Abstract
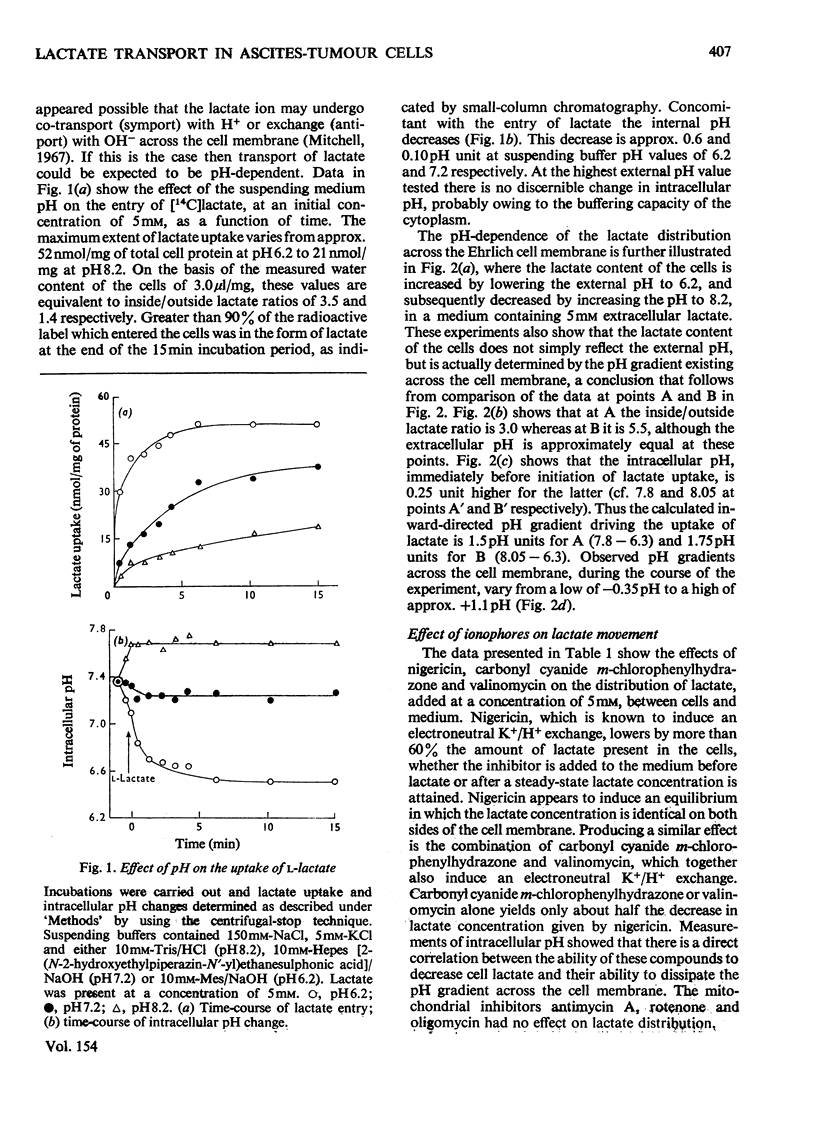
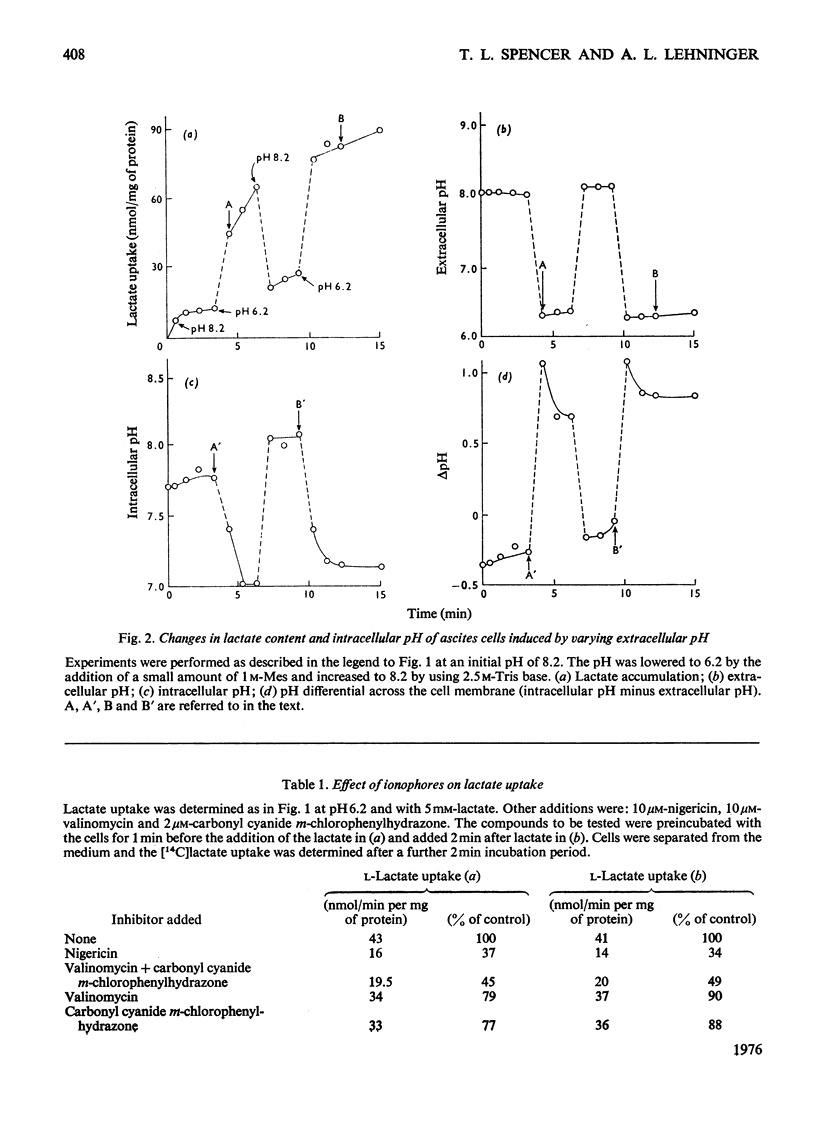
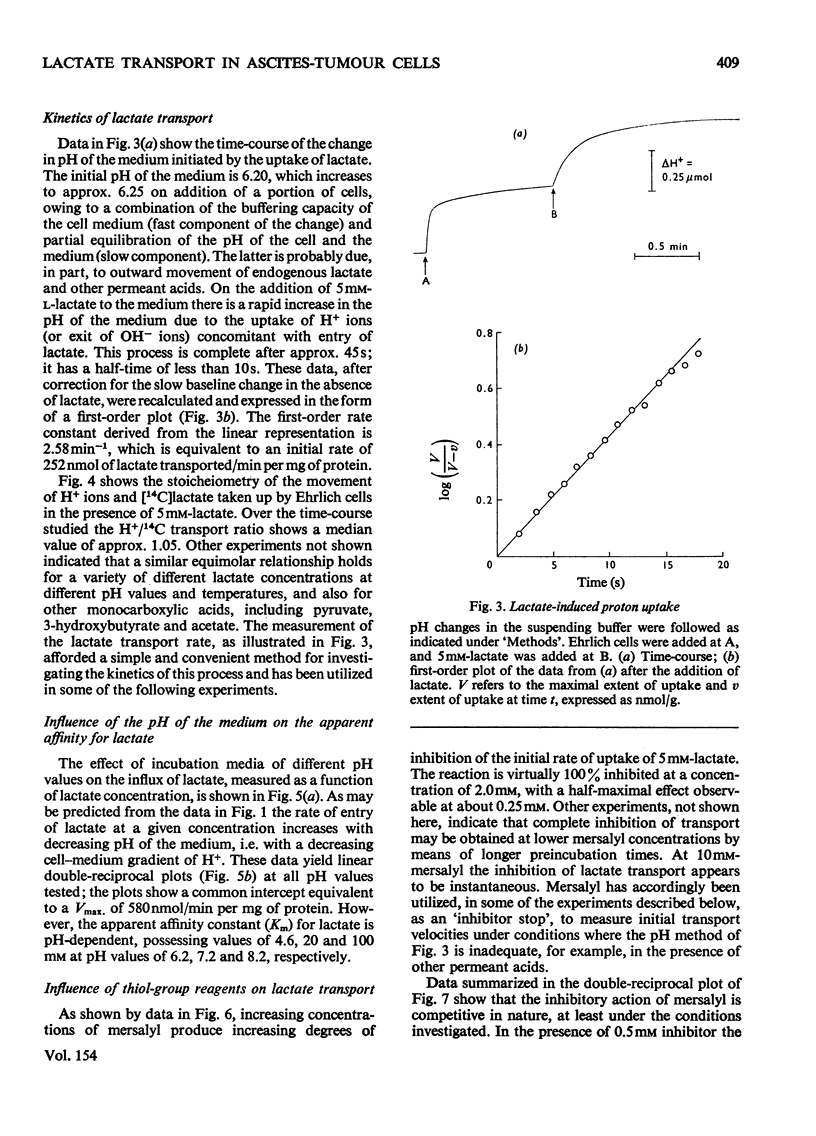
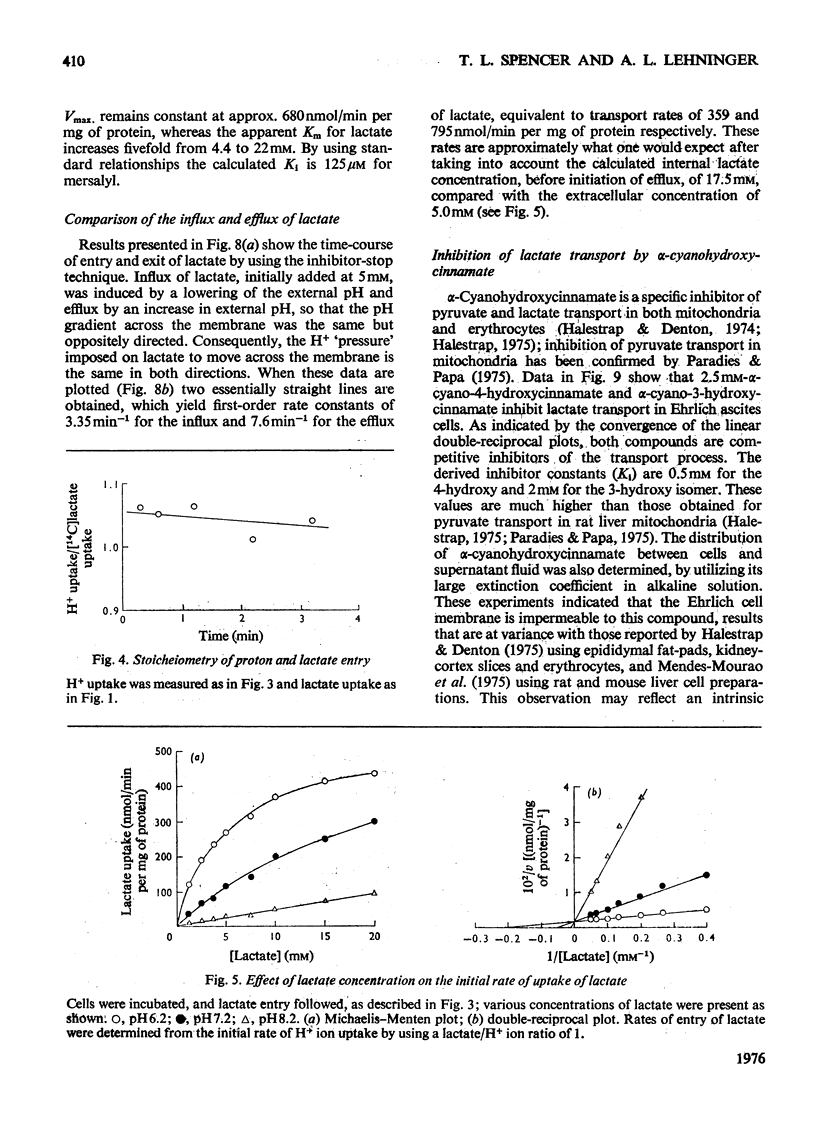
Ehrlich ascites-tumour cells were investigated with regard to their stability to transport L-lactate by measuring either the distribution of [14C]lactate or concomitant H+ ion movements. The movement of lactate was dependent on the pH difference across the cell membrane and was electroneutral, as evidenced by an observed 1:1 antiport for OH- ions or 1:1 symport with H+ ions. 2. Kinetic experiments showed that lactate transport was saturable, with an apparent Km of approx. 4.68 mM and a Vmax. as high as 680 nmol/min per mg of protein at pH 6.2 and 37 degrees C. 3. Lactate transport exhibited a high temperature dependence (activation energy = 139 kJ/mol). 