Abstract
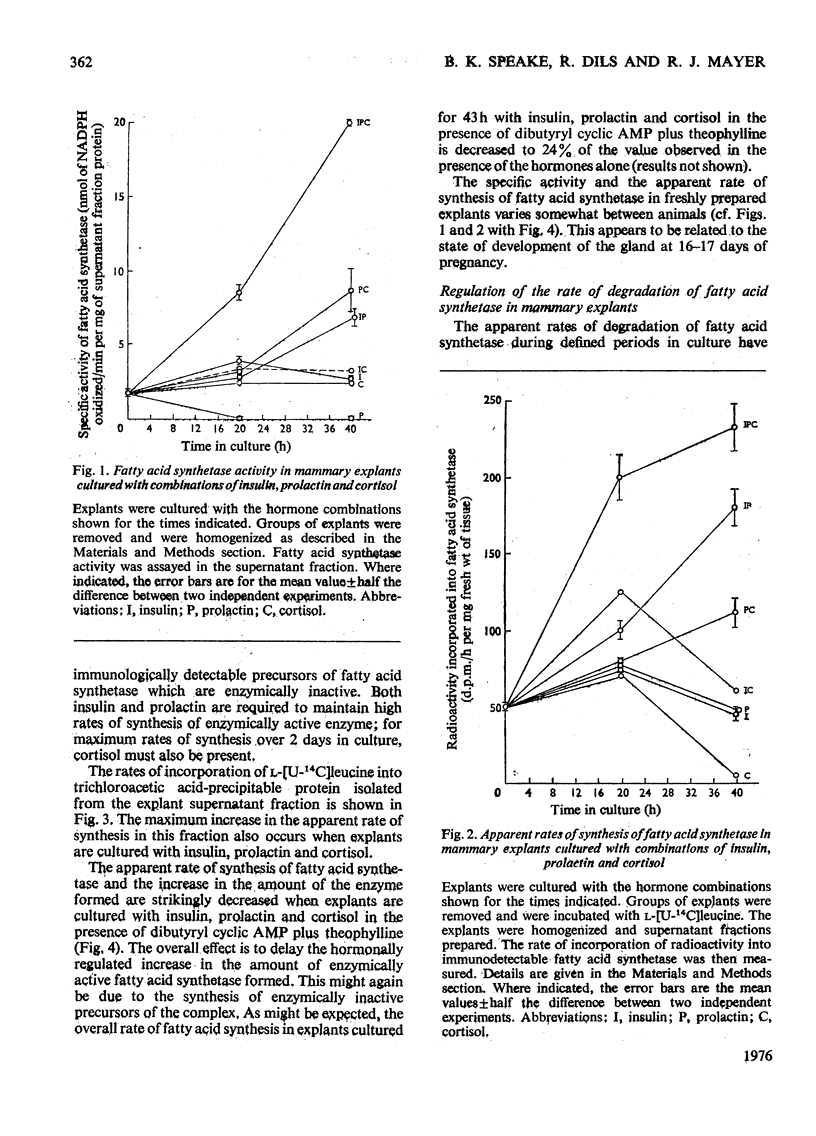
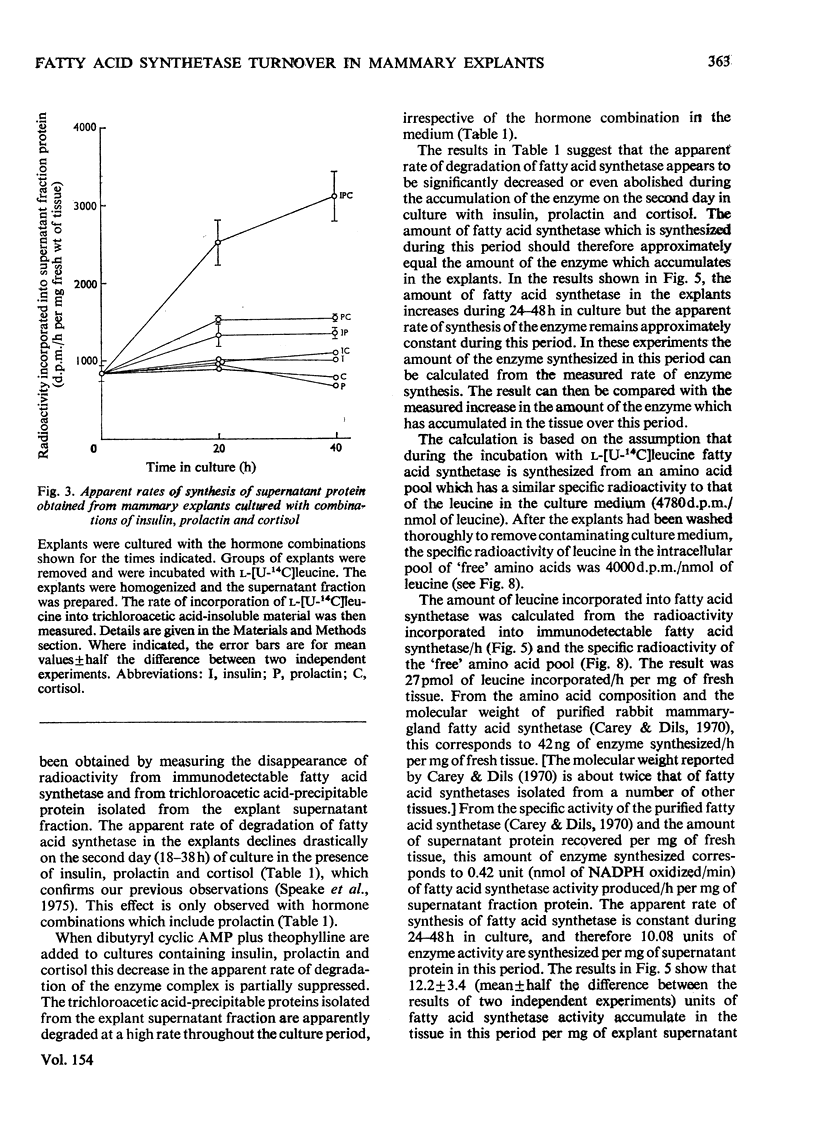
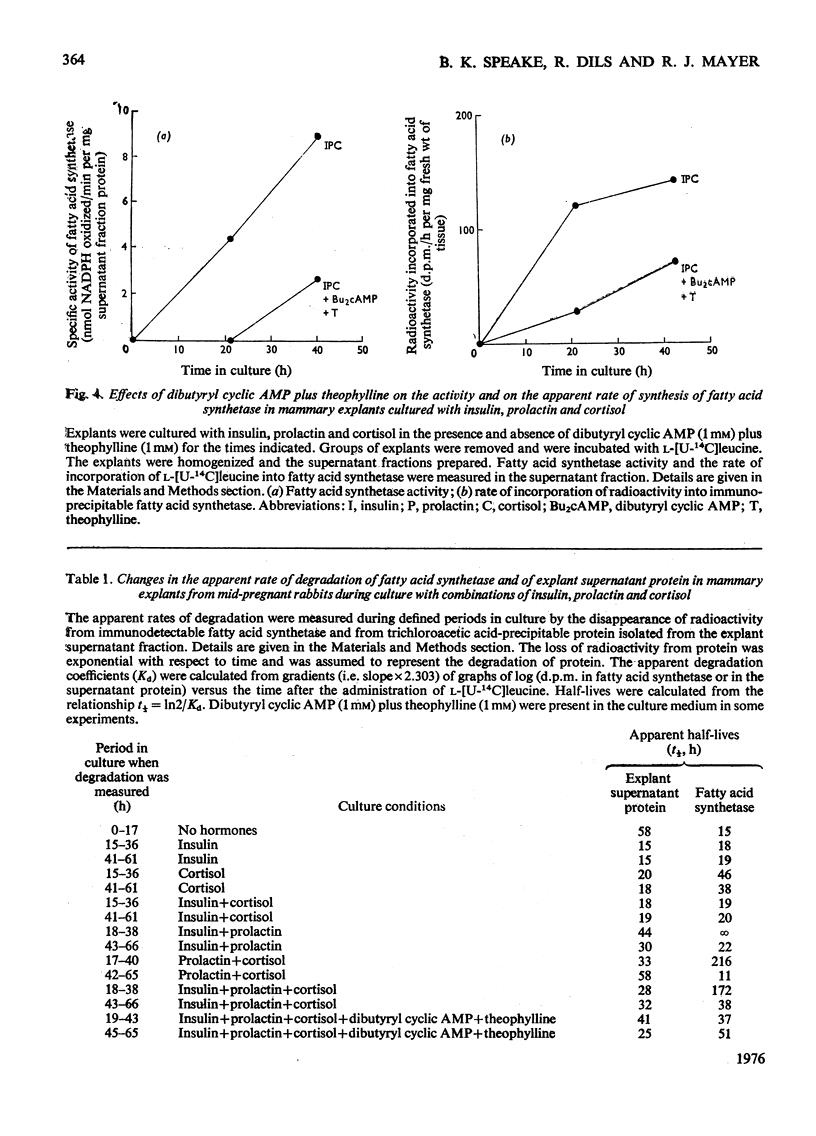
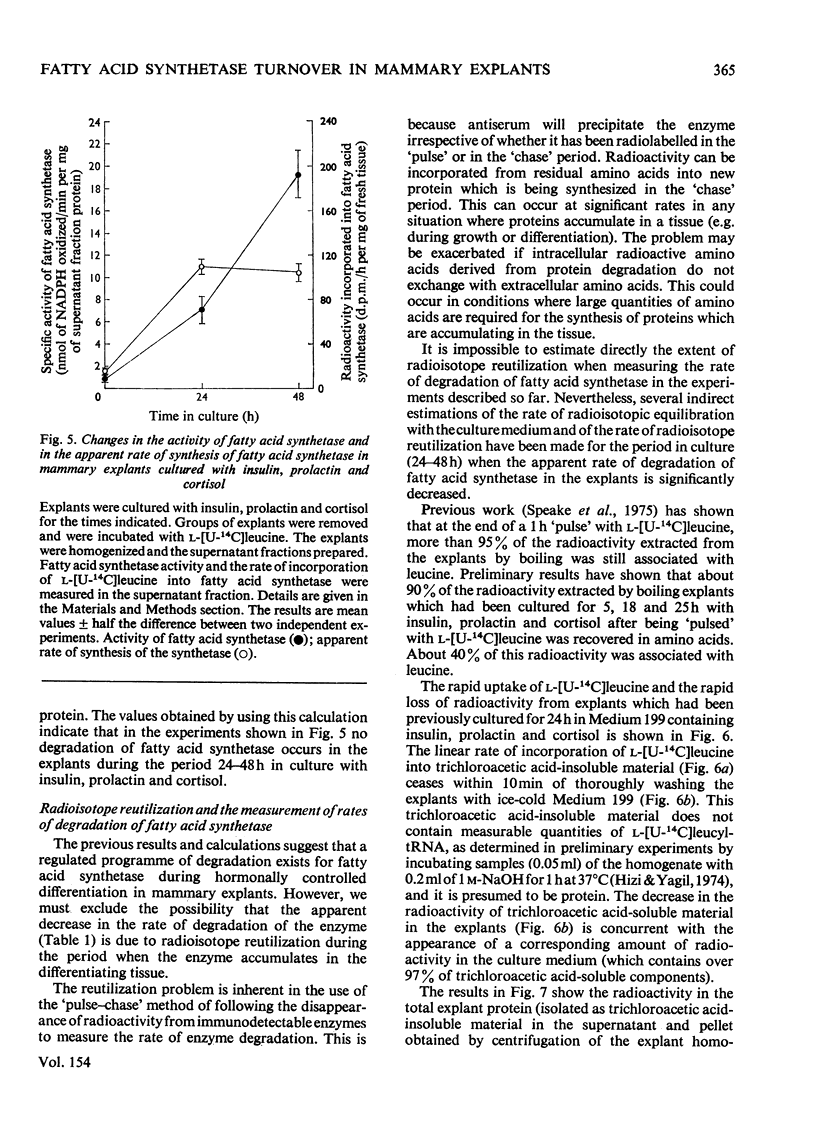
1. Explants of mammary gland from mid-pregnant rabbits were cultured in Medium 199 containing combinations of insulin, prolactin and cortisol. With hormone combinations which included prolactin, a sustained increase in the apparent rate of synthesis and in the amount of fatty acid synthetase was measurable immunologically. Maximum increase was produced with insulin, prolactin and cortisol present together. 2. With prolactin present alone, synthetase activity in the explants decreased to undetectable values after 1 day in culture, whereas the incorporation of l-[U-14C]leucine into immunodetectable material increased. Prolactin may therefore direct the synthesis of immunologically cross-reactive precursors of fatty acid synthetase which are enzymically inactive. 