Abstract
Helicobacter pylori (H. pylori) is a Gram-negative, spiral-shaped bacterium that colonizes the gastric epithelium and is associated with a range of gastrointestinal disorders, exhibiting a global prevalence of approximately 50%. Despite the availability of treatment options, H. pylori frequently reemerges and demonstrates increasing antibiotic resistance, which diminishes the efficacy of conventional therapies. Consequently, it is imperative to explore non-antibiotic treatment alternatives to mitigate the inappropriate use of antibiotics. This review examines H. pylori infection, encompassing transmission pathways, treatment modalities, antibiotic resistance, and eradication strategies. Additionally, it discusses alternative therapeutic approaches such as probiotics, anti-biofilm agents, phytotherapy, phototherapy, phage therapy, lactoferrin therapy, and vaccine development. These strategies aim to reduce antimicrobial resistance and enhance treatment outcomes for H. pylori infections. While alternative therapies can maintain low bacterial levels, they do not achieve complete eradication of H. pylori. These therapies are designed to bolster the immune response, minimize side effects, and provide gastroprotective benefits, rendering them suitable for adjunctive use alongside conventional treatments. Probiotics may serve as adjunctive therapy for H. pylori; however, their effectiveness as a monotherapy is limited. Photodynamic and phage therapies exhibit potential in targeting H. pylori infections, including those caused by drug-resistant strains, without the use of antibiotics. The development of a reliable vaccine is also critical for the eradication of H. pylori. This review identifies candidate antigens such as VacA, CagA, and HspA, along with various vaccine formulations, including vector-based and subunit vaccines. Some vaccines have demonstrated efficacy in clinical trials, while others have shown robust immune protection in preclinical studies. Nevertheless, each of the aforementioned alternative therapies requires thorough preclinical and clinical evaluation to ascertain their efficacy, side effects, cost-effectiveness, and patient compliance.
Keywords: Helicobacter pylori, disease transmission, antibiotic resistance, alternative therapies, infection control
1. Introduction
Helicobacter pylori (H. pylori) is a spiral-shaped, flagellated, Gram-negative, microaerophilic bacterium that thrives under specific growth conditions [1,2,3]. Initially isolated in 1983 from patients diagnosed with antral gastritis [4]. H. pylori has been implicated in various gastrointestinal disorders, including gastritis, peptic ulcers, and certain malignancies [5]. In 2017, the International Agency for Research on Cancer classified H. pylori as a Class I carcinogen [6]. The bacterium possesses several virulence factors that enhance its pathogenic potential, including resistance to acidic environments and antibiotics [7,8,9,10]. Notably, factors such as Cytotoxin-Associated Gene A (cagA) and Vacuolating Cytotoxin A (vacA) have been associated with the development of gastric carcinoma [7,11,12]. Research suggests that the flagella of H. pylori facilitate its penetration into the submucosa of the stomach [13,14,15], while the urease enzyme contributes to its survival in acidic conditions [16,17,18].
H. pylori infection can occur early in life via oral–oral or oral–fecal routes, with natural elimination rare without antimicrobials [19]. Approximately 4.5 billion people are infected worldwide, contributing to 9% of cancer-related deaths [20]. Infection rates are 15–25% in wealthy countries and 75–90% in underdeveloped countries [21,22]. The infection is more prevalent in impoverished regions compared to industrialized areas. Factors contributing to this gap include health issues, family finances, ethnicity, and the number of individuals affected [23]. Exposure to H. pylori increases the likelihood of infection due to prolonged tobacco use, insufficient vitamin intake, high salt consumption, and living conditions that alter stomach pH [24].
H. pylori is primarily transmitted through oral–oral and fecal–oral routes [24]. The bacterium is present in the saliva, feces, and vomit of infected individuals, facilitating transmission [25]. It often spreads within families in developing countries, especially between infected mothers and their children [26], though partner transmission is uncertain [27,28]. The exact route to the human stomach is unclear, but environmental contamination likely plays a role [24]. Poor hygiene can contaminate treated water [24], and studies indicate that water may transfer H. pylori from feces to the mouth. Infection is more common in children using external water sources or consuming raw vegetables irrigated with untreated wastewater [29,30]. Food can also become contaminated in unsanitary conditions, and milk, along with vegetables and meat, has been studied for its role in transmission [31].
The crisis of antimicrobial resistance against pathogenic bacteria is considered an urgent matter worldwide [32,33,34,35,36,37,38,39,40,41]. A key factor in the failure of H. pylori eradication programs is antibiotic resistance [42]. Proton pump inhibitors (PPIs), combined with two classes of antimicrobials and bismuth, are the standard treatment for H. pylori infection, but eradication rates have declined due to drug resistance [20]. The World Health Organization (WHO) has reported a troubling increase in antimicrobial resistance, with some antibiotics, like metronidazole and clarithromycin, showing resistance levels of 15% or more. [20]. Resistance to clarithromycin rose from 15.6% in the early 2000s to over 40% by 2020. Metronidazole resistance increased from 58% in the early 2000s to 78% in 2020, as indicated by Garvey et al. [43]. Meanwhile, Savoldi et al. [20] reported that standard triple treatment is less than 80% effective in eradicating H. pylori.
Medical authorities advise discontinuing triple antibiotic therapy if antimicrobial resistance exceeds 15%, as per the Maastricht IV/Florentine Consensus Report [44]. In these cases, quadruple therapy, which includes two antimicrobial agents, PPIs, and bismuth salts, may be used. However, since quadruple therapy still contains antibiotics, individuals resistant to these should avoid it. Due to a shortage of bismuth salts, initiating quadruple therapy with bismuth is impractical in many countries where its use is restricted [45,46]. Furthermore, Poonyam et al. [47], have shown that antimicrobial agents can cause digestive issues, including diarrhea, eating disorders, vomiting, and abdominal discomfort. There are significant safety concerns regarding antimicrobial treatment in older adults, children, and pregnant women, making it inadvisable for these populations [48].
Complementary therapies are gaining popularity for managing H. pylori infections. Using probiotics and herbal medicines alongside antibiotics can mitigate antibiotic side effects and reduce resistant organisms [49]. Lactoferrin (LF) inhibits bacterial growth by depriving bacteria of iron and enhancing membrane permeability [50,51]. LF may also help treat H. pylori infections and gastric ulcers due to its anti-inflammatory effects [52,53]. Currently, phage therapy has shown promise in treating various illnesses, including chronic conditions [54]. However, its use against H. pylori may be delayed [55], because the understanding of H. pylori phage biology is still developing. H. pylori vaccination is rapidly advancing [1], with about ten antigen types and nearly ten adjuvant types identified to enhance the immune response [56]. Various delivery technologies have been developed to improve antigen presentation, and several clinical studies are underway, offering new hope for eradicating H. pylori infection [57,58,59,60]. There is a high demand for alternative drugs to control H. pylori infections.
Therefore, this review examines H. pylori infection, focusing on transmission pathways, treatment modalities, antibiotic resistance, and eradication strategies, which include tailored therapy and potassium-competitive acid blockers. Additionally, it discusses alternative therapeutic approaches such as probiotics, anti-biofilm agents, phytotherapy, phototherapy, phage therapy, lactoferrin therapy, and vaccine development. The objective of these strategies is to mitigate antimicrobial resistance and enhance treatment outcomes for H. pylori infections.
2. Methodological Methods
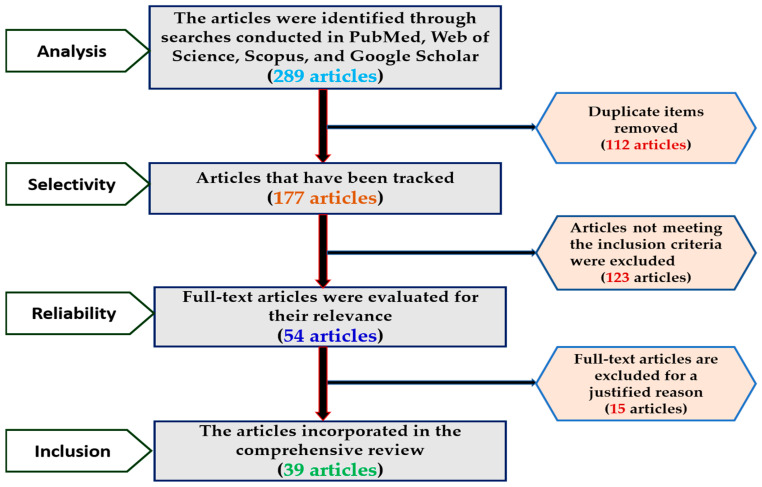
The review process was executed following a flowchart, as illustrated in Figure 1, which delineates the steps for evaluating manuscripts that have successfully undergone the screening process. This review involved a comprehensive literature analysis aimed at collecting information on H. pylori, with particular emphasis on transmission pathways, treatment failures, antimicrobial resistance, and alternative therapeutic strategies. The inclusion criteria encompassed original research articles, review papers, and clinical trials that addressed antibiotic resistance, conventional treatment modalities, and alternative interventions for H. pylori. Key topics explored included the potential applications of alternative medicine, instances of treatment failure, and vectors of infection. To ensure the relevance and timeliness of the research, only English-language publications from 1983 to 2024 were considered. The analysis specifically excluded non-research materials such as editorials and commentaries, non-English publications, duplicate studies, and research that did not pertain to H. pylori transmission mechanisms, antibiotic resistance, alternative therapies, or treatment failures. Searches were performed across databases including PubMed, Web of Science, Scopus, and Google Scholar, utilizing keywords such as “H. pylori”, “transmission routes”, “antibiotic resistance”, “alternative therapy”, “treatment failure”, “probiotics”, “phage therapy”, and “vaccine”. The quality of the included studies was evaluated based on predetermined criteria.
Figure 1.
Flow chart explaining the review process for manuscripts that have been screened.
3. The Transmission Patterns of Helicobacter pylori
The mechanisms underlying the transmission of H. pylori remain inadequately understood. Consequently, there is an urgent need to develop a more comprehensive understanding of the pathways through which H. pylori disseminates into the gastric environment, thereby enhancing human resistance to such infections. Given that H. pylori appears to have a restricted host range, it is posited that its primary host is the human gastrointestinal tract, where infection can occur [24,61]. New infections are believed to arise from environmental exposure or direct interpersonal contact. Generally, there are three principal modes through which humans may become infected with H. pylori.
3.1. Human-to-Human Transmission
H. pylori infections are primarily transmitted through person-to-person contact, with two main modes: vertical and horizontal. Horizontal transmission occurs through contact with non-family members or environmental contamination. Vertical transmission is the transfer of an infectious agent from one generation to the next within the same family [62]. Many studies have investigated the link between H. pylori infection and familial susceptibility. The majority of the studies [26,63] indicate that H. pylori infections frequently cluster in families. H. pylori can spread within families through direct transmission [1,64]. Factors such as close relationships, genetic predisposition, shared socioeconomic conditions, and common sources of infection contribute to this spread [65,66,67,68]. Yang et al. [14] highlight that transmission is especially common in households with frequent mother–child interactions. Research suggests that H. pylori infections may cluster in families, and a study by Ding et al. [69] found that children can acquire the infection from infected parents.
