Abstract
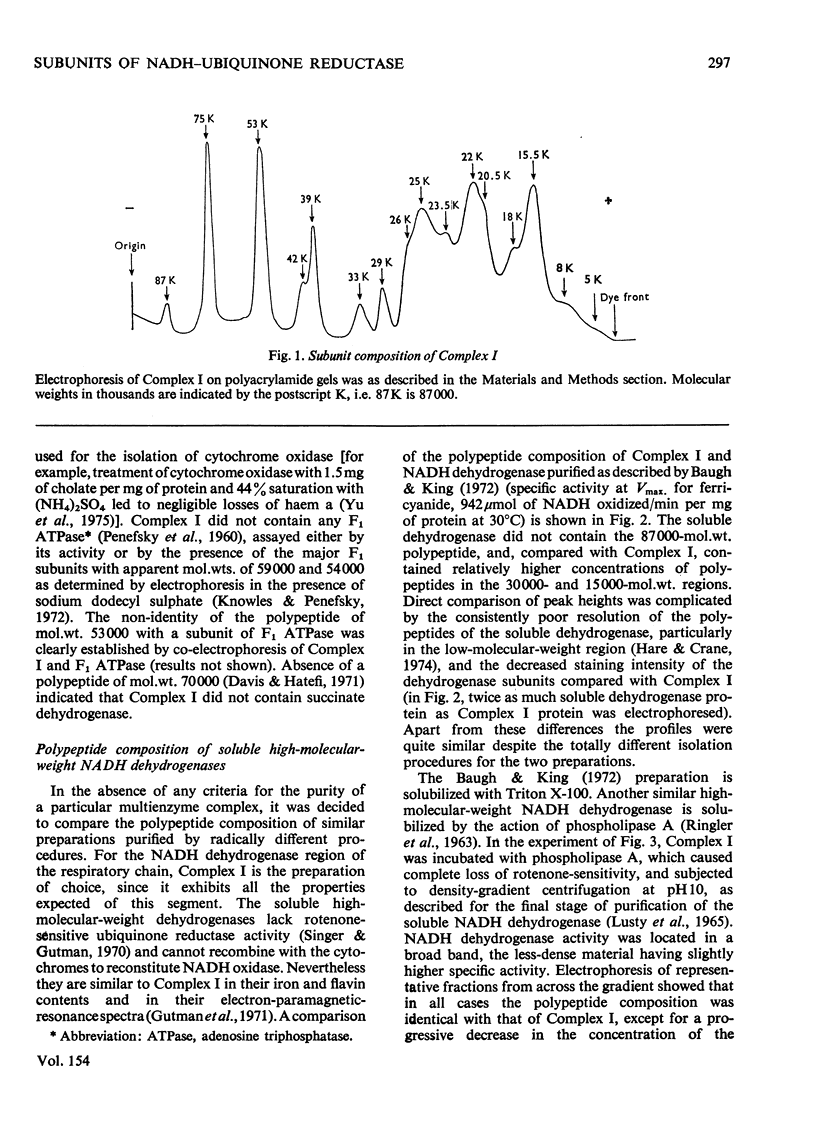
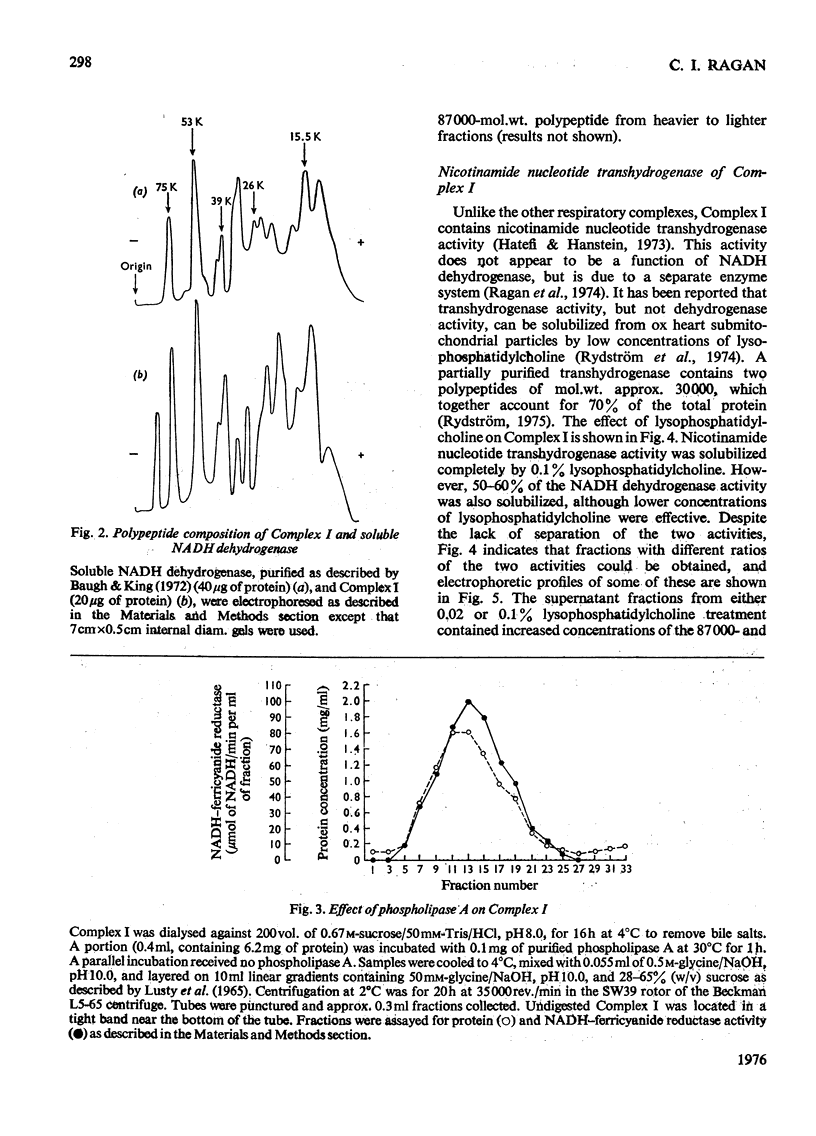
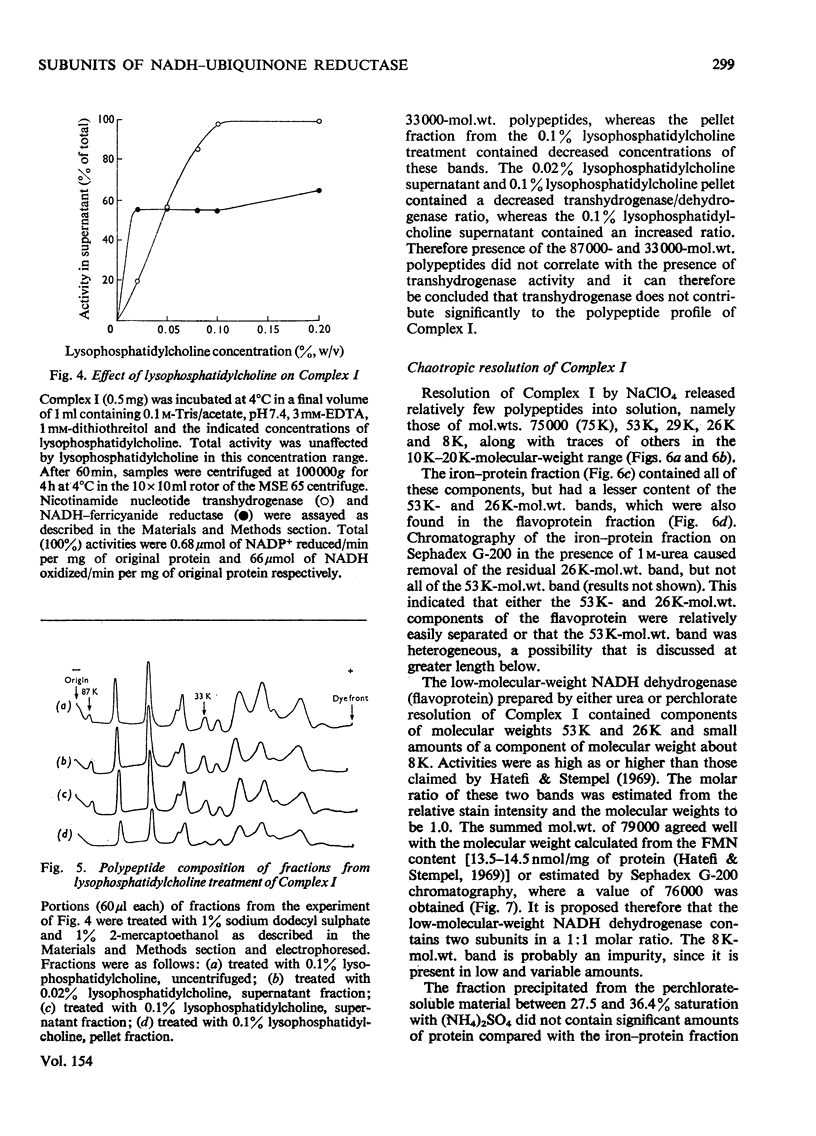
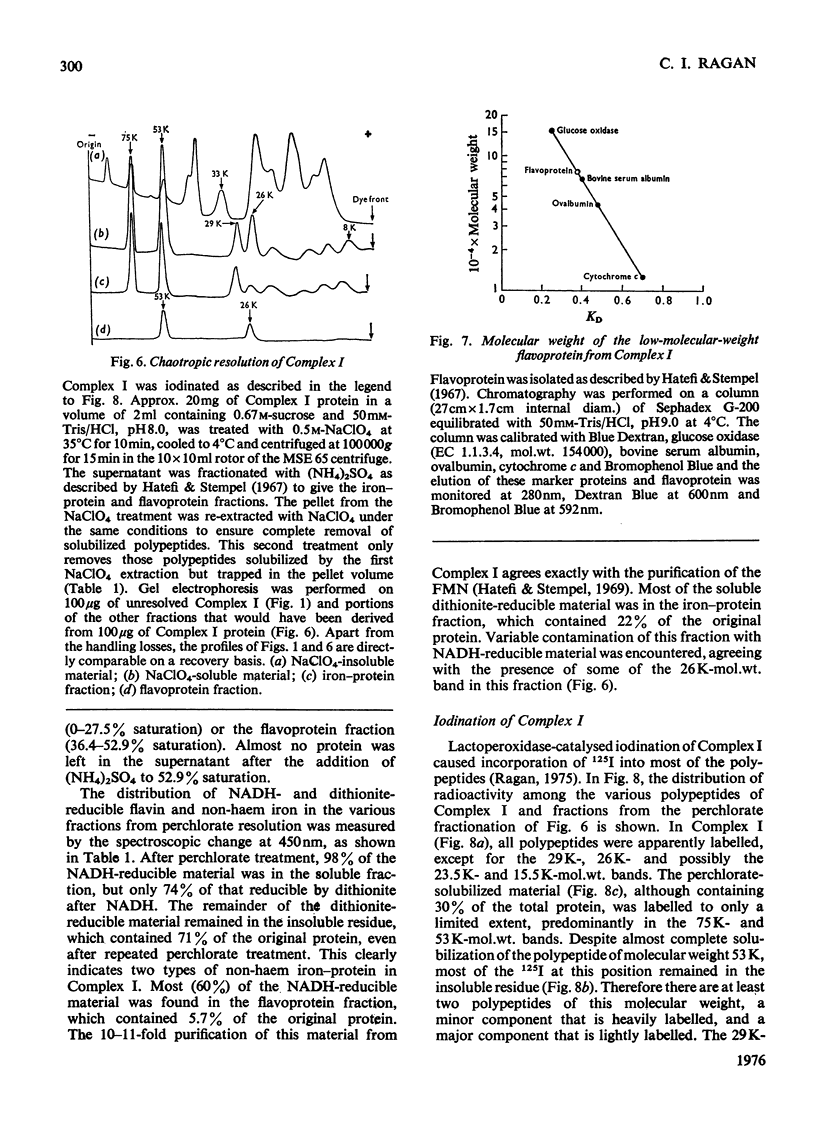
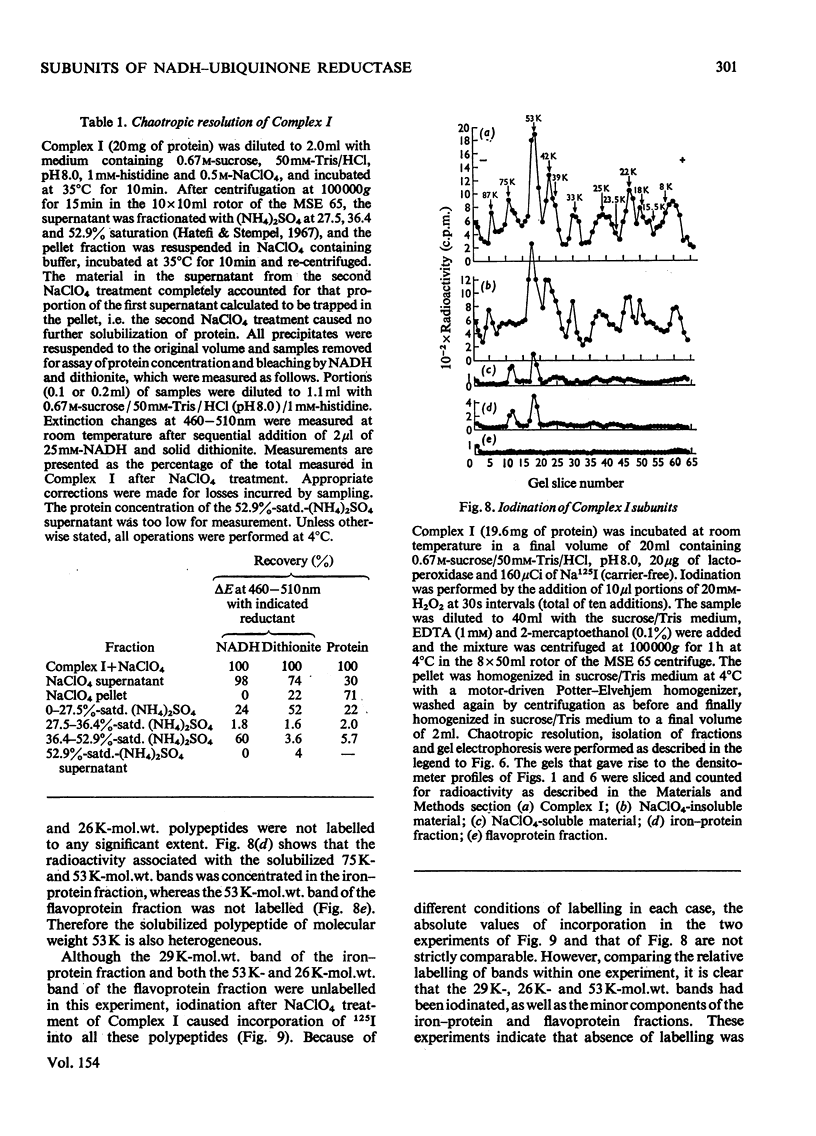
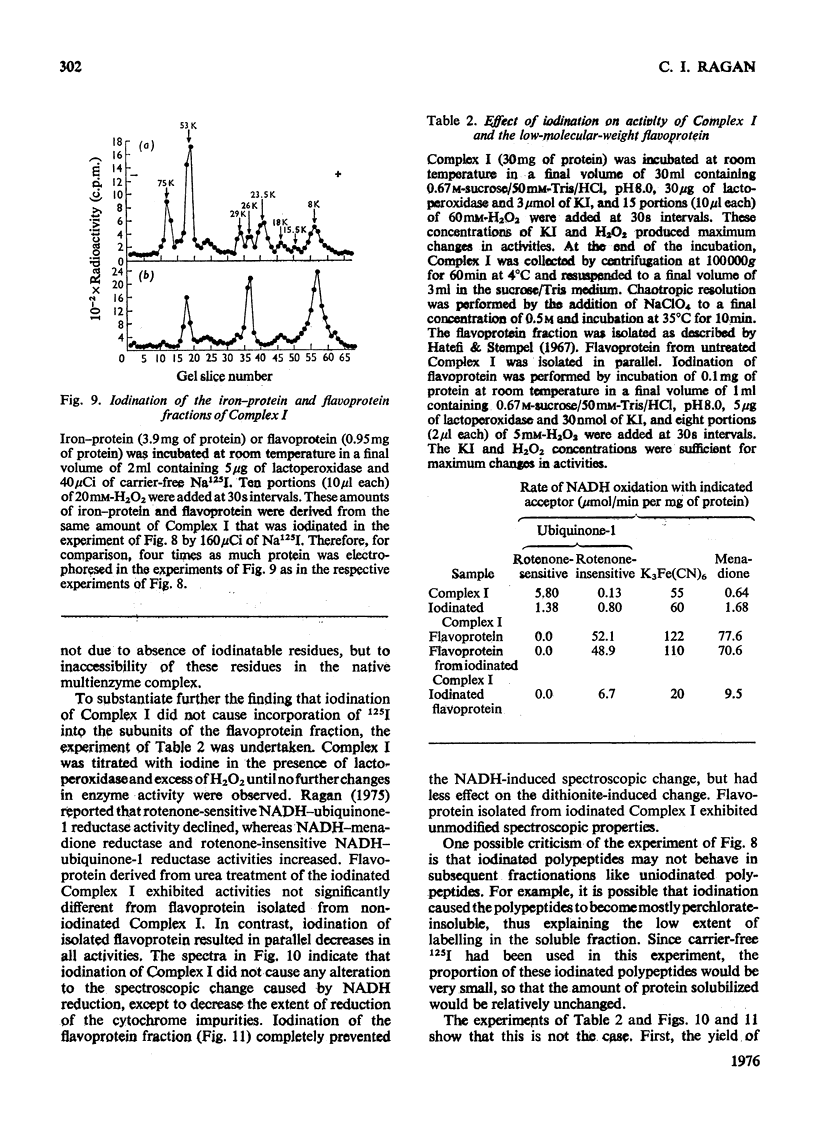
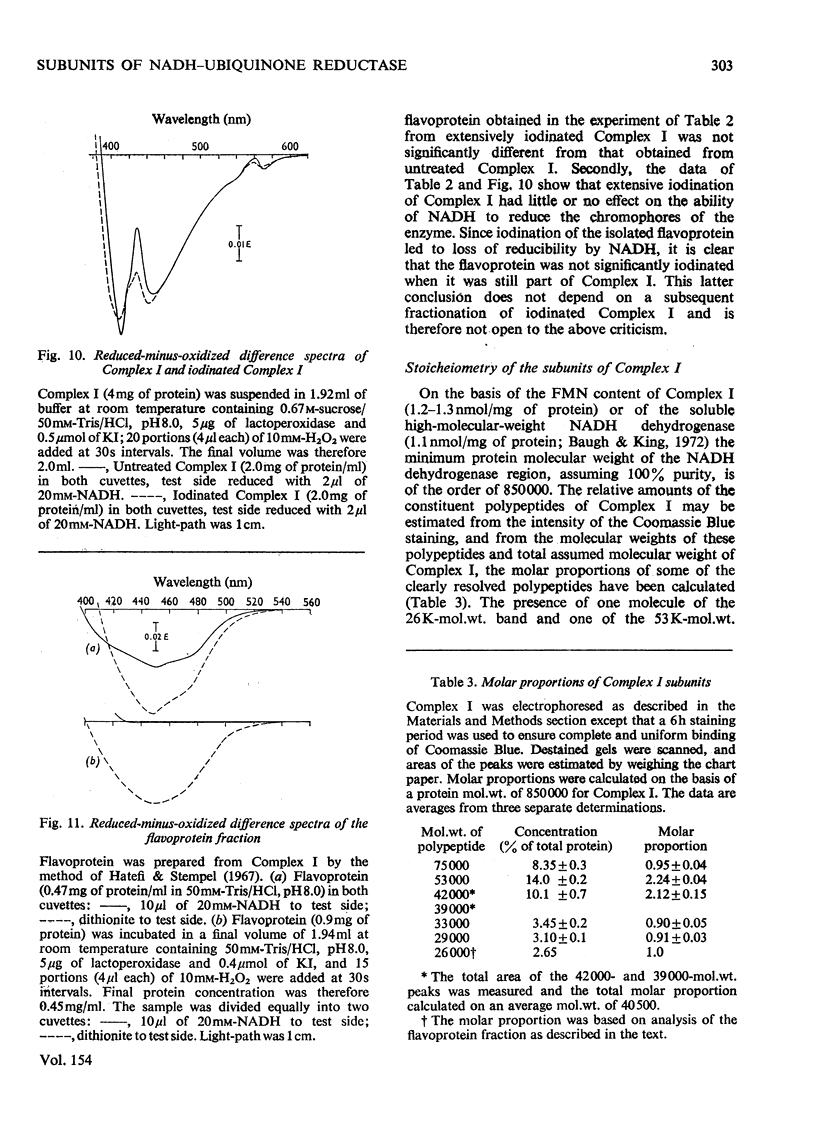
Preparations of NADH-ubiquinone reductase from bovine heart mitochondria (Complex I) were shown to contain at least 16 polypeptides by gel electrophoresis in the presence of sodium dodecyl sulphate. 2. High-molecular-weight soluble NADH dehydrogenase prepared from Triton X-100 extracts of submitochondrial particles [Baugh & King (1972) Biochem. Biophys. Res. Commun. 49, 1165-1173] was similar to Complex I in its polypeptide composition. 3. Solubilization of Complex I by phospholipase A treatment and subsequent sucrose-density-gradient centrifugation did not alter the polypeptide composition. 