Abstract
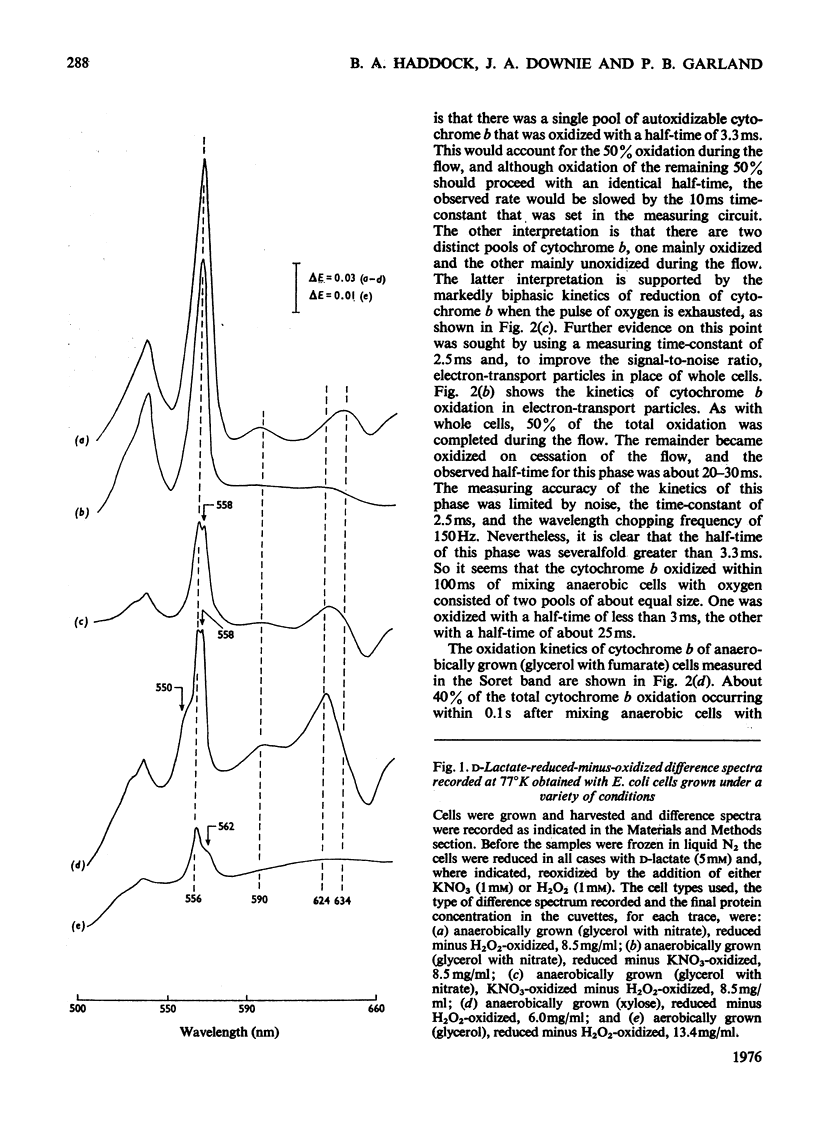
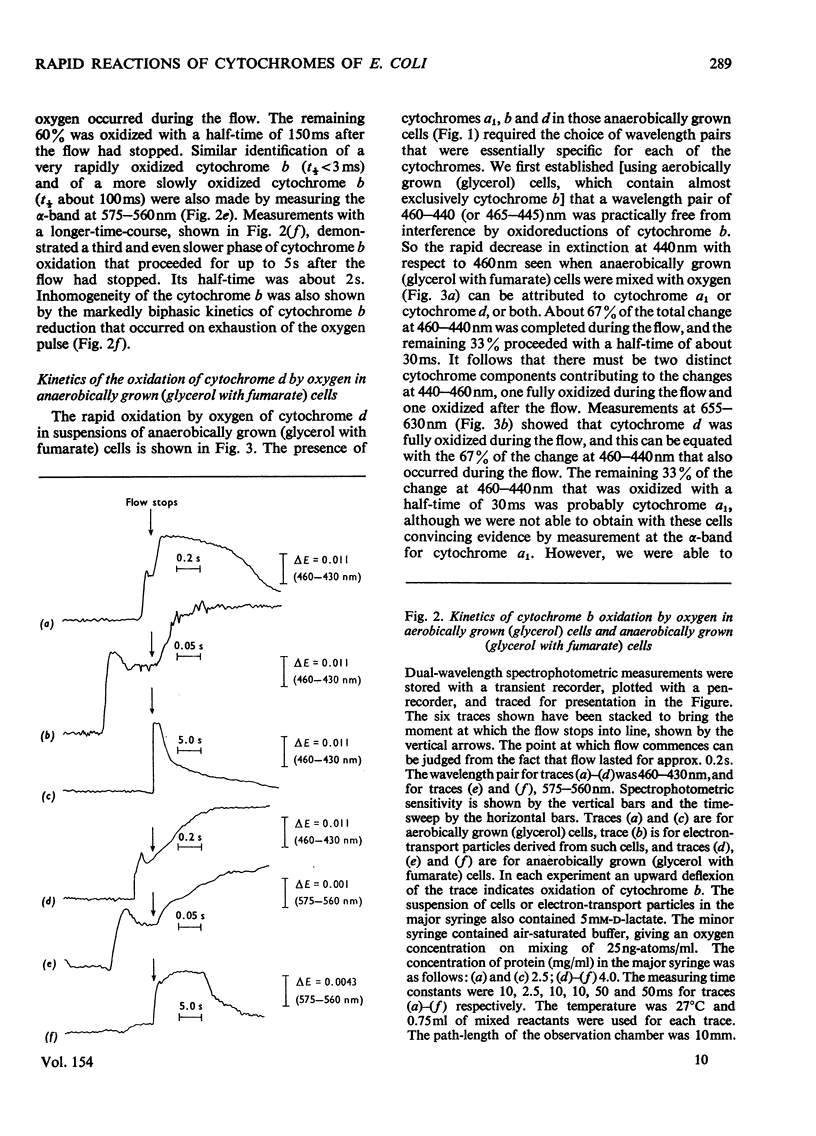
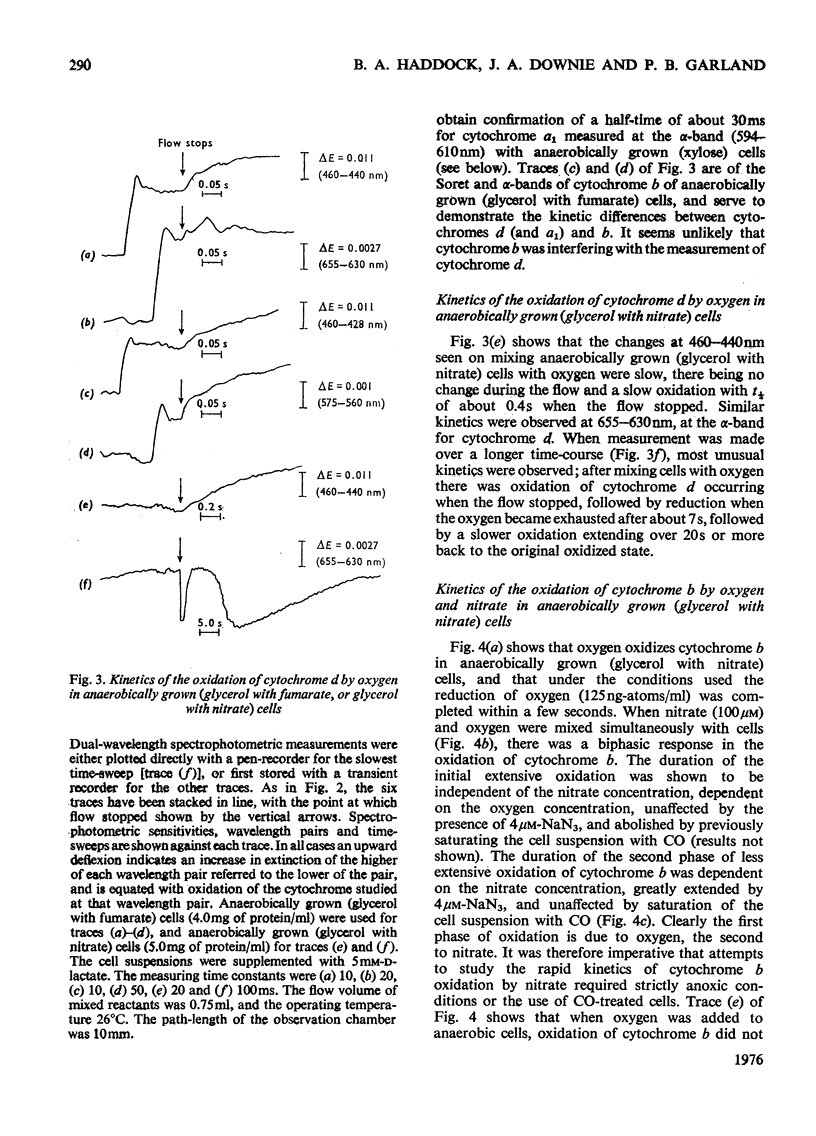
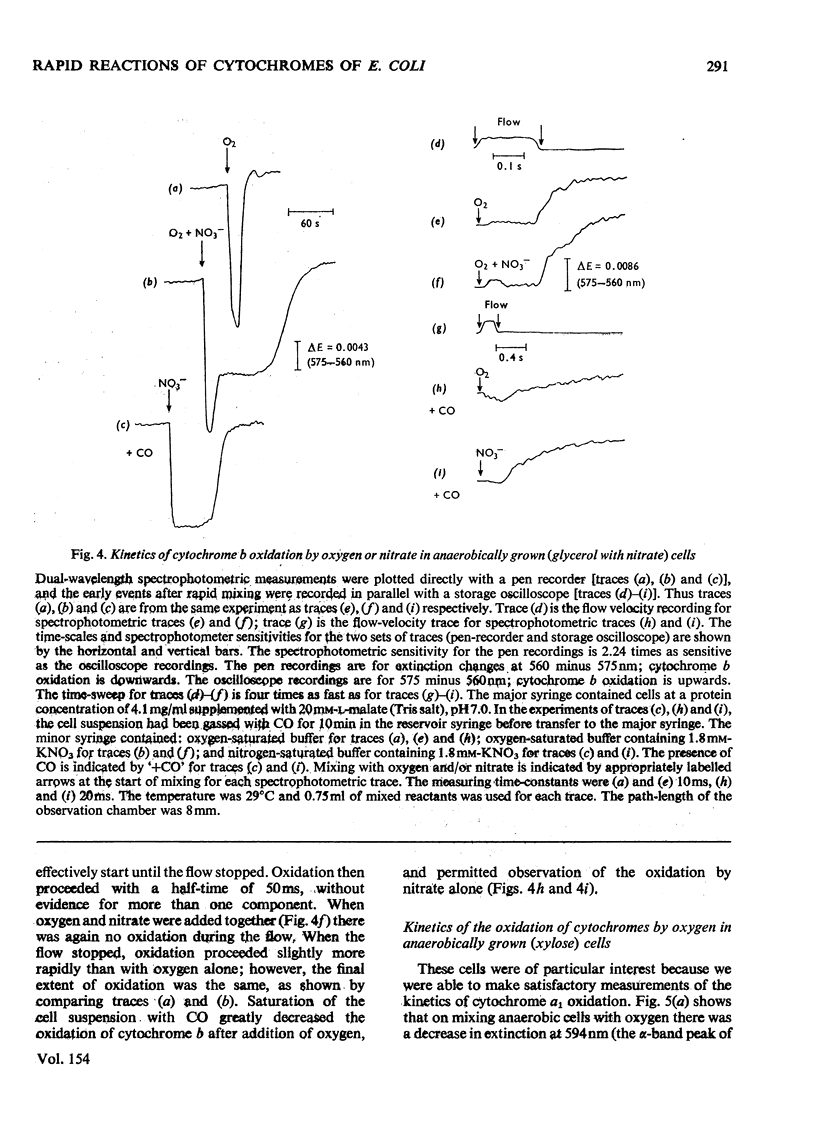
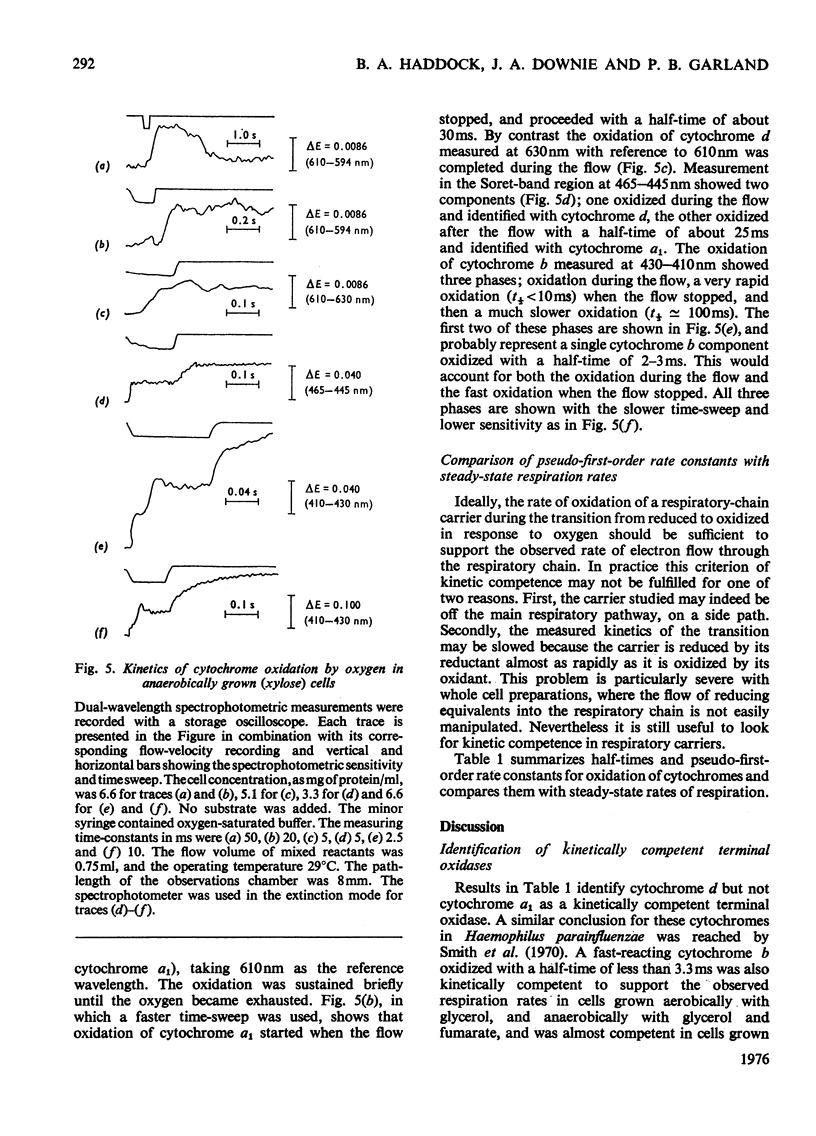
A study was made of the rapid oxidation kinetics of the cytochromes of Escherichia coli. The b-type cytochromes were kinetically heterogeneous, with one species (presumably cytochrome o) oxidized so rapidly that it could fully support observed oxidation rates. Cytochrome d but not cytochrome a1 was also kinetically competent to support respiration. However, in cells grown anaerobically in the presence of NO3-, cytochrome d exhibited slow oxidation kinetics and a red-shift in its reduced-minus-oxidized difference spectrum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft J. R., Haddock B. A. Synthesis of alternative membrane-bound redox