Childhood infection risk is significantly affected by environmental factors and family dynamics. Children in larger families with more siblings are more likely to contract H. pylori infections [70], with mothers and grandparents typically serving as primary caregivers. Young children can contract H. pylori through oral and fecal pathways by consuming food chewed by a caregiver, being kissed on the mouth, or if the caregiver fails to wash their hands after using the bathroom [71]. Thus, children with relatives who have had H. pylori infections are also at risk [72]. A 2013 study in Japan found that grandmothers significantly contribute to H. pylori transmission across generations [73]. Goodman and Correa [74] indicated that older family members are more likely to transmit H. pylori to younger siblings, especially those close in age. Fialho et al. [75] found that H. pylori can be transmitted from younger to older relatives, indicating possible sibling transmission. Patel et al. [76] discovered that children in economically disadvantaged schools in Edinburgh had a significantly higher prevalence of H. pylori infection than those in other regions, even when controlling for other risk factors. Although H. pylori transmission likelihood decreases with age, Brenner et al. [77] found that couples can still contract the infection. In a study of 670 couples in Germany, the infection prevalence was 34.9% in women and 14.5% in uninfected husbands.
According to a study conducted in the medical field, 82.4% of gastrointestinal endoscopy specialists had H. pylori infections in their stomachs. The infection rate among gastrointestinal healthcare professionals is 16.8%, whereas it can reach 70% among dental professionals, according to Kehre et al. [78]. As a result, work-related variables are important conduits through which potential H. pylori infection can spread. The spread of H. pylori-contaminated saliva can also occur through the use of shared utensils by individuals who are infected with this bacterium and healthy individuals who are not. In a study of 328 adult Chinese immigrants living in Melbourne, Australia, Chow et al. [79] examined the prevalence of H. pylori infection and found significant associations with utensil transmission in both male and female infectors. Although some evidence suggests that H. pylori can be transmitted by utensils, a study published by Leung et al. [80] found that the presence of H. pylori was only 3.7% in infected cases and 10% in salivary-infected cases, indicating low odds of transmission by utensils. A summary of the data shows that H. pylori most commonly affects children and adolescents; however, feeding utensils pose a small risk of infection.
3.2. Animals to Human’s Transmission
Helicobacter species can infect humans and domesticated animals, such as dogs, cats, pigs, and birds, as well as wild animals like monkeys [81,82,83,84]. A Helicobacter bacterium similar to those in animals with gastritis has also been found in humans with gastritis [85]. A large segment of the global population lives near domestic animals, especially dogs and cats, highlighting the importance of these findings [86]. While some animals, such as sheep and dogs, can temporarily carry H. pylori, the impact on humans is still uncertain. Factors that may increase the risk of H. pylori infection include childhood exposure to unpasteurized milk, raw vegetable consumption, and contact with pets like dogs and cats. H. pylori can be transmitted zoonotically, mainly through indirect means [87]. This infection is a key transmission pathway from animals to humans, especially in developing countries. Duan et al.’s work, published in 2023, notes that H. pylori infects both humans and animals [88]. Papież et al. [89] found higher H. pylori infection rates among sheep ranchers and their families in the Tatra Mountains of Poland (97.6% and 86%, respectively) compared to farmers without sheep (65.1%) [90]. Several studies have indicated that H. pylori can be detected in milk [91,92], poultry slaughterhouses [93], and other fresh foods.
3.3. Transmission Through Water and Food
Water is vital for human survival, posing a risk of contact with H. pylori-contaminated sources [94,95,96]. H. pylori can survive in various water types, including cold, salty, distilled, and tap water, due to its ability to modify peptidoglycans in its cell walls. Contaminated water is a primary vector for H. pylori transmission, often linked to fecal matter [97]. Klein et al. [98], found that children from high-income families using municipal water are twelve times more likely to contract H. pylori than those using well water, indicating greater contamination in city supplies. H. pylori infections can also result from contaminated food, with fecal contamination of drinking water being a primary transmission route through streams, rivers, lakes, soil, and groundwater. Studies show high levels of H. pylori in Iranian water bottles and an increasing infection rate among those using non-municipal water for toilets [99,100]. Individuals at higher risk may consume water from polluted sources. Preventing H. pylori spread requires better dietary management and rigorous water testing.
Contaminated water with H. pylori poses a serious risk, as it can taint fruits and vegetables, resulting in foodborne illnesses [101]. Hemmatinezhad et al. found that 28% of 50 fruit salads tested positive for H. pylori through molecular analysis [91]. The risk of infection can be reduced through thorough cleaning and avoiding contaminated water sources [91]. Hamada et al. [93] examined 90 samples of chicken meat, gizzards, and liver from a semi-automated slaughterhouse in Sadat City, Egypt, finding that seven samples (7.78%) tested positive for H. pylori. Similarly, Mashak et al. [102] tested 600 raw meat samples from Iranian slaughterhouses for H. pylori. Mutton contamination was 13.07%, while goat mutton was 11.53%. A study by Shaaban et al. [92] found H. pylori in 5 of 13 milk samples from farm animals. Figure 2 shows the main routes of H. pylori infection in humans, with food and water as potential sources. The risk of transmission rises with close contact between infected individuals and livestock. Regular handwashing and sanitizing are crucial for health and safety. Regularly examining water sources is crucial for identifying H. pylori infection origins and reducing transmission risk. Thoroughly clean fruits and vegetables before consumption and limit fresh meat and dairy intake.
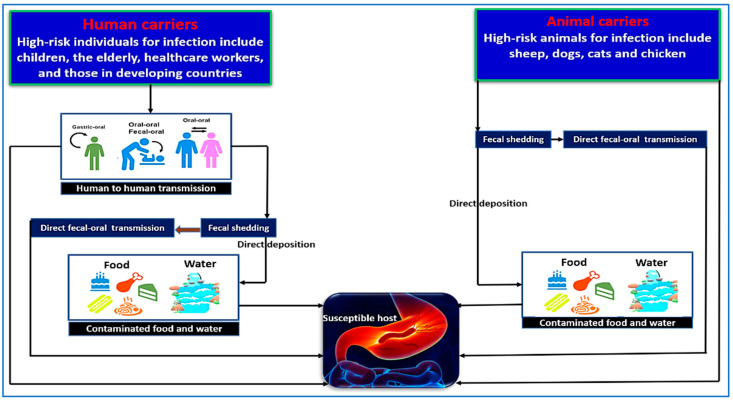
Figure 2.
The pathways through which H. pylori is transmitted. Person-to-person transmission occurs among children, the elderly, healthcare workers, and individuals living in developing countries. The bacterium can spread through oral–oral, fecal–oral, or gastric–oral routes, as well as through fecal shedding that contaminates food or water sources. Oral–oral transmission may occur when sharing food utensils or between mothers and their newborns. Additionally, H. pylori can be transmitted to animals such as sheep, dogs, cats, and chickens through fecal shedding or direct fecal–oral contact. Food and water sources contaminated with H. pylori can also directly transmit the bacteria to susceptible individuals.
4. H. pylori Infection: Standard Therapy, Antimicrobial Resistance, and Failure of Treatment
When choosing the optimal therapy, it is essential to consider regional antibiotic resistance and antimicrobial susceptibility testing results [103,104]. In some countries, a recommended treatment plan may include mixed therapy, administering multiple medications simultaneously for two weeks or more [105,106,107]. Triple therapy, combining amoxicillin, clarithromycin, and a PPI like omeprazole, has historically been the first-line treatment for H. pylori [13,107,108]. However, a 2016 study by Thung et al. [109] revealed significant antibiotic resistance, leading to the recommendation of second-line treatments. In the U.S. and Europe, quadruple therapy with metronidazole, tetracycline, omeprazole, and bismuth is now advised [47,110]. Clarithromycin’s minimal effect on stomach pH and effective mucosal diffusion make it essential in combination therapy for H. pylori infections [111]. The global prevalence of H. pylori and its related diseases is largely due to clarithromycin’s reduced effectiveness and recurrence in countries with poor healthcare infrastructure [112]. Therefore, the use of antimicrobial agents for H. pylori infections should be limited.
A meta-analysis by Boyanova et al. [113], found that H. pylori strains in Bulgaria had 30% resistance to clarithromycin and 42% to metronidazole. Savoldi et al. [20], reported that clarithromycin resistance in Europe was about 18%, compared to 33% in the western Pacific and 34% in the Mediterranean [114]. Antimicrobial resistance rates differ significantly between industrialized and developing nations [20,109,115,116,117,118,119,120]. Resistance to metronidazole and clarithromycin is notably higher than for other antibiotics [119]. In China, clarithromycin resistance has increased from 14.8% to 52.6% [109]. Over the past century, H. pylori has increasingly shown resistance to antibiotics like clarithromycin, amoxicillin, and metronidazole [121,122]. Figure 3 illustrates studies from Asia, Africa, Europe, and America that examined resistance rates for clarithromycin [123,124,125,126], metronidazole [66,125,126,127], levofloxacin [124,125,126,127], and amoxicillin [124,125,126,127] from 2001 to 2022, 2007 to 2017, 2013 to 2021, and 2011 to 2021, respectively.
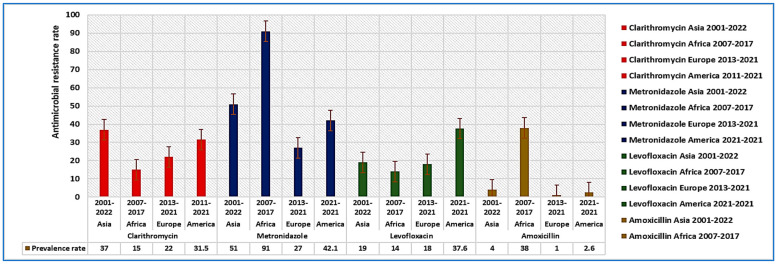
Figure 3.
The prevalence rates of multidrug-resistant H. pylori across various regions, including Asia (2001–2022), Africa (2007–2017), Europe (2013–2021), and America (2011–2022). The resistance rates to clarithromycin were found to be 37% in Asia, 15% in Africa, 22% in Europe, and 31.5% in America. For metronidazole, the resistance rates were 51% in Asia, 91% in Africa, 27% in Europe, and 42.1% in America. The resistance rates to levofloxacin were reported as 19% in Asia, 14% in Africa, 18% in Europe, and 37.6% in America. Lastly, amoxicillin resistance rates were recorded at 4% in Asia, 38% in Africa, 1% in Europe, and 2.6% in America.Resistance mechanisms primarily stem from mutations that alter pharmacological targets. Drug-resistant genotypes are linked to mutations affecting membrane permeability, biofilm formation, and efflux pumps [120,128]. Amoxicillin resistance mainly arises from changes in membrane permeability and mutations in the penicillin-binding protein gene [120]. H. pylori strains often resist clarithromycin due to point mutations in 23S rRNA. A study at Peking University utilized next-generation sequencing to identify genetic factors enhancing resistance to levofloxacin and clarithromycin [129]. Key mutation sites for clarithromycin resistance include peptidyl transferases in the 23S rRNA, with A2143G and A2142G being the most common. Mutations in the DNA gyrase (gyrA) gene (N87K, D91N, D91G) were linked to levofloxacin resistance [129]. Reduced drug influx due to structural changes in lipopolysaccharide (LPS) membranes also contributes to resistance. Mutations in the rfaF (LPS heptosyltransferase II) gene lead to deep, coarse LPS drug absorption [130] and causing slight resistance to chloramphenicol, along with cross-resistance to amoxicillin, tetracycline, and clarithromycin [131]. Increased expression of tolC homolog genes (hefA) in patients with gastrointestinal disorders in Iran [132] was linked to efflux pump induction, as shown by real-time PCR in metronidazole and clarithromycin-resistant bacteria. The multidrug-resistant phenotype was found in 9.5% of cases. A genome-wide analysis identified prevalent mutations, including A2143G in 23S rRNA (63.1%) and alterations in the rdxA gene (85.5%).
