Abstract
1. A rapid method for the isolation of hormonally sensitive rat fat-cell plasma membranes was developed by using immunological techniques. 2. Rabbit anti-(rat erythrocyte) sera were raised and shown to cross-react with isolated rat fat-cells. 3. Isolated rat fat-cells were coated with rabbit anti-(rat erythrocyte) antibodies, homogenized and the homogenate made to react with an immunoadsorbent prepared by covalently coupling donkey anti-(rabbit globulin) antibodies to aminocellulose. Uptake of plasma membrane on to the immunoadsorbent was monitored by assaying the enzymes adenylate cyclase and 5'-nucleotidase and an immunological marker consisting of a 125I-labelled anti-(immunoglobulin G)-anti-cell antibody complex bound to the cells before fractionation. Contamination of the plasma-membrane preparation by other subcellular fractions was also investigated. 4. By using this technique, a method was developed allowing 25-40% recovery of plasma membrane from fat-cell homogenates within 30 min of homogenization. 5. Adenylate cyclase in the isolated plasma-membrane preparation was stimulated by 5 mum-adrenaline.
Full text
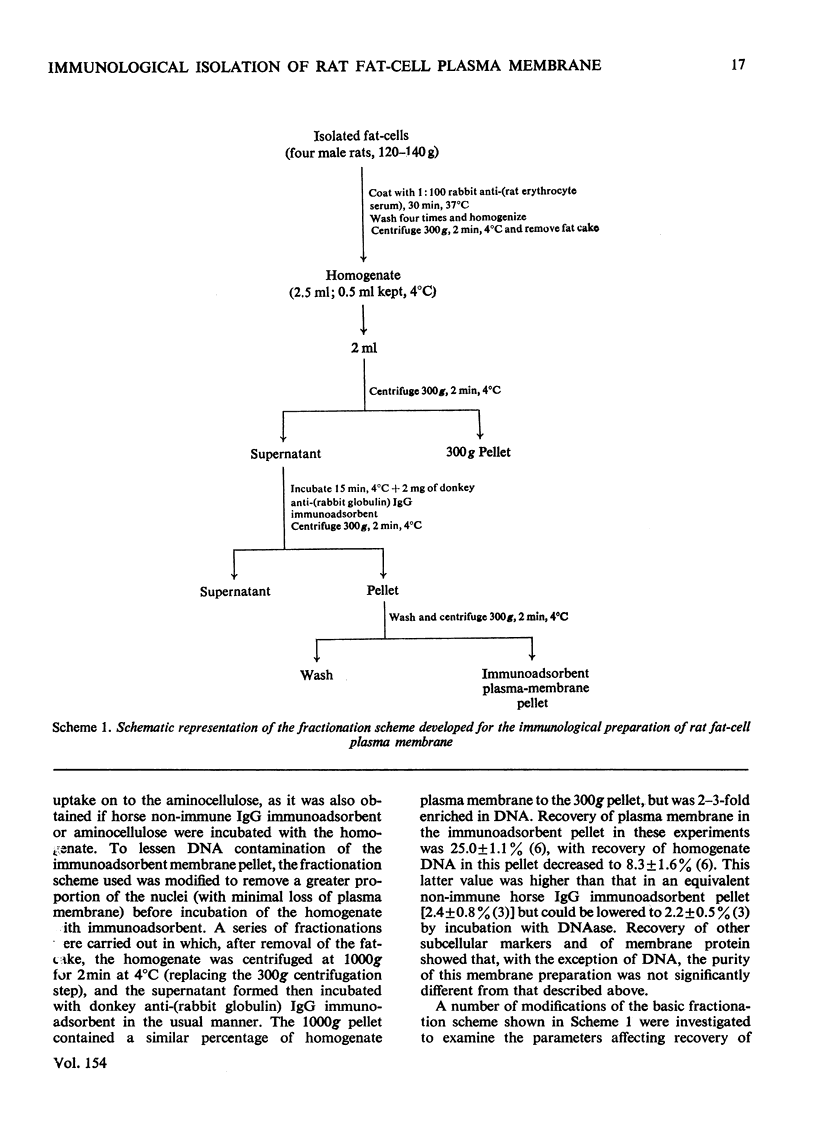
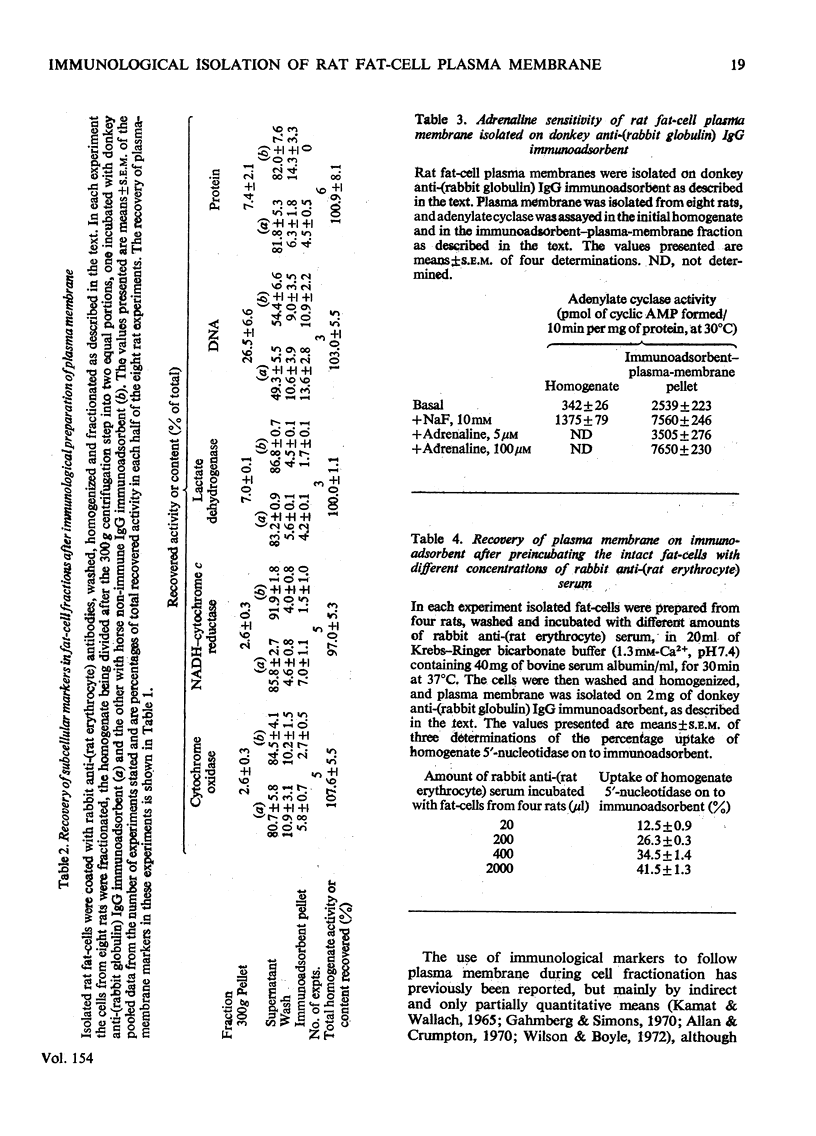
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Beck P., Nicholas H. Immunoassay of serum polypeptide hormones by using 125I-labelled anti(-immunoglobulin G) antibodies. Biochem J. 1975 Mar;145(3):607–616. doi: 10.1042/bj1450607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C. W., Ford L. E., Bond H. E., Stuart D. C., Lorenz D. Isolation of plasma membrane fragments from HeLa cells. J Cell Biol. 1969 May;41(2):378–392. doi: 10.1083/jcb.41.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Chang K. J., Bennett V., Cuatrecasas P. Membrane receptors as general markers for plasma membrane isolation procedures. The use of 125-I-labeled wheat germ agglutinin, insulin, and cholera toxin. J Biol Chem. 1975 Jan 25;250(2):488–500. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- Gahmberg C. G., Simons K. Isolation of plasma membrane fragments from BHK21 cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(2):176–182. doi: 10.1111/j.1699-0463.1970.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Hales C. N. Immunological techniques in diabetes research. Diabetologia. 1972 Aug;8(4):229–235. doi: 10.1007/BF01225565. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Luzio J. P., Chandler J. A., Herman L. Localization of calcium in the smooth endoplasmic reticulum of rat isolated fat cells. J Cell Sci. 1974 Jun;15(1):1–15. doi: 10.1242/jcs.15.1.1. [DOI] [PubMed] [Google Scholar]