3. Culture with dibutyryl cyclic AMP plus theophylline in the presence of insulin, prolactin and cortisol delayed the increase in the rate of synthesis and accumulation of the synthetase. These compounds may also prevent the apparent decrease in the rate of degradation of the synthetase which occurs on day 2 of culture. 4. A large decrease in the apparent rate of degradation of the synthetase on day 2 of culture occurs during culture with hormone combinations which include prolactin. The protein obtained by centrifugation of explant homogenates for 6min at 14000gav. is degraded continuously throughout the culture period. 5. This decrease in the apparent rate of degradation of the synthetase was measured by radio-immunological precipitation. It is probably part of a regulated programme of enzyme degradation and not a reflexion of the reutilization of radioactive amino acids for the following reasons. (a) The calculated increase in the amount of the synthetase in explants on day 2 of culture with insulin, prolactin and cortisol was approximately equal to the measured increase of the enzyme complex which accumulates in the explants. This suggests little or no enzyme degradation has occurred. (b) Explants were cultured for 24h with insulin, prolactin and cortisol. They were then incubated with l-[U-14C]leucine, washed and incubated again for up to 4½h. l-[U-14C]Leucine rapidly equilibrated with the intracellular amino acid pool. Within 10min of incubation after washing explants to remove endogenous l-[U-14C]leucine the previously linear incorporation of l-[U-14C]-leucine into total explant protein ceased. This suggests that protein is synthesized from an amino acid pool which rapidly equilibrates with amino acids in the culture medium. (c) Explants were cultured for 24h as described in (b) but after washing they were cultured with insulin, prolactin and cortisol for 24h. Approx. 90% of the radioactivity lost from the `free' intracellular amino acid pool and from amino acids derived from the degradation of explant protein in this period was detected in the culture medium. This suggests that the `free' intracellular amino acids and amino acids derived from protein degradation can equilibrate with amino acids in the medium. A residual `free' radioactive amino acid pool was present in the tissue. (d) Casein represents approx. 20% of the protein synthesized after 1 day in culture with insulin, prolactin and cortisol. Histological evidence suggests that on day 2 of culture, casein is unlikely to be degraded in the tissue. No increase in the radioactivity incorporated into casein can be measured in the 23h after incubation of explants with l-[U-14C]leucine as described in (b). This suggests that the incorporation of radioactivity into proteins during culture after incubation with l-[U-14C]leucine is minimal. (e) Inhibition of protein synthesis in explants by cycloheximide after incubation with l-[U-14C]leucine does not reveal a latent continuous degradation of fatty acid synthetase on day 2 of culture which might have been masked by the high rates of protein synthesis and therefore the accumulation of the enzyme. 6. The conclusion is discussed that there is a real decrease (or even cessation) in the rate of degradation of fatty acid synthetase during the period when the enzyme accumulates in explants cultured with hormone combinations which contain prolactin.