Hou et al. [133] found that H. pylori’s resistance to antimicrobial agents is the main factor in biofilm development (Figure 4). Extracellular polymeric substances (EPSs) coat microbial surfaces and, due to their negative charge, hinder the penetration of antimicrobial agents, making microbes up to a thousand times more resistant to antibiotics than planktonic bacteria [133,134,135]. Administering antibiotics to H. pylori during biofilm formation is ineffective, as the antibiotics cannot penetrate the biofilm, resulting in unsuccessful therapy. Biofilms also protect H. pylori from the immune system, increasing antibiotic resistance [133]. Patients requiring repeated therapy for H. pylori often need a second treatment long after the first. H. pylori can switch from a spiral to a spherical shape, entering a viable but nonculturable state (VBNC), which cannot be cultivated [3,136,137]. Microbes can endure stressful conditions, such as sub-inhibitory drug dosages or unfavorable environments, without damage [97]. Chaput et al. [138] noted a significant alteration in the peptidoglycan of spherical H. pylori cells, allowing them to evade immune recognition while still stimulating IL-8 production in the stomach epithelium. This enables H. pylori to avoid or modulate the host immune response in a viable but non-culturable (VBNC) state, facilitating long-term survival in the stomach.
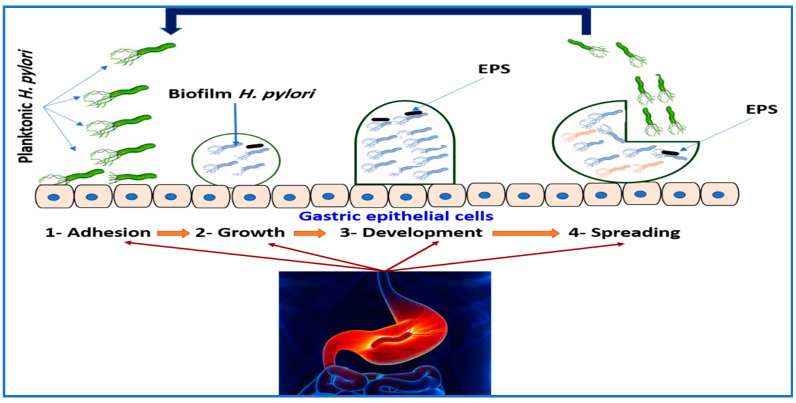
Figure 4.
An overview of the biofilm formation process: (1) Attachment stage of biofilm formation involves reversible and irreversible processes. Reversible attachment occurs when planktonic cells adhere to surfaces via chemical interactions, aided by virulence factors like adhesins and pili, triggering biofilm formation and increasing microbial susceptibility to antimicrobials. (2) Growth (irreversible attachment) leads to microbial proliferation and colony establishment, enhancing adherence through transcriptional changes. This phase promotes substrate exchange, metabolic product distribution, and byproduct excretion. H. pylori secrete EPS, which lower biofilm cell susceptibility to host defenses and antimicrobials. (3) Development features an increasing extracellular matrix around microcolonies, driven by EPS production and quorum-sensing communication, both vital for resistance. Mature biofilms have high EPS content and interstitial spaces for nutrient, water, and planktonic cell movement. (4) Spreading occurs when detachment due to nutrient depletion prompts cells to seek new surfaces through erosion and sloughing.
Wang and Wang [139] developed a population of spherical H. pylori by treating these cells with a sublethal dose of antimicrobial agents. Researchers confirmed the pathogenicity of spherical H. pylori cells by analyzing sequences from various strains. The study found a complete cagA gene in the bacteria, with about 99% similarity to the original sequence of vegetative forms. These results indicate that phenotypic changes are crucial for maintaining H. pylori’s “health” and survival throughout its life cycle [140]. The polymer substances and coccoid formation of H. pylori, along with the efflux pump on its membrane, contribute to drug resistance [133]. The efflux pump expels antimicrobial agents, reducing their intracellular concentrations [128,141,142]. In H. pylori, efflux pumps are key players in multidrug resistance [143]. Biofilms exposed to clarithromycin show significantly higher resistance than planktonic organisms, with increased expression of efflux pump genes [144,145]. Microorganisms producing biofilms are more likely to express the efflux pump genes Hp605, Hp971, Hp1327, Hp1489, Hp118, and Hp1174 than those forming planktonic structures [144,146]. Efflux pumps and biofilms work together to enhance drug resistance.
5. Alternative Therapies
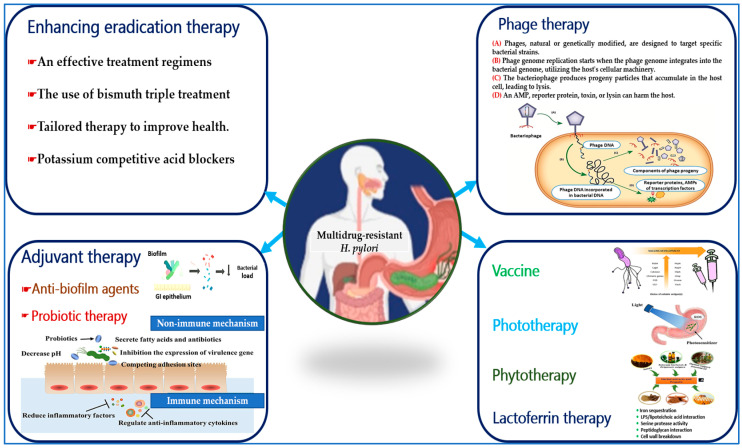
The rise of antimicrobial resistance in H. pylori has complicated treatment. If eradication fails, multiple rounds of different antimicrobial combinations may be needed. Physicians are now tasked with finding effective alternatives to declining traditional therapies, which often have higher pill burdens and side effects [147]. Expert guidelines have shifted, now favoring quadruple therapy with bismuth as the initial treatment over the previous triple therapy with clarithromycin [148]. With limited options for antibiotic-resistant strains, innovative treatments, including non-antibiotic approaches, are urgently needed to address this issue (Figure 5).
Figure 5.
A range of alternative therapeutic approaches has been developed to combat the antimicrobial resistance exhibited by H. pylori. Probiotic therapy employs non-immune mechanisms to counteract H. pylori by competing for attachment sites, inhibiting the expression of virulence genes, and inducing cell death. Additionally, it reduces inflammatory mediators and regulates anti-inflammatory cytokines through immune mechanisms. Photodynamic therapy effectively eradicates H. pylori by generating ROS using a light source in conjunction with a photosensitizer. Phage therapy involves the production of progeny particles from bacteriophages that lyse host cells. The development of vaccines utilizing virulence antigens is crucial for reducing colonization and eradicating H. pylori. Phytotherapy can inhibit urease activity, prevent bacterial adhesion, and enhance membrane permeation against H. pylori infection. Lactoferrin therapy sequesters iron, interacts with lipopolysaccharides and lipoteichoic acids, modulates serine protease activity, and engages with peptidoglycan, ultimately leading to the collapse of the cellular wall.
5.1. Enhancing Eradication Therapy
H. pylori eradication regimens, developed by gastrointestinal specialists, aim to eliminate this bacterium. However, global success rates have declined, and antimicrobial resistance has increased [149]. A more targeted approach using specific antibiotics is needed to improve outcomes [105]. Customizing therapy strategies to regional sensitivity profiles is essential for addressing varying antimicrobial resistance trends [105,150]. However, the lack of reliable statistics on antibiotic-resistant bacteria in local communities hinders decision-makers from selecting effective empirical eradication strategies. Regions with high metronidazole- and clarithromycin-resistant H. pylori are advised to use bismuth triple therapy for infection elimination [151,152,153]. Adding bismuth to certain protocols and extending treatment to two weeks can boost eradication rates by up to 30% in resistant strains [154]. Botija et al. [155] conducted an evaluation of the efficacy of colloidal bismuth subcitrate (CBS) therapy in eradicating H. pylori among patients aged 5 to 8 years. They utilized data from a national pediatric registry comprising 682 patients. Among these patients, 38 (5.6%) received CBS treatment, with 50% of this group having experienced prior unsuccessful eradication attempts. A follow-up assessment of 32 patients revealed an eradication rate of 93.8% for those treated with CBS, compared to an 86.7% eradication rate for patients who did not receive CBS treatment (p < 0.05). Recent meta-analyses show that first-line regimens with bismuth have higher eradication rates than those without [156]. Nijevitch and colleagues [157] assessed the effectiveness of a triple therapy of nifuratel, amoxicillin, and bismuth for pediatric H. pylori gastritis. After endoscopy for dyspeptic symptoms, 73 children aged 9 to 14 received a 10-day course of treatment. H. pylori was eradicated in 63 participants (86%; 95% CI: 76.6–93.2). There were no withdrawals due to side effects, and no severe adverse reactions occurred.
The rising rates of resistance and multidrug-resistant H. pylori underscore the need for better detection of antimicrobial susceptibility and treatment [158]. Tailored therapy effectively increases eradication rates while reducing unnecessary antibiotic use [159]. This approach allows for drug selection based on susceptibility to antimicrobial agents determined by drug composition. In 2022, Nyssen et al. [160] conducted a meta-analysis examining the empirical and susceptibility-guided treatment approaches for H. pylori, which encompassed 54 studies involving a total of 6705 patients in the empirical treatment cohort and 7895 patients in the susceptibility-guided cohort. The results indicated that the eradication rates of H. pylori were significantly higher in the susceptibility-guided group, achieving an 86% success rate, in contrast to a 76% success rate observed in the empirical treatment group. Gingold-Belfer et al. [161] performed a meta-analysis of 16 randomized controlled trials to compare susceptibility-guided therapy and empirical therapy for H. pylori infection. The study involved 2451 patients receiving empirical treatment and 2374 receiving susceptibility-guided therapy. The findings revealed no significant difference in effectiveness, with a relative risk (RR) of 1.02 (95% confidence interval: 0.92–1.13; p = 0.759; I2 = 80%). Although empirical regimens effectively eradicate H. pylori, the advantages of tailored therapy may not be clear. Challenges include the absence of H. pylori cultures and antibiotic susceptibility testing. More research is needed to promote the widespread use of tailored treatments for H. pylori elimination.
While proton pump inhibitors with triple therapy are effective for acid reflux, there is growing interest in acid-suppressing medications [162]. The dual and triple use of potassium-competitive acid blockers (P-CABs) is an innovative and effective method for eradicating H. pylori [163]. Acid suppression is vital in H. pylori treatment, as a higher stomach pH fosters bacterial growth and increases susceptibility to antibiotics [164]. Lowering stomach pH stabilizes medications like clarithromycin and amoxicillin, which require acid suppression to prevent excessive acidity [165]. Vonoprazan, a P-CAB, is more effective than proton pump inhibitors and provides longer acid suppression [166]. Elazazi et al. [167] conducted a clinical investigation to evaluate the efficacy of H. pylori eradication protocols using Vonoprazan compared to proton pump inhibitors. The study involved 232 treatment-naïve participants, split into two groups: Arm 1 (58 patients) received clarithromycin, amoxicillin, and vonoprazan, while Arm 2 (58 patients) received clarithromycin, amoxicillin, and esomeprazole. Group II included treatment-experienced patients in Arm 3 (intervention) and Arm 4 (comparator), each with 58 participants. Arm 3 received levofloxacin, vonoprazan, nitazoxanide, and doxycycline, while Arm 4 received levofloxacin, esomeprazole, nitazoxanide, and doxycycline. All participants followed their treatment regimens for 14 days, with H. pylori eradication assessed four weeks later. Arm 3 had a 50% eradication rate, compared to 43.1% in Arm 4. Arm 1 achieved 58.6%, and Arm 2 recorded 50%. Regimens containing P-CABs were acceptable, with few adverse events. This therapy is beneficial with amoxicillin or amoxicillin plus clarithromycin for eradicating H. pylori. Japan and several other countries have approved a triple-treatment regimen including vonoprazan for H. pylori eradication [168,169].