4. Lysophosphatidylcholine treatment of Complex I caused some selective solubilization of a polypeptide of mol.wt. 33000 previosuly postulated to be the transmembrane component of Complex I in the mitochondrial membrane [Ragan (1975) in Energy Transducing Membranes: Structure, Function and Reconstitution (Bennun, Bacila & Najjar, eds.), Junk, The Hague, in the press]. 5. Chaotropic resolution of Complex I caused solubilization of polypeptides of molecular weights 75000, 53000, 29000, 26000 and 15500 and traces of others in the 10000-20000-mol.wt.range. 6. The major components of the iron-protein fraction from chaotropic resolution had molecular weights of 75000, 53000 and 29000, whereas the flavoprotein contained polypeptides of molecular weights 53000 and 26000 in a 1:1 molar ratio. 7. Iodination of Complex I by lactoperoxidase indicated that the water-soluble polypeptides released by chaotropic resolution, in particular those of the flavoprotein fraction, were largely buried in the intact Complex. 8. The polypeptides of molecular weights 75000, 53000, 42000, 39000, 33000, 29000 and 26000 were present in 1:2:1:1:1:1:1 molar proportions. The two subunits of molecular weight 53000 are probably non-identical.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh R. F., King T. E. Purification, properties and reconstitutive activity of a DPHN dehydrogenase. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1165–1173. doi: 10.1016/0006-291x(72)90591-8. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A. Identification of the major enzymic activities of the mitochondrial inner membrane in terms of their migration in sodium dodecyl sulfate polyacrylamide gel electrophoresis. Arch Biochem Biophys. 1974 Jul;163(1):99–105. doi: 10.1016/0003-9861(74)90459-7. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Komai H., Hunter D. R. Isolation of a major hydrophobic protein of the mitochondrial inner membrane. Biochem Biophys Res Commun. 