4. Lactate transport was inhibited competitively by (a) a variety of other substituted monocarboxylic acids (e.g. pyruvate, Ki = 6.3 mM), which were themselves transported, (b) the non-transportable analogues alpha-cyano-4-hydroxycinnamate (Ki = 0.5 mM), alpha-cyano-3-hydroxycinnamate (Ki = 2mM) and DL-p-hydroxyphenyl-lactate (Ki = 3.6 mM) and (c) the thiol-group reagent mersalyl (Ki = 125 muM). 5. Transport of simple monocarboxylic acids, including acetate and propionate, was insensitive to these inhibitors; they presumably cross the membrane by means of a different mechanism. 6. Experiments using saturating amounts of mersalyl as an "inhibitor stop" allowed measurements of the initial rates of net influx and of net efflux of [14C]lactate. Influx and efflux of lactate were judged to be symmetrical reactions in that they exhibited similar concentration dependence. 7. It is concluded that lactate transport in Ehrlich ascites-tumour cells is mediated by a carrier capable of transporting a number of other substituted monocarboxylic acids, but not unsubstituted short-chain aliphatic acids.
Full text
PDF

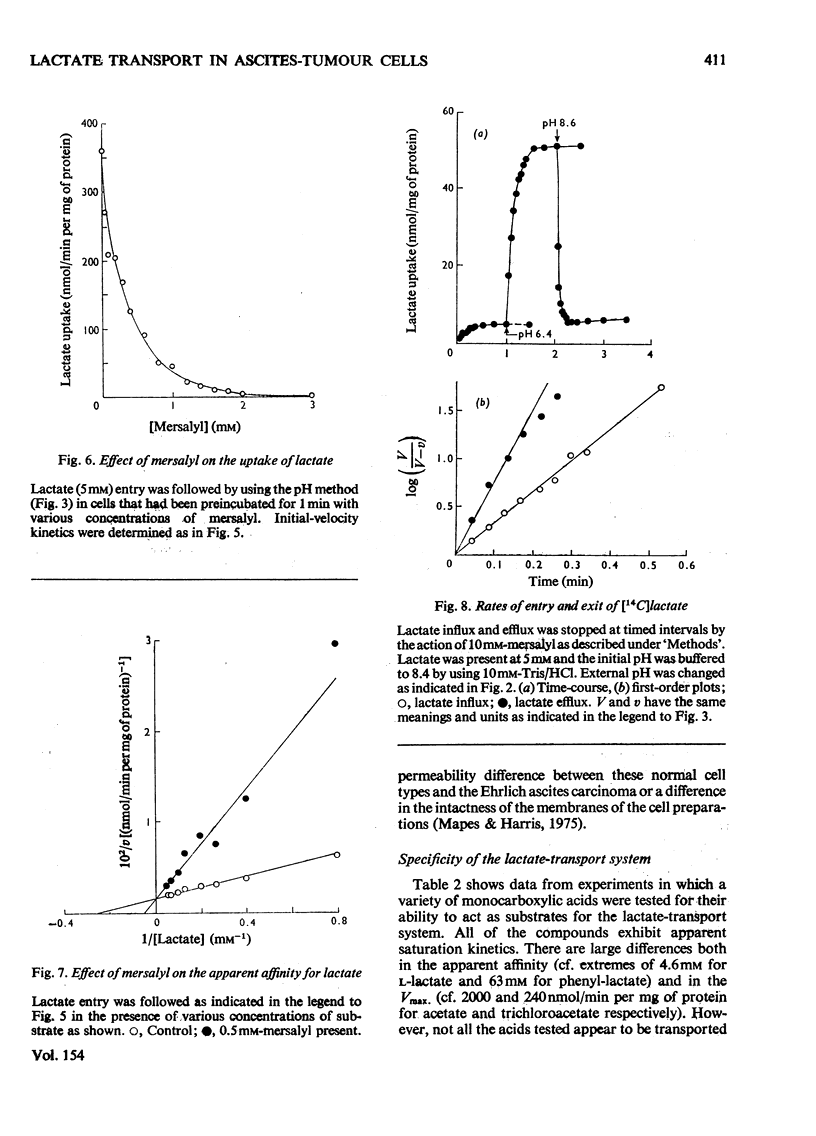
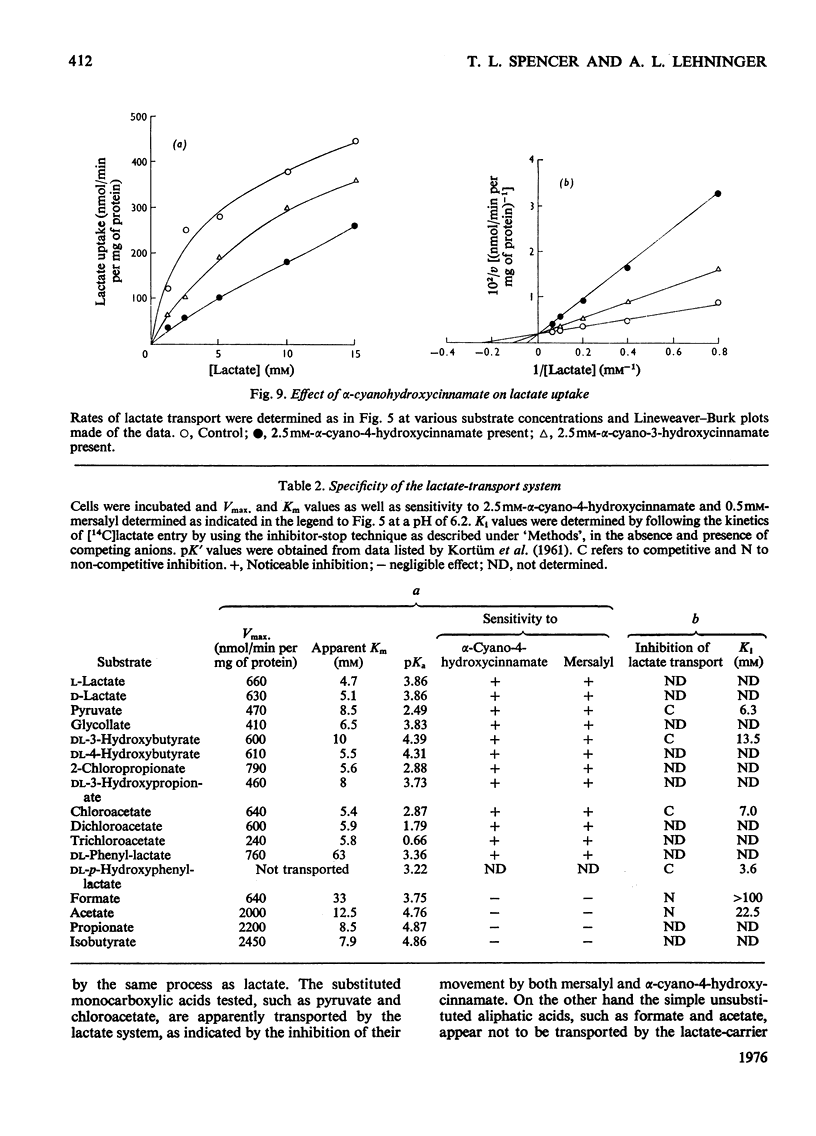
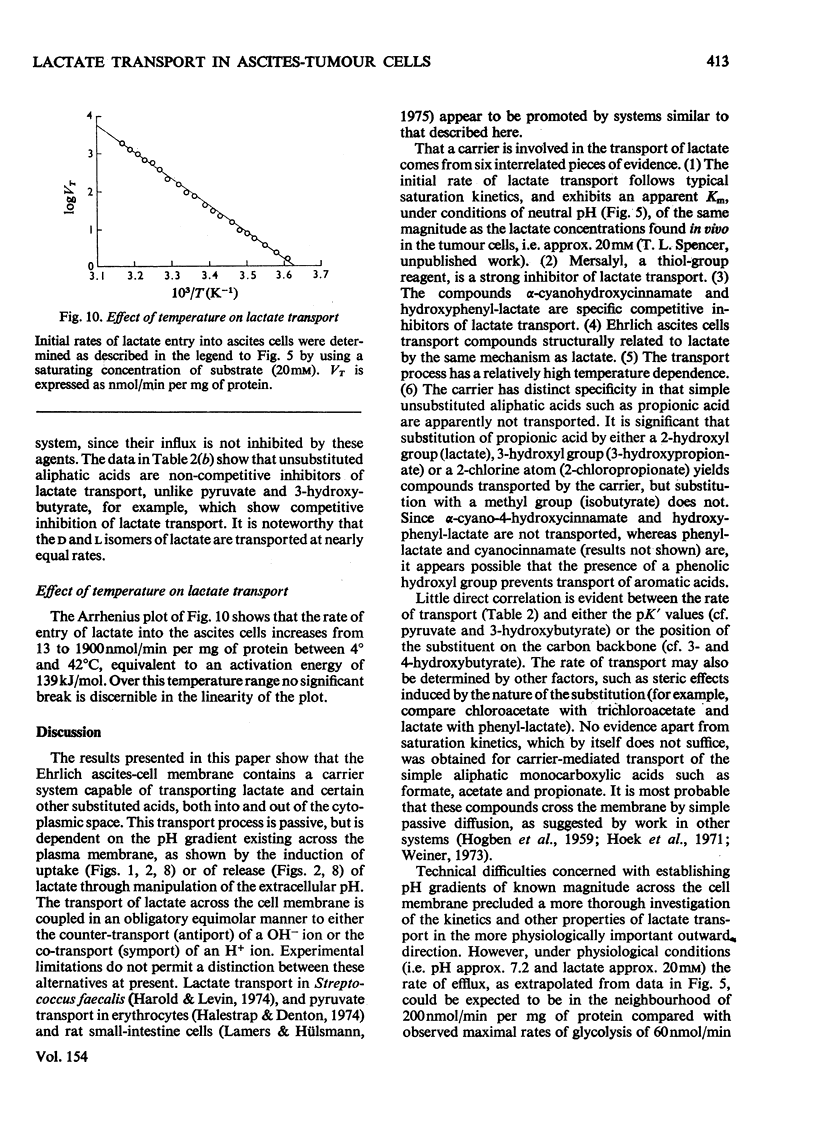

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- BUSCH H. Studies on the metabolism of acetate-1-C in tissues of tumor-bearing rats. Cancer Res. 1953 Nov;13(11):789–794. [PubMed] [Google Scholar]