5.2. Adjuvant Therapies (Probiotics and Anti-Biofilm Agents)
Adjuvant medicines aim to enhance antimicrobial therapy by combating antibiotic resistance or modifying the host response [147]. Probiotics, defined by the WHO and FAO, are live organisms that confer health benefits when administered in adequate amounts [170]. Beneficial probiotics in clinical settings include Lactobacillus, Bifidobacterium, Bacillus, Streptococcus, and Escherichia coli, which produce lactic acid [171]. Once inside the human body, these microorganisms produce antimicrobial compounds like lactic acid, hydrogen peroxide, and bacteriocins that kill bacteria. Lactic acid inhibits the urease activity of H. pylori [172], and reactive oxygen species (ROS) from probiotics can damage bacterial cell walls and membranes [173]. A recent meta-analysis found that most probiotics in triple therapy improved outcomes with standard eradication therapy [174,175]. Mohtasham et al. [174] conducted a double-blind, randomized controlled trial with 450 participants to assess probiotics as an adjuvant to quadruple therapy for H. pylori eradication. Participants received a 14-day treatment of bismuth subcitrate, pantoprazole, amoxicillin, and clarithromycin, along with either a probiotic (Lactobacillus ruteri, 100 mg) or a placebo. After eight weeks, the urea breath test showed slightly higher eradication rates in the probiotic group (per-protocol: 80.1% vs. 75.2%; intention-to-treat: 78.7% vs. 72%), though not statistically significant. However, only 69.7% of the probiotic group reported side effects, compared to 98.6% in the placebo group (p < 0.001), and they experienced fewer gastrointestinal adverse effects, except for constipation (p < 0.001).
The application of probiotic therapy has shown greater effectiveness in eradicating H. pylori infections [170]. Probiotics have the ability to reduce H. pylori colonization by strengthening the stomach’s mucosal barrier and competing with pathogenic bacteria for adherence [176]. This could potentially help manage diseases linked to H. pylori. Numerous studies have suggested that probiotics have minimal adverse effects on patients’ digestive systems, increasing the chances of compliance [170,177]. Probiotics may inhibit H. pylori colonization, maintain the gastric mucosal barrier, and reduce gastric inflammation. They can also modulate the host’s immune response to infection [147]. Probiotic supplements can help restore intestinal microbiota balance disrupted by antibiotics [178,179]. Yuan et al. [180] studied the effects of H. pylori eradication and probiotics on gastric microbiota in young adults. The study included 95 H. pylori-positive participants and 56 negative controls, aged 19 to 30, assigned to probiotics monotherapy, probiotics-supplemented quadruple therapy, or quadruple therapy alone. Gastric mucosal samples were collected before treatment and two months later for 16S rRNA gene sequencing. Two months post-eradication, the gastric microbial composition differed significantly from H. pylori-negative participants, with decreased alpha diversity in gastric juice and increased diversity in gastric mucosa. Probiotic-assisted eradication improved microbial diversity compared to quadruple therapy, increasing Bifidobacterium and Lactobacillus while reducing harmful bacteria like Fusobacterium and Campylobacter. Probiotic monotherapy had limited effects on H. pylori and beneficial bacteria but significantly altered gastric microbiota diversity, leading to an increase in potentially harmful bacteria post-treatment.
Probiotics exert diverse molecular effects based on their characteristics and chemical composition, leading to beneficial outcomes through various mechanisms [181]. They interact directly with gastrointestinal cells, releasing bioactive compounds that act as signaling molecules in the interactions among intestinal immune cells, gut microbiota, and epithelial cells [182,183]. Key molecular effectors include proteins, low molecular weight peptides, amino acids, bacterial DNA, and short-chain fatty acids (SHFAs) [184]. Probiotic antigens can penetrate the intestinal barrier and trigger immune responses. They enhance intestinal barrier selectivity by increasing mucin, immunoglobulin A (IgA), and defensins, while also boosting the synthesis of vitamins, minerals, SCFAs, and growth regulators [176,185]. Probiotics further promote antiangiogenic factors, cytokines like interleukin-2 (IL-2) and interleukin-12 (IL-12), and antioxidants, which help lower intestinal pH. Lastly, they regulate apoptosis and cell differentiation by inhibiting harmful pathways such as tyrosine kinase [170]. Further research is needed to fully understand the mechanisms and functions of probiotics in eradicating H. pylori.
Another treatment option targets bacterial biofilms with anti-biofilm agents, primarily derived from natural products such as phytochemicals, biosurfactants, antimicrobial peptides, and microbial enzymes [186,187]. Probiotics and quorum-sensing inhibitors also effectively inhibit biofilm growth [188,189]. Almost all natural substances can act as antibacterial agents against H. pylori biofilms [133]. Natural products show anti-biofilm and antibacterial properties against H. pylori strains resistant to multiple antimicrobial agents [190,191,192]. N-acetylcysteine (NAC), a dietary supplement with anti-inflammatory and antioxidant effects, effectively treats H. pylori infections [193,194] and can reduce bacterial load while enhancing eradication rates [195,196,197]. NAC treatment before antibiotics improves H. pylori clearance, as shown in a clinical trial [198]. However, the exact mechanism of NAC’s effects on biofilm disruption and antimicrobial resistance in H. pylori is still unknown. Moreover, combining antimicrobial agents with rhamnolipid, a glycolipid biosurfactant that disrupts biofilms and may reduce bacterial adhesion in vitro, effectively inhibits biofilm development [199,200].
6. Other Developing Therapies
6.1. Lactoferrin Therapy
Lactoferrin (LF) is an iron-binding protein in the transferrin family [201] with antiviral, antibacterial, antioxidant, and anti-inflammatory properties [51]. LF levels rise significantly during H. pylori infections, correlating with gastric mucosal inflammation [51]. It is crucial for maintaining iron balance and aids in iron absorption in the intestinal tract [202,203]. LF inhibits bacterial growth by depriving them of essential iron and increasing membrane permeability [50]. Yamazaki et al. [204] conducted a study on the antibacterial effects of lactoferrin and Lactoferricin® against H. pylori in vitro. Bovine Lactoferricin® was found to be effective at concentrations above 5.0 mg/L, while human and bovine lactoferrins had minimum bactericidal concentrations of 1.25 to 2.50 mg/mL. Both compounds showed dose-dependent effects during exponential growth. Bovine Lactoferricin® exhibited modest activity in brucella broth but had rapid effects in 1% Bacto-peptone medium at concentrations of 0.1 to 1.0 mg/mL. Iron-saturated lactoferrin did not inhibit growth, but bovine Lactoferricin® reduced H. pylori urease activity. These findings suggest that H. pylori is susceptible to both compounds, with the effectiveness of lactoferrin depending on the bacterium’s iron status and growth phase, unlike Lactoferricin®.
Wada et al. [205] studied the effects of bovine lactoferrin (bLF) on germ-free BALB/c mice infected with H. pylori. After oral inoculation, the mice received daily bLF for two to four weeks. Results showed that 10 mg of bLF increased H. pylori presence tenfold while significantly reducing its attachment to the gastric epithelium. Consequently, serum antibody titers for H. pylori became undetectable, indicating a weakened immune response. These findings suggest that bLF has a direct antibacterial effect and can detach H. pylori from the stomach epithelium. Ciccaglione et al. [206] found that combining bovine LF with levofloxacin, amoxicillin, and a proton pump inhibitor provided an additional 21% therapeutic effect in patients from areas with high antibiotic resistance. Other studies have shown that bovine LF inhibits H. pylori growth at pH 6, both in vivo [207] and in vitro [208].
Yuan et al. [53] examined the efficacy of goat-derived transgenic recombinant human LF against H. pylori in vitro and in vivo. Their in vitro findings showed that recombinant LF reduced the virulence factors cagA and vacA and inhibited H. pylori development. Lu et al. [209] also studied the effects of H. pylori infection on host LF levels using animal models. The study revealed that H. pylori-infected stomachs had LF levels 9.3 times higher than healthy stomachs. More recent research by Imoto et al. [51] found that bovine LF inhibits H. pylori growth in vitro at concentrations of 25.2 to 50.0 mg/mL. LF is often combined with antibiotics to treat H. pylori infections effectively [44] and has been shown to improve treatment success rates. In the future, LF combined with antibiotics may replace traditional triple therapy as a more effective option.
6.2. Herbal Therapy (Phytotherapy)
Herbal therapy, or phytotherapy, involves using plants and their extracts for medicinal purposes [210,211]. Various plant parts—leaves, stems, flowers, roots, and seeds—are used to create raw or processed herbal products [212]. Health regulations classify herbs as nutritional additives, allowing them to be sold without prior safety or efficacy evaluations [213]. The effectiveness of herbal therapies relies mainly on empirical evidence due to limited scientific research [214]. Controlled clinical trials are vital for assessing the efficacy of herbal medicines and improving their quality and safety [215]. Li et al. [216] examined the effects of Banxia Xiexin Decoction (BXXXT), a traditional Chinese medicine prescription, on drug-resistant H. pylori-induced gastritis in mice using both in vivo and in vitro methods. The aqueous extract (BXXXT) was prepared through water decoction. In vitro tests demonstrated BXXXT’s inhibitory effects on H. pylori, while an acute gastritis model was established in vivo. Treated mice were assessed for H. pylori colonization, gastric mucosal repair, inflammation, and apoptosis. The minimum inhibitory concentration (MIC) of BXXXT was found to be 256–512 μg/mL, with a dosage of 28 mg/kg proving more effective than standard triple therapy. The extract consisted of at least 11 compounds, including berberine and quercetin, which exhibited synergistic effects and enhanced immune function in CD3+ and CD4+ T cells. Certain plants and fruits contain compounds such as flavonoids, terpenoids, and alkaloids that may effectively treat H. pylori infections [217,218].
Fahmy et al. [219] found that flavonoids from Erythrina speciosa (Fabaceae) had the lowest minimum inhibitory concentration (MIC) against H. pylori. Zardast et al. [220] reported that raw garlic significantly reduced H. pylori growth in the stomach mucosa within 72 h. The ethyl acetate extract from this plant showed the highest antimicrobial activity, with an MIC of 62.5 µg/mL. Ayoub et al. [221] evaluated the essential oils and methanol extracts of Pimenta racemosa (P. racemosa) leaves and stems for their inhibitory activities against H. pylori, both in vitro and in silico. The essential oil from the stems showed significant antibacterial activity with a MIC of 3.9 µg/mL, comparable to clarithromycin’s MIC of 1.95 µg/mL. In silico studies suggested that compounds such as decanal, eugenol, terpineol, delta-cadinene, and amyl vinyl may inhibit H. pylori urease, indicated by strong binding affinity scores. These results highlight the therapeutic potential of P. racemosa, particularly in its stems, which are often considered agro-industrial waste. Shmuely et al. [222] noted that plant extracts inhibit urease, prevent adhesion, and permeate membranes to combat H. pylori. Fahmy and his colleagues [219] reported significant antimicrobial activity in plant extracts at MICs below 100 µg/mL, supporting the use of plants for treating H. pylori infections. These agents have proven effective in eliminating H. pylori and preventing related gastrointestinal disorders.