Full text
PDF




Selected References
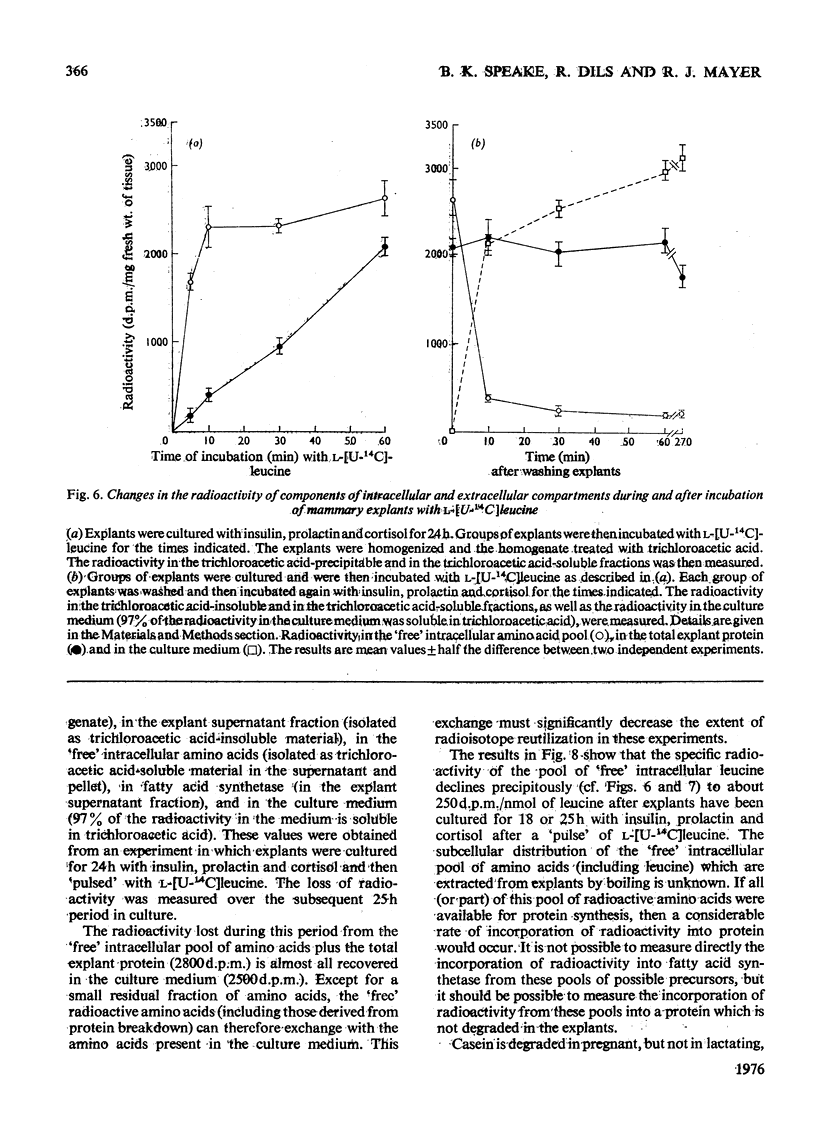
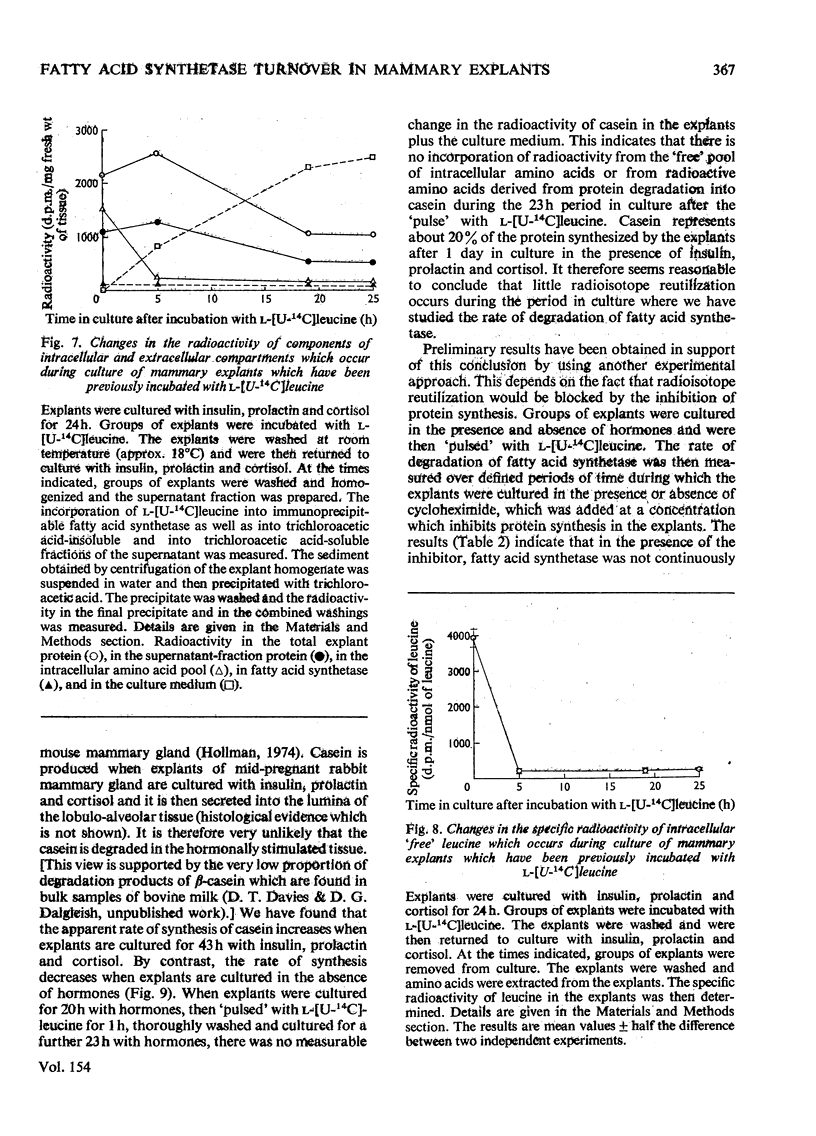