- HOGBEN C. A., TOCCO D. J., BRODIE B. B., SCHANKER L. S. On the mechanism of intestinal absorption of drugs. J Pharmacol Exp Ther. 1959 Apr;125(4):275–282. [PubMed] [Google Scholar]
- HUCKABEE W. E. Relationship of pyruvate and lactate during anaerobic metabolism. V. Coronary adequacy. Am J Physiol. 1961 Jun;200:1169–1176. doi: 10.1152/ajplegacy.1961.200.6.1169. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-Cyano-4-hydroxycinnamate and related compounds. Biochem J. 1975 Apr;148(1):97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975 Apr;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Levin E. Lactic acid translocation: terminal step in glycolysis by Streptococcus faecalis. J Bacteriol. 1974 Mar;117(3):1141–1148. doi: 10.1128/jb.117.3.1141-1148.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. H., Craig R. J., Gorlin R., Sonnenblick E. H. Lactate and pyruvate kinetics in isolated perfused rat hearts. Am J Physiol. 1969 Dec;217(6):1752–1756. doi: 10.1152/ajplegacy.1969.217.6.1752. [DOI] [PubMed] [Google Scholar]
- Hohorst H. J., Arese P., Bartels H., Stratmann D., Talke H. L(+) lactic acid and the steady state of cellular red/ox-systems. Ann N Y Acad Sci. 1965 Jul 31;119(3):974–994. doi: 10.1111/j.1749-6632.1965.tb47456.x. [DOI] [PubMed] [Google Scholar]
- Lamers J. M., Hülsmann W. C. Inhibition of pyruvate transport by fatty acids in isolated cells from rat small intestine. Biochim Biophys Acta. 1975 Jun 11;394(1):31–45. doi: 10.1016/0005-2736(75)90202-3. [DOI] [PubMed] [Google Scholar]