Herbal medicine offers numerous advantages, including widespread availability, affordability, and a significant presence among consumers who perceive it as a safer alternative to synthetic pharmaceuticals, particularly in regions with a longstanding tradition of herbal use [223]. The application of natural products may pose fewer risks compared to conventional treatments that often involve multiple antibiotics, although it is important to acknowledge that herbal remedies can also have side effects [224,225]. Research indicates that the integration of conventional therapy with ethnomedicine results in higher eradication rates and a reduction in adverse effects [226]. Furthermore, combination therapy has proven effective in alleviating symptoms of gastritis associated with Helicobacter pylori through a holistic approach [227]. Additionally, herbal therapy may mitigate antibiotic resistance due to its multitarget effects [227]. Patients who are unable to tolerate high doses of antibiotics may be particularly well-suited for herbal therapy.
6.3. Photodynamic Therapy
Photodynamic therapy (PDT) is a proposed method for eliminating harmful bacteria [228]. It generates ROS through the oxidation of biomolecules when a photosensitizer (PS) is exposed to laser light [229,230]. Unlike traditional antibiotics, PDT poses no risk of drug resistance, making it a promising alternative [231,232,233]. However, its application for treating H. pylori is still in early development, necessitating a PS that specifically targets H. pylori to protect normal cells from phototoxicity [234,235]. H. pylori produces sialic acid binding adhesin (SabA), which binds specifically to 2,3-linked sialic acid on sialyl-dimeric Lewis X antigens in the gastric epithelium. This binding promotes strong adhesion and colonization of the gastric mucosa [236,237]. The presence of 2,3-linked sialic acids in 3′-sialyl lactose (3SL) suggests it may effectively target H. pylori, as human cells lack 3SL receptors, making it highly selective for this bacterium [228,235,238].
The fundamental principle underlying PDT involves the generation of high ROS through the interaction of a PS, molecular oxygen, and visible light at an appropriate wavelength [239]. This process leads to the oxidation of various cellular components, resulting in rapid cell inactivation [240]. Numerous studies have identified potential targets for ROS generated by PDT within biofilms, particularly the EPS matrix [241], which comprises proteins [242], lipids [243], DNA [244], and extracellular polysaccharides [245]. Damage to proteins and DNA induced by PDT significantly diminishes the metabolic activity of the biofilm and may lead to structural disruption [246]. Phototherapy can effectively eliminate bacterial biofilms in the stomach, providing a therapeutic benefit against antibiotic-resistant bacteria [247]. Qiao et al. [248] studied the antibacterial effects of phototherapy on multidrug-resistant H. pylori using a near-infrared photosensitizer, T780T-Gu, created by combining guanidinium (Gu) with T780T. The results showed that T780T-Gu has synergistic effects in photothermal and photodynamic treatments against biofilms and MDR strains of H. pylori, potentially enhanced by structural deficits and reduced metabolism. Im et al. [228] developed a photomedicine called multiple 3SL-conjugated poly-L-lysine-based photomedicine (p3SLP) for targeted PDT against H. pylori. In C57BL/6 mice, oral administration of p3SLP followed by laser irradiation effectively inactivated H. pylori by targeting sabA on the bacteria’s membrane, without harming host cells. p3SLP shows potential as an endoscopic antibacterial PDT method for treating H. pylori.
6.4. Phage Therapy
Phage therapy, which uses bacteriophages to treat bacterial infections, has gained attention due to advancements in genetic engineering, metagenomics, high-throughput genome sequencing, and biotechnology [249,250,251]. Bacteriophages infect and destroy bacteria by attaching to specific receptors, introducing their genetic material, multiplying, and causing the bacterial cell to rupture, releasing more phages in the lytic cycle [252,253]. The destruction of bacterial cells helps maintain host cell health by eliminating pathogenic bacteria. Engineered phages can enhance their ability to target specific bacteria. Phage therapy may treat antibiotic-resistant infections in diverse patient populations and reduce antibiotic use in livestock [251].
Interest in phage therapy for H. pylori infections has grown [232]. This method employs bacteriophages to target and eliminate H. pylori [254]. Ferreira et al. [255] isolated the novel podovirus prophage HPy1R using H. pylori strains using UV radiation. It has a genomic length of 31,162 base pairs and encodes 36 predicted proteins, including 17 structural proteins. Phage particles remained stable at 37 °C and pH 3–11 for 24 h. In an in vitro stomach digestion model, only a slight reduction occurred during the gastric phase, indicating adaptation. This phage also reduced H. pylori levels for up to 24 h post-infection at multiplicities of infection of 0.01, 0.1, and 1 microaerophilic condition, suggesting its potential for phage therapy in the absence of exclusively lytic phages. Cuomo et al. [256] studied the effectiveness of H. pylori-specific lytic phage (H. pylori φ) alone and with lactoferrin adsorbed on hydroxyapatite (LF-HA) nanoparticles (H. pylori φ + LF-HA) in preventing H. pylori infection. The bacteria were obtained from human stomach biopsies and cultured in brain heart infusion (BHI) broth with 10% horse serum at 37 °C and 5% CO2 for phage isolation. The study found that LF-HA significantly enhances H. pylori φ activity, indicating that phages complexed with LF can selectively eliminate H. pylori without harming host cells, making it a promising therapeutic option. The H. pylori φ φ + LF-HA combination showed potential efficacy when administered at the onset of infection, but the minimum effective doses were not established.
A study examined the effects of lactoferrin on hydroxyapatite nanoparticles combined with a lytic phage, showing improved antibacterial effects in human gastric cancer cells [257]. Nonetheless, knowledge about phage-H. pylori interactions in the stomach microenvironment are still lacking. The limited availability of sequenced phage genomes restricts our understanding of H. pylori phages, including their pathogenicity, antimicrobial resistance genes, and toxins [232]. H. pylori phages lack endolysins [258], proteins that dissolve bacterial cell walls, and could serve as alternative treatments in phage therapy. Endolysins are host-specific, with no known bacterial resistance [259]. However, the protective outer layer of Gram-negative pathogens like H. pylori complicates treatment. Lysins can penetrate outer membranes when combined with mild acids or engineering techniques [260,261]. Despite the potential of phage therapy, more research is needed before it can be widely implemented for H. pylori.
6.5. Vaccination Against H. pylori: Potential Uses
An effective H. pylori vaccine could transform infection control and reduce future antibiotic use. While few candidates have shown promise in generating a protective immune response [56,262,263], several are under evaluation. The stomach’s acidic pH and the continuous renewal of mucosa allow H. pylori to evade the immune system. Even after eradication, patients may not remain protected [1]. A vaccine could prevent or reduce the frequency and severity of stomach infections [264]. To improve the effectiveness of preventive or therapeutic vaccinations, it is crucial to select appropriate adjuvants and immunogenic bacterial antigens [265]. Antigens such as Cytotoxin-associated gene A (CagA), vacuolating cytotoxin A (VacA), blood group antigen-binding adhesin (BabA), H. pylori adhesin A (HpaA), neutrophil-activating protein (NapA), outer inflammatory protein A (OipA), gamma-glutamyl transpeptidase (GGT), heat shock protein A (HspA), outer membrane proteins (Omp), and flagellar cap protein (FliD) have been linked to vaccinations [266]. Vaccines targeting four virulence proteins (FVPE) [267] and the multi-epitope vaccine (CTB-UE) [268] contain adjuvants and antigens expressed on CD4+ and CD8+ cells. Cholera toxin and E. coli enterotoxin are used as mucosal adjuvants to boost the immunogenicity of whole-cell and subunit vaccines. Furthermore, intramuscular H. pylori subunit vaccines with aluminum hydroxide adjuvants and oral live vector vaccines expressing H. pylori antigens are recommended for long-lasting protection [269].
In 2017, Guo and colleagues [270] developed the multivalent epitope-based vaccine CFAdE, using antigenic fragments from four H. pylori adhesins: ure, Lpp20, HpaA, and cagL. They assessed its specificity, immunogenicity, and ability to generate neutralizing antibodies in BALB/c mice, as well as its therapeutic efficacy and protective immune responses in H. pylori-infected Mongolian gerbils. CFAdE induces high levels of specific antibodies against urease, Lpp20, HpaA, and cagL. Oral vaccination with CFAdE and polysaccharide adjuvant (PA) significantly reduces H. pylori colonization compared to ure and PA immunization, with protection linked to IgG, sIgA antibodies, and antigen-specific CD4+ T cells. A multivalent epitope-based vaccine targeting multiple adhesins in H. pylori is more effective than a urease-targeting single epitope vaccine, offering a promising treatment for H. pylori infection. Adding a polysaccharide adjuvant to the multivalent vaccine dramatically reduced H. pylori levels in mice compared to the monovalent vaccine group [268]. As a result, multivalent vaccinations are becoming more popular. The vaccine developed by Guo and his colleagues targets H. pylori using bacterial attachment molecules, including urease, lipoprotein (Lpp20), H. pylori adhesins (HpaA), and CagL. Testing in experimental models showed increased antibody production against adhesion molecules in vaccinated mice [270]. Reports indicate that the deactivated H. pylori whole-cell vaccine enhances gastrointestinal immunity and reduces H. pylori severity [57].
In 2023, Katsande et al. [271] modified Bacillus subtilis spores to display H. pylori antigens, urease subunit A (ureA), and subunit B (ureB). They evaluated immunity and colonization in mice challenged with H. pylori after oral administration of these spores. Vaccination with ureA or ureB-expressing spores induced antigen-specific mucosal responses (fecal sIgA), seroconversion, and hyperimmunization, reducing H. pylori colonization by up to 1 log. This study highlights the potential of Bacillus spores for mucosal immunization against H. pylori, given their thermal stability and probiotic properties. Zeng et al. [272] conducted a Phase 3 clinical study in China to evaluate a three-dose oral recombinant H. pylori vaccine in healthy children aged six to fifteen. Participants without prior H. pylori infection were randomly assigned to receive the vaccine or a placebo, with the primary outcome being the incidence of infection within one year (ClinicalTrials.gov: NCT02302170). From 2 December 2004 to 19 March 2005, 4464 individuals were assigned to the vaccination (n = 2232) or placebo group (n = 2232), with 4403 (99%) completing the regimen. In the first year, 64 infections were reported: 14 in the vaccination group and 50 in the placebo group, resulting in a vaccine effectiveness of 71.8% (95% CI: 48.2–85.6). Adverse reactions occurred in 157 individuals (7%) in the vaccination group and 161 (7%) in the placebo group, with major events in five (<1%) and seven (<1%) individuals, none linked to the vaccine. While vaccination could help prevent H. pylori infections globally, no vaccine candidates have yet proven clinically relevant [232,273].
A comprehensive summary of various therapeutic studies aimed at eradicating H. pylori is presented in Table 1 below. This table includes detailed information regarding each potential therapy, encompassing the type of study (preclinical, clinical, in vitro, etc.) along with the results and outcomes associated with each investigation.