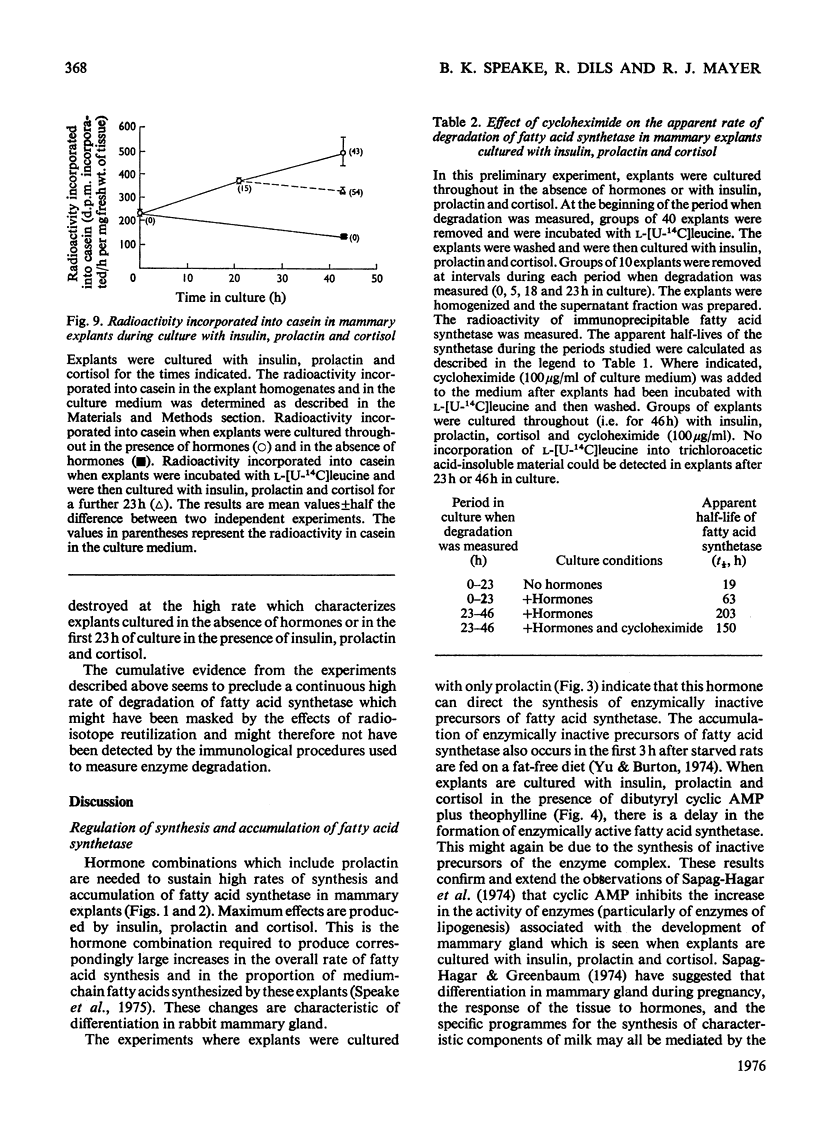
These references are in PubMed. This may not be the complete list of references from this article.