- Lee I. Y., Strunk R. C., Coe E. L. Coordination among rate-limiting steps of glycolysis and respiration in intact ascites tumor cells. J Biol Chem. 1967 May 10;242(9):2021–2028. [PubMed] [Google Scholar]
- Lofrumento N. E., Hoek J. B., Meyer A. J., Tager J. M. Phosphate transport in rat-liver mitochondria. Biochim Biophys Acta. 1971 Mar 2;226(2):297–308. doi: 10.1016/0005-2728(71)90096-x. [DOI] [PubMed] [Google Scholar]
- Mapes J. P., Harris R. A. On the oxidation of succinate by parenchymal cells isolated from rat liver. FEBS Lett. 1975 Mar 1;51(1):80–83. doi: 10.1016/0014-5793(75)80858-1. [DOI] [PubMed] [Google Scholar]
- Mendes-Mourāo J., Halestrap A. P., Crisp D. M., Pogson C. I. The involvement of mitochondrial pyruvate transport in the pathways of gluconeogenesis from serine and alanine in isolated rat and mouse liver cells. FEBS Lett. 1975 Apr 15;53(1):29–32. doi: 10.1016/0014-5793(75)80674-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Paradies G., Papa S. The transport of monocarboxylic oxoacids in rat liver mitochondria. FEBS Lett. 1975 Mar 15;52(1):149–152. doi: 10.1016/0014-5793(75)80659-4. [DOI] [PubMed] [Google Scholar]
- Poole D. T. Intracellular pH of the Ehrlich ascites tumour cell as it is affected by sugars and sugar derivatives. J Biol Chem. 1967 Aug 25;242(16):3731–3736. [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Wilhelm Gerhard, Schulz Jürgen, Hofmann Eberhard. pH-dependence of aerobic glycolysis in ehrlich ascites tumour cells. FEBS Lett. 1971 Sep 15;17(1):158–162. doi: 10.1016/0014-5793(71)80587-2. [DOI] [PubMed] [Google Scholar]