Table 1.
Summary of therapeutic studies contributing to the eradication of multidrug-resistant H. pylori infection.
| Therapy | Type of Study | Study Description | Outcomes and Endpoints | References |
|---|---|---|---|---|
| Triple therapy plus colloidal bismuth subcitrate (CBS) therapy | Clinical | The study included children aged 5 to 18 with H. pylori infection identified by endoscopy in the Spanish Registry. It analyzed patients who received CBS treatment between 2020 and 2023, with 38 patients (5.6%) treated out of 682 registered. |
|
[155] |
| Clinical | Seventy-three pediatric outpatients (48 males, 25 females; ages 9–14) diagnosed with H. pylori-associated chronic gastritis and dyspeptic symptoms participated in the study. They underwent endoscopic evaluation and received a 10-day treatment of bismuth subcitrate (8 mg/kg/day), nifuratel (30 mg/kg/day), and amoxicillin (50 mg/kg/day), given four times daily. H. pylori infection status was evaluated before and 4 to 6 weeks after treatment using modified Giemsa staining. |
|
[157] | |
| Tailored therapy | Clinical | A meta-analysis assessed empirical and susceptibility-guided treatment approaches for H. pylori, involving 54 studies with 6705 patients in the empirical cohort and 7895 in the susceptibility-guided cohort. |
|
[160] |
| Clinical | This meta-analysis reviewed 16 randomized controlled trials comparing susceptibility-guided therapy and empirical therapy for H. pylori infection, involving 2451 patients on empirical treatment and 2374 on susceptibility-guided therapy. |
|
[161] | |
| Potassium-competitive acid blockers (P-CABs) | Clinical | The study included 232 treatment-naïve participants divided into two groups: Arm 1 (58 patients) received clarithromycin, amoxicillin, and vonoprazan, while Arm 2 (58 patients) received clarithromycin, amoxicillin, and esomeprazole. Treatment-experienced patients were in Group II, consisting of Arm 3 (intervention) and Arm 4 (comparator), each with 58 participants. Arm 3 received levofloxacin, vonoprazan, nitazoxanide, and doxycycline, while Arm 4 received levofloxacin, esomeprazole, nitazoxanide, and doxycycline. All participants followed their treatment for 14 days, with H. pylori eradication assessed four weeks later. |
|
[167] |
| Probiotics | Clinical | This double-blind, randomized controlled trial enrolled 450 patients with H. pylori infection. Participants received a 14-day quadruple treatment of bismuth subcitrate, pantoprazole, amoxicillin, and clarithromycin, and were randomly assigned to either a probiotic (Lactobacillus ruteri, 100 mg) or a placebo. Eight weeks post-therapy, a urea breath test assessed H. pylori eradication rates, the primary outcome, while side effects were evaluated as a secondary outcome. |
|
[174] |
| Clinical | The study involved 95 H. pylori-positive participants and 56 negative controls, aged 19 to 30, assigned to probiotics monotherapy, probiotics-supplemented quadruple therapy, or quadruple therapy alone. Gastric mucosal samples were collected before treatment and two months later for 16S rRNA gene sequencing. Two months after eradication, the gastric microbial composition significantly differed from that of H. pylori-negative participants, with decreased alpha diversity in gastric juice and increased diversity in gastric mucosa. |
|
[180] | |
| Lactoferrin therapy | Preclinical in vitro | An investigation was conducted to evaluate the antibacterial properties of lactoferrin and Lactoferricin®, an antimicrobial peptide derived from lactoferrin, against H. pylori. |
|
[204] |
| Preclinical in vivo | The impact of bovine lactoferrin (bLF) on germ-free BALB/c mice infected with H. pylori was examined. After oral inoculation with H. pylori, the mice were given bLF daily for either two or four weeks. The mice were then euthanized to evaluate serum antibody levels and bacterial counts in the stomach. To isolate H. pylori attached to the gastric epithelium, the stomachs were agitated in phosphate-buffered saline. |
|
[205] | |
| Phytotherapy | Preclinical in vitro and in vivo | This study examines the effects of Banxia Xiexin Decoction (BXXXT), a traditional Chinese medicine prescription, on drug-resistant H. pylori-induced gastritis in mice using in vivo and in vitro methods. The aqueous extract of BXXXT was prepared by water decoction. In vitro tests indicated that BXXXT inhibits H. pylori. An acute gastritis model was established in vivo to assess H. pylori colonization, gastric mucosal repair, inflammation, and apoptosis in treated mice. |
|
[216] |
| Preclinical In vitro and in silico | The essential oils and methanol extracts of Pimenta racemosa (P. racemosa) leaves and stems were studied for their potential inhibitory activities against H. pylori both in vitro and in silico. The antibacterial activity of the essential oils and methanol extracts against H. pylori was evaluated using the micro-well dilution technique. |
|
[221] | |
| Phototherapy | Preclinical In vitro | A bacteria-targeted near-infrared (NIR) photosensitizer, designated T780T-Gu, has been developed through the combination of positively charged guanidinium (Gu) and the effective phototherapeutic agent T780T. |
|
[248] |
| Preclinical In vivo | The authors have developed a poly-L-lysine-based photomedicine conjugated with multiple 3SL (p3SLP). They proposed a targeted PDT strategy utilizing an endoscopic laser system for the treatment of H. pylori. The antibacterial efficacy of p3SLP was evaluated in C57BL/6 mice infected with H. pylori. |
|
[228] | |
| Phage therapy | Preclinical In vitro | Prophage isolation using H. pylori strains and UV radiation led to the identification of HPy1R, a new podovirus with a genome of 31,162 bp and a GC content of 37.1%. It encodes 36 predicted proteins, 17 of which are structural. The phage remains stable at 37 °C and pH levels from 3 to 11 for 24 h. |
|
[255] |
| Preclinical In vitro | The effectiveness of H. pylori-specific lytic phage (H. pylori φ) alone and with lactoferrin adsorbed on hydroxyapatite (LF-HA) nanoparticles (H. pylori φ + LF-HA) in preventing H. pylori infection. The bacteria were obtained from human stomach biopsies and cultured in brain heart infusion (BHI) broth with 10% horse serum at 37 °C and 5% CO2 for phage isolation. |
|
[256] | |
| Vaccine development | Preclinical in vivo | The multivalent epitope-based vaccine CFAdE was developed from antigenic fragments of four Helicobacter pylori adhesins: urease, Lpp20, HpaA, and cagL. Its specificity, immunogenicity, and ability to generate neutralizing antibodies were tested in BALB/c mice, followed by evaluations in H. pylori-infected Mongolian gerbils. |
|
[270] |
| Preclinical In vivo |
Bacillus subtilis spores were engineered to display potential H. pylori protective antigens, urease subunit A (ureA), and subunit B (ureB), on the spore surface. Immunity and colonization in mice challenged with H. pylori after orally administering these spores were tested. |
|
[271] | |
| Clinical Phase 3 trial) | A phase 3 clinical study in China evaluated a three-dose oral recombinant H. pylori vaccine’s effectiveness, safety, and immunogenicity in healthy children aged six to fifteen. Participants without prior infection were randomly assigned to receive the vaccine or a placebo, with the primary outcome being the incidence of infection within one year. Registered with ClinicalTrials.gov (NCT02302170), the trial enrolled 4464 individuals from 2 December 2004, to 19 March 2005, with 4403 (99%) completing the regimen. |
|
[272] |
7. Conclusions
H. pylori infection poses a significant global health challenge, with gastric cancer as a common complication. Rising antibiotic resistance has led to interest in alternative treatments. This review clarifies transmission pathways, treatment failure, antimicrobial resistance, and emerging therapies. H. pylori is primarily transmitted through saliva and contaminated food or water. Developing countries are especially susceptible due to poor water treatment and hygiene. The infection spreads mainly among family members, particularly affecting children. Triple therapy with amoxicillin, clarithromycin, and a PPI like omeprazole has been the first-line treatment for H. pylori. Due to multidrug resistance, quadruple therapy with metronidazole, tetracycline, omeprazole, and bismuth is now recommended as a second-line option. Clarithromycin, metronidazole, levofloxacin, and amoxicillin are often linked to H. pylori drug resistance in developing countries due to altered membrane permeability, biofilm formation, and efflux pump activity. Alternative therapies, such as adjuvant therapy (probiotics and antibiofilm agents), phage therapy, phototherapy, phytotherapy, lactoferrin therapy, and vaccine development, are essential for treating H. pylori infection. Probiotics fight H. pylori by competing for attachment sites, inducing cell death, and regulating inflammatory cytokines. Phytotherapy inhibits urease activity and improves membrane permeability, though it is still in early development. Both probiotics and herbal therapies are effective second-line treatments due to their safety and lack of resistance. Phage therapy uses bacteriophages to lyse host cells. Photodynamic therapy generates ROS to eliminate H. pylori. Lactoferrin therapy sequesters iron and disrupts bacterial cell walls, making it a safe alternative. The development of vaccines targeting virulence antigens, such as cagA and vacA, is crucial for reducing H. pylori colonization and enhancing eradication strategies. However, further clinical evidence is needed to validate their practical implementation. Although vaccines, probiotics, and phages offer promising therapeutic alternatives, additional research is necessary to clarify the underlying mechanisms and assess their efficacy through rigorous clinical trials.
