What This Study Adds
As PLHIV with known diabetes mellitus, obesity, and hypothyroidism were excluded from our study, the prevalence of MASLD observed in our study underestimates the real prevalence of MASLD in this specific population.
The prevalence of MASLD in PLHIV in our study was 16.6%. This is a large number, considering the exclusion criteria of other predisposing conditions like obesity, T2DM, hypothyroidism, and concurrent hepatitis B/C. This underscores the requirement of a screening strategy as part of the national program for PLHIV to detect MASLD early.
However, in light of the existing evidence of association of DTG with significant weight gain, and the recent inclusion of DTG in the first-line ART regimen nationally, robust surveillance and large-scale studies are recommended to study the contribution of DTG to MASLD in PLHIV, if any.
Acknowledgments
None.
Footnotes
Authors’ Note: Participants: PLHIV attending the ART center and Department of Medicine, Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi.
Author Contributions: All authors contributed to conceptualization and carrying out the study. They also were involved in the drafting of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: The study was approved by the MAMC Institutional Ethics Committee vide F.1/IEC/MAMC/170/05/2019/No 442/1-11-19.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Informed written consent was obtained from all patients.
ORCID iDs: Anuradha Subramanian https://orcid.org/0000-0001-9116-7233
Rajeshwari K. https://orcid.org/0000-0002-7144-9871
References
- 1.The World Health Organisation. HIV/AIDS [cited 2021 Oct 19]. https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
- 2.Seth A, Sherman KE. Fatty liver disease in persons with HIV infection. Top Antivir Med. 2019;27(2):75–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 4.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 6.Anand D, Puri S. Anthropometric and nutritional profile of people living with HIV and AIDS in India: an assessment. Indian J Community Med. 2014;39(3):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thuppal SV, Jun S, Cowan A, Bailey RL. The nutritional status of HIV-infected US adults. Curr Dev Nutr. 2017;1(10):e001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS. 2017;31(11):1621–1632. [DOI] [PubMed] [Google Scholar]
- 9.Macías J, González J, Tural C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28(9):1279–1287. [DOI] [PubMed] [Google Scholar]
- 10.Vuille-Lessard É, Lebouché B, Lennox L, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS. 2016;30(17):2635–2643. [DOI] [PubMed] [Google Scholar]
- 11.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–257. [DOI] [PubMed] [Google Scholar]
- 12.Nishijima T, Gatanaga H, Shimbo T, et al. Traditional but not HIV-related factors are associated with nonalcoholic fatty liver disease in Asian patients with HIV-1 infection. PLoS One. 2014;9(1):e87596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lui G, Wong VW-S, Wong GL-H, et al. Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment Pharmacol Ther. 2016;44(4):411–421. [DOI] [PubMed] [Google Scholar]
- 14.Amarapurkar D, Kamani P, Patel N, et al. Prevalence of non alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6(3):161–163. [PubMed] [Google Scholar]
- 15.Singh SP, Nayak S, Swain M, et al. Prevalence of nonalcoholic fatty liver disease in coastal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25(2):76–79. [PubMed] [Google Scholar]
- 16.Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS. Prevalence of non alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84(1):84–91. [DOI] [PubMed] [Google Scholar]
- 17.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26(7):856–863. [DOI] [PubMed] [Google Scholar]
- 18.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(4):1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e676. [DOI] [PubMed] [Google Scholar]
- 21.Jimba S, Nakagami T, Takahashi M, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22(9):1141–1145. [DOI] [PubMed] [Google Scholar]
- 22.Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47(2):455–460. [DOI] [PubMed] [Google Scholar]
- 23.Sumida Y, Yoneda M, Hyogo H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong VW-S, Vergniol J, Wong GL-H, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–462. [DOI] [PubMed] [Google Scholar]