- Aisbitt R. P., Barry J. M. Stimulation by insulin of ornithine decarboxylase activity in cultured mammary tissue. Biochim Biophys Acta. 1973 Oct 5;320(3):610–616. doi: 10.1016/0304-4165(73)90140-2. [DOI] [PubMed] [Google Scholar]
- Beckerton A., Buttery P. J., Bailey F. J., Bolton N. Improved method for the separation of lysine from N-epsilon-monomethyllysine in plasma using cation-exchange chromatography. J Chromatogr. 1975 Jan 29;104(1):170–171. doi: 10.1016/s0021-9673(01)85502-3. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Dils R. Fatty acid biosynthesis. V. Purification and characterisation of fatty acid synthetase from lactating-rabbit mammary gland. Biochim Biophys Acta. 1970 Sep 8;210(3):371–387. doi: 10.1016/0005-2760(70)90033-0. [DOI] [PubMed] [Google Scholar]
- Every D., Ashworth J. M. Rates of degradation and synthesis of glycosidases de novo during growth and differentiation of Dictyostelium discoideum. Biochem J. 1975 May;148(2):169–177. doi: 10.1042/bj1480169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M., Malamud D. Decreased protein catabolism during stimulated growth. FEBS Lett. 1974 Sep 15;46(1):308–311. doi: 10.1016/0014-5793(74)80394-7. [DOI] [PubMed] [Google Scholar]
- Hizi A., Yagil G. The use of anti-immunoglobulin for the immunoprecipitation of labeled proteins from tissue homogenates. Anal Biochem. 1974 Dec;62(2):386–399. doi: 10.1016/0003-2697(74)90171-7. [DOI] [PubMed] [Google Scholar]
- Juergens W. G., Stockdale F. E., Topper Y. J., Elias J. J. Hormone-dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci U S A. 1965 Aug;54(2):629–634. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Walther B. T., Rutter W. J. Protein synthesis during the secondary developmental transition of the embryonic rat pancreas. J Biol Chem. 1972 Jun 25;247(12):3941–3952. [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels D. E., Li J. B., Morgan H. E., Jefferson L. S. Evaluation of hormone effects on protein turnover in isolated perfused organs. Methods Enzymol. 1975;37:238–250. doi: 10.1016/s0076-6879(75)37020-1. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Sapag-Hagar M., Greenbaum A. L., Lewis D. J., Hallowes R. C. The effects of di-butyryl cAMP on enzymatic and metabolic changes in explants of rat mammary tissue. Biochem Biophys Res Commun. 1974 Jul 10;59(1):261–268. doi: 10.1016/s0006-291x(74)80201-9. [DOI] [PubMed] [Google Scholar]
- Sapag-Hagar M., Greenbaum A. L. The role of cyclic nucleotides in the development and function of rat mammary tissue. FEBS Lett. 1974 Sep 15;46(1):180–183. doi: 10.1016/0014-5793(74)80363-7. [DOI] [PubMed] [Google Scholar]
- Scornik O. A. Decreased in vivo disappearance of labelled liver protein after partial hepatectomy. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1063–1066. doi: 10.1016/0006-291x(72)90941-2. [DOI] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differention. Studies on the effects of hormones on the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1975 May;148(2):309–320. doi: 10.1042/bj1480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]