Author Contributions
Conceptualization, A.E., A.A. (Adil Abalkhail), N.A. (Nuha Anajirih), F.A. (Fahad Alkhamisi), M.A. (Mohammed Aldamegh), A.A. (Abdullah Alramzi), R.A., N.A. (Naif Alotaibi), A.A. (Abdullah Aljuaid), H.A., F.A. (Feras Alzaben), M.R., M.I., M.H.A., N.I.R., M.E.A.M., M.R.A. and H.M.E.; Data curation, A.E., A.A. (Adil Abalkhail), N.A. (Nuha Anajirih), F.A. (Fahad Alkhamisi), M.A. (Mohammed Aldamegh), A.A. (Abdullah Alramzi), R.A., N.A. (Naif Alotaibi), A.A. (Abdullah Aljuaid), H.A., F.A. (Feras Alzaben), M.R., M.I., M.H.A., N.I.R., M.E.A.M., M.R.A. and H.M.E.; Formal analysis, A.E., F.A. (Feras Alzaben) and M.A. (Mubarak Alqahtani); Investigation, A.E. and F.A. (Feras Alzaben); Methodology, A.E., A.A. (Adil Abalkhail), N.A. (Nuha Anajirih), F.A. (Fahad Alkhamisi), M.A. (Mohammed Aldamegh), A.A. (Abdullah Alramzi), R.A., N.A. (Naif Alotaibi), A.A. (Abdullah Aljuaid), H.A., F.A. (Feras Alzaben), M.R., M.I., M.H.A., N.I.R., M.E.A.M., M.R.A., H.M.E. and M.A. (Mubarak Alqahtani); Recourses, A.E. and F.A. (Feras Alzaben); Validation, M.A. (Mubarak Alqahtani); Visualization, A.E., A.A. (Adil Abalkhail), N.A. (Nuha Anajirih), F.A. (Fahad Alkhamisi), M.A. (Mohammed Aldamegh), A.A. (Abdullah Alramzi), R.A., N.A. (Naif Alotaibi), A.A. (Abdullah Aljuaid), H.A., F.A. (Feras Alzaben), M.R., M.I., M.H.A., N.I.R., M.E.A.M., M.R.A., H.M.E. and M.A. (Mubarak Alqahtani); Writing—original draft, A.E., A.A. (Adil Abalkhail), N.A. (Nuha Anajirih), F.A. (Fahad Alkhamisi), M.A. (Mohammed Aldamegh), A.A. (Abdullah Alramzi), R.A., N.A. (Naif Alotaibi), A.A. (Abdullah Aljuaid), H.A., F.A. (Feras Alzaben), M.R., M.I., M.H.A., N.I.R., M.E.A.M., M.R.A. and H.M.E.; Writing—review & editing, A.E., A.A. (Adil Abalkhail), N.A. (Nuha Anajirih), F.A. (Fahad Alkhamisi), M.A. (Mohammed Aldamegh), A.A. (Abdullah Alramzi), R.A., N.A. (Naif Alotaibi), A.A. (Abdullah Aljuaid), H.A., F.A. (Feras Alzaben), M.R., M.I., M.H.A., N.I.R., M.E.A.M., M.R.A., H.M.E. and M.A. (Mubarak Alqahtani). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Elbehiry A., Marzouk E., Aldubaib M., Abalkhail A., Anagreyyah S., Anajirih N., Abu-Okail A. Helicobacter pylori infection: Current status and future prospects on diagnostic, therapeutic and control challenges. Antibiotics. 2023;12:191. doi: 10.3390/antibiotics12020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burz C., Pop V., Silaghi C., Lupan I., Samasca G. Helicobacter pylori Infection in Patients with Gastric Cancer: A 2024 Update. Cancers. 2024;16:1958. doi: 10.3390/cancers16111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng T., Boneca I.G. The shapeshifting Helicobacter pylori: From a corkscrew to a ball. Mol. Microbiol. 2024;121:260–274. doi: 10.1111/mmi.15218. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson P., Wright D.H. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::AID-CNCR2820520813>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Sharndama H.C., Mba I.E. Helicobacter pylori: An up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022;53:33–50. doi: 10.1007/s42770-021-00675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogiatzi P., Cassone M., Luzzi I., Lucchetti C., Otvos L., Jr., Giordano A. Helicobacter pylori as a class I carcinogen: Physiopathology and management strategies. J. Cell. Biochem. 2007;102:264–273. doi: 10.1002/jcb.21375. [DOI] [PubMed] [Google Scholar]
- 7.Ansari S., Yamaoka Y. Helicobacter pylori virulence factor cytotoxin-associated gene A (CagA)-mediated gastric pathogenicity. Int. J. Mol. Sci. 2020;21:7430. doi: 10.3390/ijms21197430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukri A., Hanafiah A., Mohamad Zin N., Kosai N.R. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. Apmis. 2020;128:150–161. doi: 10.1111/apm.13034. [DOI] [PubMed] [Google Scholar]
- 9.Kolinjivadi A.M., Sankar H., Choudhary R., Tay L.S., Tan T.Z., Murata-Kamiya N., Ito Y. The H. pylori CagA oncoprotein induces DNA double strand breaks through Fanconi Anemia pathway downregulation and replication fork collapse. Int. J. Mol. Sci. 2022;23:1661. doi: 10.3390/ijms23031661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadvar N., Akrami S., Mousavi Sagharchi S.-M.-A., Askandar R.H., Merati A., Aghayari M., Kashfi M. A review for non-antibiotic treatment of Helicobacter pylori: New insight. Front. Microbiol. 2024;15:1379209. doi: 10.3389/fmicb.2024.1379209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brasil-Costa I., Souza C.d.O., Monteiro L.C.R., Santos M.E.S., Oliveira E.H.C.D., Burbano R.M.R. H. pylori infection and virulence factors cagA and vacA (s and m regions) in gastric adenocarcinoma from Pará State, Brazil. Pathogens. 2022;11:414. doi: 10.3390/pathogens11040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nejati S., Karkhah A., Darvish H., Validi M., Ebrahimpour S., Nouri H.R. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb. Pathog. 2018;117:43–48. doi: 10.1016/j.micpath.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Ali A., AlHussaini K.I. Helicobacter pylori: A contemporary perspective on pathogenesis, diagnosis and treatment strategies. Microorganisms. 2024;12:222. doi: 10.3390/microorganisms12010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H., Wang L., Zhang M., Hu B. The role of adhesion in Helicobacter pylori persistent colonization. Curr. Microbiol. 2023;80:185. doi: 10.1007/s00284-023-03264-6. [DOI] [PubMed] [Google Scholar]
- 15.Afra L.G., Afkhami H., Khaledi M., Fathi J., Taghadosi R., Hoseini M.H.M., Heidari M. Detection of H. pylori in tissues with benign prostatic hyperplasia isolates from hospitalized patient in Qom, Iran. Gene Rep. 2021;23:101193. doi: 10.1016/j.genrep.2021.101193. [DOI] [Google Scholar]
- 16.Ansari S., Yamaoka Y. Survival of Helicobacter pylori in gastric acidic territory. Helicobacter. 2017;22:e12386. doi: 10.1111/hel.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheok Y.Y., Lee C.Y.Q., Cheong H.C., Vadivelu J., Looi C.Y., Abdullah S., Wong W.F. An overview of Helicobacter pylori survival tactics in the hostile human stomach environment. Microorganisms. 2021;9:2502. doi: 10.3390/microorganisms9122502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes V.E. Helicobacter pylori and its role in gastric cancer. Microorganisms. 2023;11:1312. doi: 10.3390/microorganisms11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelsberger V., Gerhard M., Mejías-Luque R. Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk. Front. Cell. Infect. Microbiol. 2024;14:1339750. doi: 10.3389/fcimb.2024.1339750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savoldi A., Carrara E., Graham D.Y., Conti M., Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y., Fan C., Xie H. Effect of Helicobacter pylori infection on the risk of acute coronary syndrome: A systematic review and meta-analysis. Medicine. 2019;98:e18348. doi: 10.1097/MD.0000000000018348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abo-Amer Y.E.-E., Sabal A., Ahmed R., Hasan N.F.E., Refaie R., Mostafa S.M., Abd-Elsalam S. Relationship between Helicobacter pylori infection and nonalcoholic fatty liver disease (NAFLD) in a developing country: A cross-sectional study. Diabetes Metab. Syndr. Obes. 2020;13:619–625. doi: 10.2147/DMSO.S237866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoder G., Muhammad J.S., Mahmoud I., Soliman S.S., Burucoa C. Prevalence of Helicobacter pylori and its associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens. 2019;8:44. doi: 10.3390/pathogens8020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefano K., Marco M., Federica G., Laura B., Barbara B., Gioacchino L., Gian L.d.A. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Bio Medica Atenei Parm. 2018;89((Suppl. 8)):72. doi: 10.23750/abm.v89i8-S.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsonnet J., Shmuely H., Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. 1999;282:2240–2245. doi: 10.1001/jama.282.23.2240. [DOI] [PubMed] [Google Scholar]
- 26.Weyermann M., Rothenbacher D., Brenner H. Acquisition of Helicobacter pylori infection in early childhood: Independent contributions of infected mothers, fathers, and siblings. Off. J. Am. Coll. Gastroenterol. ACG. 2009;104:182–189. doi: 10.1038/ajg.2008.61. [DOI] [PubMed] [Google Scholar]
- 27.Luman W., Zhao Y., Ng H., Ling K. Helicobacter pylori infection is unlikely to be transmitted between partners: Evidence from genotypic study in partners of infected patients. Eur. J. Gastroenterol. Hepatol. 2002;14:521–528. doi: 10.1097/00042737-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Kivi M., Tindberg Y., Sörberg M., Casswall T.H., Befrits R., Hellström P.M., Granstrom M. Concordance of Helicobacter pylori strains within families. J. Clin. Microbiol. 2003;41:5604–5608. doi: 10.1128/JCM.41.12.5604-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins R.J., Vial P.A., Ferreccio C., Ovalle J., Prado P., Sotomayor V., Morris J.G., Jr. Seroprevalence of Helicobacter pylori in Chile: Vegetables may serve as one route of transmission. J. Infect. Dis. 1993;168:222–226. doi: 10.1093/infdis/168.1.222. [DOI] [PubMed] [Google Scholar]
- 30.Bizri A.R.N., Nuwayhid I.A., Hamadeh G.N., Steitieh S.W., Choukair A.M., Musharrafieh U.M. Association between hepatitis A virus and Helicobacter pylori in a developing country: The saga continues. J. Gastroenterol. Hepatol. 2006;21:1615–1621. doi: 10.1111/j.1440-1746.2006.04268.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y.Y., Xia H.X., Zhuang Z.H., Zhong J. ‘true’re-infection of Helicobacter pylori after successful eradication–worldwide annual rates, risk factors and clinical implications. Aliment. Pharmacol. Ther. 2009;29:145–160. doi: 10.1111/j.1365-2036.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- 32.Elbehiry A., Marzouk E., Moussa I., Mushayt Y., Algarni A.A., Alrashed O.A., Alghamdi K.S., Almutairi N.A., Anagreyyah S.A., Alzahrani A., et al. The prevalence of multidrug-resistant Acinetobacter baumannii and its vaccination status among healthcare providers. Vaccines. 2023;11:1171. doi: 10.3390/vaccines11071171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elbehiry A., Marzouk E., Moussa I.M., Dawoud T.M., Mubarak A.S., Al-Sarar D., Alsubki R.A., Alhaji J.H., Hamada M., Zahran R.N. Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:1158–1166. doi: 10.1016/j.sjbs.2020.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elbehiry A., Marzouk E., Aldubaib M., Moussa I., Abalkhail A., Ibrahem M., Hamada M., Sindi S., Alzaben F., Mohammad A., et al. Pseudomonas species prevalence, protein analysis, and antibiotic resistance: An evolving public health challenge. Amb Express. 2022;12:53. doi: 10.1186/s13568-022-01390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbehiry A., Al-Dubaib M., Marzouk E., Moussa I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. Microbiologyopen. 2019;8:e00698. doi: 10.1002/mbo3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbehiry A., Marzouk E., Abdeen E., Al-Dubaib M., Alsayeqh A., Ibrahem M., Hemeg H.A. Proteomic characterization and discrimination of Aeromonas species recovered from meat and water samples with a spotlight on the antimicrobial resistance of Aeromonas hydrophila. Microbiologyopen. 2019;8:e782. doi: 10.1002/mbo3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbehiry A., Marzouk E., Abalkhail A., El-Garawany Y., Anagreyyah S., Alnafea Y., Draz A. The development of technology to prevent, diagnose, and manage antimicrobial resistance in healthcare-associated infections. Vaccines. 2022;10:2100. doi: 10.3390/vaccines10122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abalkhail A., AlYami A.S., Alrashedi S.F., Almushayqih K.M., Alslamah T., Alsalamah Y.A., Elbehiry A., editors. The prevalence of multidrug-resistant Escherichia coli producing ESBL among male and female patients with urinary tract infections in Riyadh Region, Saudi Arabia. Healthcare. 2022;10:1778. doi: 10.3390/healthcare10091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abalkhail A., Elbehiry A. Methicillin-resistant Staphylococcus aureus in diabetic foot infections: Protein profiling, virulence determinants, and antimicrobial resistance. Appl. Sci. 2022;12:10803. doi: 10.3390/app122110803. [DOI] [Google Scholar]