- Jarett L., Reuter M., McKeel D. W., Smith R. M. Loss of adenyl cyclase hormone receptors during purification of fat cell plasma membranes. Endocrinology. 1971 Nov;89(5):1186–1190. doi: 10.1210/endo-89-5-1186. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M., Crespin S. R. Epinephrine binding to rat adipocytes and their subcellular fractions. Endocrinology. 1974 Mar;94(3):719–729. doi: 10.1210/endo-94-3-719. [DOI] [PubMed] [Google Scholar]
- Jarett L. Subcellular fractionation of adipocytes. Methods Enzymol. 1974;31:60–71. doi: 10.1016/0076-6879(74)31007-5. [DOI] [PubMed] [Google Scholar]
- KAMAT V. B., WALLACH D. F. SEPARATION AND PARTIAL PURIFICATION OF PLASMA-MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA MICROSOMES. Science. 1965 Jun 4;148(3675):1343–1345. doi: 10.1126/science.148.3675.1343. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Krug F., Desbuquois B., Cuatrecasas P. Glucagon affinity absorbents: selective binding of receptors of liver cell membranes. Nat New Biol. 1971 Dec 29;234(52):268–270. doi: 10.1038/newbio234268a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L. E., Hales C. N. The preparation and properties of purified 125-I-labelled antibodies to insulin. Biochem J. 1968 Jul;108(4):611–618. doi: 10.1042/bj1080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Luzio J. P., Hales C. N. The properties and extracellular location of 5'-nucleotidase of the rat fat-cell plasma membrane. Biochem J. 1975 Mar;146(3):625–633. doi: 10.1042/bj1460625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest. 1974 Jun;33(4):291–306. doi: 10.1080/00365517409082499. [DOI] [PubMed] [Google Scholar]
- Siddle K., Hales C. N. The relationship between the concentration of adenosine 3':5'-cyclic monophosphate and the anti-lipolytic action of insulin in isolated rat fat-cells. Biochem J. 1974 Jul;142(1):97–103. doi: 10.1042/bj1420097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle K., Kane-Maguire B., Campbell A. K. The effects of glucagon and insulin on adenosine 3':5'-cyclic monophosphate concentrations in an organ culture of mature rat liver. Biochem J. 1973 Apr;132(4):765–773. doi: 10.1042/bj1320765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- Wilson L. A., Boyle W. A model system for study of subcellular distribution of cell surface antigens. J Immunol. 1972 Feb;108(2):460–466. [PubMed] [Google Scholar]