1973 Dec 10;55(3):655–659. doi: 10.1016/0006-291x(73)91194-7. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Gellerfors P., Nelson B. D. Analysis of the peptide composition of purified beef-heart complex III by dodecylsulfate electrophoresis. Eur J Biochem. 1975 Apr 1;52(3):433–443. doi: 10.1111/j.1432-1033.1975.tb04011.x. [DOI] [PubMed] [Google Scholar]
- Gutman M., Singer T. P. EPR studies on the iron-sulfur centers of DPNH dehydrogenase during the redox cycle of the enzyme. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1572–1578. doi: 10.1016/s0006-291x(71)80266-8. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XLI. Reduced coenzyme Q (QH2)-cytochrome c reductase. J Biol Chem. 1962 May;237:1681–1685. [PubMed] [Google Scholar]
- Harmon H. J., Hall J. D., Crane F. L. Structure of mitochondrial cristae membranes. Biochim Biophys Acta. 1974 Sep 16;344(2):119–155. doi: 10.1016/0304-4157(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Interactions of reduced and oxidized triphosphopyridine nucleotides with the electron-transport system of bovine heart mitochondria. Biochemistry. 1973 Aug 28;12(18):3515–3522. doi: 10.1021/bi00742a026. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Resolution of complex I (DPNH-coenzyme Q reductase) of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1967 Feb 8;26(3):301–308. doi: 10.1016/0006-291x(67)90122-2. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L., Galante Y., Hanstein W. G. Mitochondrial ATP-Pi exchange complex. Biochem Biophys Res Commun. 1974 Nov 6;61(1):313–321. doi: 10.1016/0006-291x(74)90568-3. [DOI] [PubMed] [Google Scholar]
- Huang P. C., Pharo R. L. A simple method for the purification of the mitochondrial NADH dehydrogenase. Biochim Biophys Acta. 1971 Aug 6;245(1):240–244. doi: 10.1016/0005-2728(71)90029-6. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem. 1972 Oct 25;247(20):6617–6623. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUSTY C. J., MACHINIST J. M., SINGER T. P. STUDIES ON THE RESPIRATORY CHAIN-LINKED REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE DEHYDROGENASE. VII. "LABILE" SULFIDE GROUPS IN THE DEHYDROGENASE AND IN RELATED PROTEINS. J Biol Chem. 1965 Apr;240:1804–1810. [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., WAGNER R. P., SNELL E. E. Biosynthesis of valine and i43soleucine, 3. alpha-Keto-beta-hydroxy acid reductase and alpha-hydroxy-beta-Keto acid reductoisomerase. J Biol Chem. 1960 Aug;235:2322–2331. [PubMed] [Google Scholar]
- RINGLER R. L., MINAKAMI S., SINGER T. P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. II. Isolation and molecular properties of the enzyme from beef heart. J Biol Chem. 1963 Feb;238:801–810. [PubMed] [Google Scholar]
- Ragan C. I., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. IV. The reconstitution of rotenone-sensitive reduced nicotinamide adenine dinucleotide-ubiquinone reductase from reduced nicotinamide adenine dinucleotide dehydrogenase and phospholipids. J Biol Chem. 1973 Oct 10;248(19):6876–6884. [PubMed] [Google Scholar]
- Ragan C. I., Widger W. R., King T. E. Pyridine nucleotide transhydrogenase activity of soluble cardiac NADH dehydrogenase and particulate NADH-ubiquinone reductase. Biochem Biophys Res Commun. 1974 Oct 8;60(3):894–900. doi: 10.1016/0006-291x(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Rydström J., Hoek J. B., Hundal T. Selective solubilization of nicotinamide nucleotide transhydrogenase from the mitochondrial inner membrane. Biochem Biophys Res Commun. 1974 Sep 9;60(1):448–455. doi: 10.1016/0006-291x(74)90224-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yu C., Yu L., King T. E. Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J Biol Chem. 1975 Feb 25;250(4):1383–1392. [PubMed] [Google Scholar]