carriers during aerobic growth of Escherichia coli in the presence of potassium cyanide. Biochem J. 1975 May;148(2):349–352. doi: 10.1042/bj1480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- CHANCE B. The spectra of the enzyme-substrate complexes of catalase and peroxidase. Arch Biochem Biophys. 1952 Dec;41(2):404–415. doi: 10.1016/0003-9861(52)90469-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem. 1955 Nov;217(1):429–438. [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. Studies on soluble cytochromes in Enterobacteriaceae. II. Cytochromes b-562 and c-550. J Biochem. 1966 Sep;60(3):329–334. doi: 10.1093/oxfordjournals.jbchem.a128440. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Littleford S. J., Haddock B. A. A stopped-flow dual-wavelength spectrophotometer suitable for the study of respiratory chains. Biochem J. 1976 Feb 15;154(2):277–284. doi: 10.1042/bj1540277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A. The reconstitution of oxidase activity in membranes derived from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. Biochem J. 1973 Dec;136(4):877–884. doi: 10.1042/bj1360877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Towne D. W., Shrager R. I. Redox properties of beta-type cytochromes in Escherichia coli and rat liver mitochondria and techniques for their analysis. Biochim Biophys Acta. 1975 Jan 31;376(1):42–62. doi: 10.1016/0005-2728(75)90203-0. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Kauffman H. F., van Gelder B. F. The respiratory chain of Azotobacter vinelandii. I. Spectral properites of cytochrome d. Biochim Biophys Acta. 1973 May 30;305(2):260–267. doi: 10.1016/0005-2728(73)90174-6. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Jones C. W. Reactivity with oxygen of bacterial cytochrome oxidases a1, aa3 and o. FEBS Lett. 1973 Jun 15;33(1):101–105. doi: 10.1016/0014-5793(73)80169-3. [DOI] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- OTA A., YAMANAKA T., OKUNUKI K. OXIDATIVE PHOSPHORYLATION COUPLED WITH NITRATE RESPIRATION. II. PHOSPHORYLATION COUPLED WITH ANAEROBIC NITRATE REDUCTION IN A CELL-FREE EXTRACT OF ESCHERICHIA COLI. J Biochem. 1964 Feb;55:131–135. [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp W. S. Cytochromes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):459–472. doi: 10.1016/0003-9861(72)90063-x. [DOI] [PubMed] [Google Scholar]
- Smith L., White D. C., Sinclair P., Chance B. Rapid reactions of cytochromes of Hemophilus parainfluenzae on addition of substrates or oxygen. J Biol Chem. 1970 Oct 10;245(19):5096–5100. [PubMed] [Google Scholar]