- 40.Osman K.M., Badr J., Orabi A., Elbehiry A., Saad A., Ibrahim M.D., Hanafy M.H. Poultry as a vector for emerging multidrug resistant Enterococcus spp.: First report of vancomycin (van) and the chloramphenicol–florfenicol (cat-fex-cfr) resistance genes from pigeon and duck faeces. Microb. Pathog. 2019;128:195–205. doi: 10.1016/j.micpath.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Edrees H.M., Elbehiry A., Elmosaad Y.M. Hypoglycemic and anti-inflammatory effect of gold nanoparticles in streptozotocin-induced type 1 diabetes in experimental rats. Nanotechnology. 2017;3:16–23. [Google Scholar]
- 42.Van Khien V., Thang D.M., Hai T.M., Duat N.Q., Khanh P.H., Ha D.T., Yamaoka Y. Management of antibiotic-resistant Helicobacter pylori infection: Perspectives from Vietnam. Gut Liver. 2019;13:483. doi: 10.5009/gnl18137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garvey E., Rhead J., Suffian S., Whiley D., Mahmood F., Bakshi N., Robinson K. High incidence of antibiotic resistance amongst isolates of Helicobacter pylori collected in Nottingham, UK, between 2001 and 2018. J. Med. Microbiol. 2023;72:001776. doi: 10.1099/jmm.0.001776. [DOI] [PubMed] [Google Scholar]
- 44.Liu M., Gao H., Miao J., Zhang Z., Zheng L., Li F., Zhuo S., Zhang Z., Li S., Sun J., et al. Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: A review. Front. Microbiol. 2024;14:1330029. doi: 10.3389/fmicb.2023.1330029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez de Santiago E., Martín de Argila de Prados C., Marcos Prieto H.M., Jorge Turrión M.Ã., Barreiro Alonso E., Flores de Miguel A., Albillos Martinez A. Limited effectiveness with a 10-day bismuth-containing quadruple therapy (Pylera®) in third-line recue treatment for Helicobacter pylori infection. A real-life multicenter study. Helicobacter. 2017;22:e12423. doi: 10.1111/hel.12423. [DOI] [PubMed] [Google Scholar]
- 46.Li H., Xia X.-J., Zhang L.-F., Chi J.-S., Liu P., Wu H., Xu C.X. Comparative study of allicin-containing quadruple therapy vs. bismuth-containing quadruple therapy for the treatment of Helicobacter pylori infection: A prospective randomized study. Eur. J. Gastroenterol. Hepatol. 2021;33:194–200. doi: 10.1097/MEG.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 47.Poonyam P., Chotivitayatarakorn P., Vilaichone R.-K. High effective of 14-day high-dose PPI-bismuth-containing quadruple therapy with probiotics supplement for Helicobacter pylori eradication: A double blinded-randomized placebo-controlled study. Asian Pac. J. Cancer Prev. APJCP. 2019;20:2859. doi: 10.31557/APJCP.2019.20.9.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng C., Hu Y., Ge Z.-M., Zou Q.-M., Lyu N.-H. Diagnosis and treatment of Helicobacter pylori infections in children and elderly populations. Chronic Dis. Transl. Med. 2019;5:243–251. doi: 10.1016/j.cdtm.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji J., Yang H. Using probiotics as supplementation for Helicobacter pylori antibiotic therapy. Int. J. Mol. Sci. 2020;21:1136. doi: 10.3390/ijms21031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno-Expósito L., Illescas-Montes R., Melguizo-Rodríguez L., Ruiz C., Ramos-Torrecillas J., de Luna-Bertos E. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018;195:61–64. doi: 10.1016/j.lfs.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Imoto I., Yasuma T., D’Alessandro-Gabazza C.N., Oka S., Misaki M., Horiki N., Gabazza E.C. Antimicrobial effects of lactoferrin against Helicobacter pylori infection. Pathogens. 2023;12:599. doi: 10.3390/pathogens12040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asaad G.F., Mostafa R.E. Lactoferrin mitigates ethanol-induced gastric ulcer via modulation of ROS/ICAM-1/Nrf2 signaling pathway in Wistar rats. Iran. J. Basic Med. Sci. 2022;25:1522. doi: 10.22038/IJBMS.2022.66823.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Y., Wu Q., Cheng G., Liu X., Liu S., Luo J., Zhang A., Bian L., Chen J., Lv J., et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. Int. J. Mol. Med. 2015;36:363–368. doi: 10.3892/ijmm.2015.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abedon S.T. Use of phage therapy to treat long-standing, persistent, or chronic bacterial infections. Adv. Drug Deliv. Rev. 2019;145:18–39. doi: 10.1016/j.addr.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Muñoz A.B., Stepanian J., Trespalacios A.A., Vale F.F. Bacteriophages of Helicobacter pylori. Front. Microbiol. 2020;11:549084. doi: 10.3389/fmicb.2020.549084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikuse T., Blanchard T.G., Czinn S.J. Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori. Springer; Berlin/Heidelberg, Germany: 2019. Inflammation, immunity, and vaccine development for the gastric pathogen Helicobacter pylori; pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Li X., Shan B., Zhang H., Zhao L. Perspectives from recent advances of Helicobacter pylori vaccines research. Helicobacter. 2022;27:e12926. doi: 10.1111/hel.12926. [DOI] [PubMed] [Google Scholar]
- 58.Sukri A., Hanafiah A., Patil S., Lopes B.S. The potential of alternative therapies and vaccine candidates against Helicobacter pylori. Pharmaceuticals. 2023;16:552. doi: 10.3390/ph16040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Das S., Banerjee S., Halder P., Dutta S., Mukhopadhyay A., Koley H. A review for the prevention and management of Helicobacter pylori induced gastritis through development of novel vaccine candidates. Microbe. 2024;4:100114. doi: 10.1016/j.microb.2024.100114. [DOI] [Google Scholar]
- 60.Friedrich V., Gerhard M. Vaccination against Helicobacter pylori–An approach for cancer prevention? Mol. Asp. Med. 2023;92:101183. doi: 10.1016/j.mam.2023.101183. [DOI] [PubMed] [Google Scholar]
- 61.Schwarz S., Morelli G., Kusecek B., Manica A., Balloux F., Owen R.J., Graham D.Y., van der Merwe S., Achtman M., Suerbaum S. Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog. 2008;4:e1000180. doi: 10.1371/journal.ppat.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waskito L.A., Yamaoka Y. Helicobacter Pylori in Human Diseases: Advances in Microbiology, Infectious Diseases and Public Health Volume 11. Springer; Berlin/Heidelberg, Germany: 2019. The story of Helicobacter pylori: Depicting human migrations from the phylogeography; pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 63.Mladenova I. Clinical relevance of Helicobacter pylori infection. J. Clin. Med. 2021;10:3473. doi: 10.3390/jcm10163473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazar D. Helicobacter pylori Infection: An Up to Date on the Pathogenic Mechanisms, Diagnosis and Clinical Management. BoD–Books on Demand; Norderstedt, Germany: 2024. [Google Scholar]
- 65.Kotilea K., Bontems P., Touati E. Helicobacter Pylori in Human Diseases: Advances in Microbiology, Infectious Diseases and Public Health Volume 11. Springer; Berlin/Heidelberg, Germany: 2019. Epidemiology, diagnosis and risk factors of Helicobacter pylori infection; pp. 17–33. [DOI] [PubMed] [Google Scholar]
- 66.Borka Balas R., Meliț L.E., Mărginean C.O. Worldwide prevalence and risk factors of Helicobacter pylori infection in children. Children. 2022;9:1359. doi: 10.3390/children9091359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arias M.J.B., Velecela Á.J.F., Díaz V.A.V., Jerez A.M.G., Sailema J.S.T., Verdezoto M.A.D. Helicobacter pylori infection and its association with digestive diseases: A comprehensive review. Medicina. 2024;9:4. [Google Scholar]
- 68.Nguyen J., Kotilea K., Bontems P., Miendje Deyi V.Y. Helicobacter pylori infections in Children. Antibiotics. 2023;12:1440. doi: 10.3390/antibiotics12091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding S.-Z., Du Y.-Q., Lu H., Wang W.-H., Cheng H., Chen S.-Y., Chen M.-H., Chen W.-C., Chen Y., Fang J.-Y., et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 edition) Gut. 2022;71:238–253. doi: 10.1136/gutjnl-2021-325630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ueno T., Suzuki H., Hirose M., Shida T., Ikezawa K., Matsui H., Yanaka A. Influence of living environment during childhood on Helicobacter pylori infection in Japanese young adults. Digestion. 2020;101:779–784. doi: 10.1159/000502574. [DOI] [PubMed] [Google Scholar]
- 71.Kim N. Helicobacter pylori. Springer; Berlin/Heidelberg, Germany: 2024. Prevalence and transmission routes of H. pylori; pp. 3–21. [Google Scholar]
- 72.Konno M., Yokota S.-I., Suga T., Takahashi M., Sato K., Fujii N. Predominance of mother-to-child transmission of Helicobacter pylori infection detected by random amplified polymorphic DNA fingerprinting analysis in Japanese families. Pediatr. Infect. Dis. J. 2008;27:999–1003. doi: 10.1097/INF.0b013e31817d756e. [DOI] [PubMed] [Google Scholar]
- 73.Urita Y., Watanabe T., Kawagoe N., Takemoto I., Tanaka H., Kijima S., Urita C. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J. Paediatr. Child Health. 2013;49:394–398. doi: 10.1111/jpc.12191. [DOI] [PubMed] [Google Scholar]
- 74.Goodman K.J., Correa P. Transmission of Helicobacter pylori among siblings. Lancet. 2000;355:358–362. doi: 10.1016/S0140-6736(99)05273-3. [DOI] [PubMed] [Google Scholar]
- 75.Fialho A.M., Braga A.B., Neto M.B.B., Carneiro J.G., Rocha A.M., Rodrigues M.N., Queiroz D.M., Braga L.L. Younger siblings play a major role in Helicobacter pylori transmission among children from a low-income community in the Northeast of Brazil. Helicobacter. 2010;15:491–496. doi: 10.1111/j.1523-5378.2010.00791.x. [DOI] [PubMed] [Google Scholar]
- 76.Patel P., Mendall M., Khulusi S., Northfield T., Strachan D. Helicobacter pylori infection in childhood: Risk factors and effect on growth. BMJ. 1994;309:1119–1123. doi: 10.1136/bmj.309.6962.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brenner H., Weyermann M., Rothenbacher D. Clustering of Helicobacter pylori infection in couples: Differences between high-and low-prevalence population groups. Ann. Epidemiol. 2006;16:516–520. doi: 10.1016/j.annepidem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Kheyre H., Morais S., Ferro A., Costa A.R., Norton P., Lunet N., Peleteiro B. The occupational risk of Helicobacter pylori infection: A systematic review. Int. Arch. Occup. Environ. Health. 2018;91:657–674. doi: 10.1007/s00420-018-1315-6. [DOI] [PubMed] [Google Scholar]
- 79.Chow T.K.F., Lambert J.R., Wahlqvist M.L. Hsu-Hage BHH. Helicobacter pylori in Melbourne Chinese immigrants: Evidence for oral-oral transmission via chopsticks. J. Gastroenterol. Hepatol. 1995;10:562–569. doi: 10.1111/j.1440-1746.1995.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 80.Leung W., Sung J.J., Ling T.K., Siu K.L., Cheng A.F. Does the use of chopsticks for eating transmit Helicobacter pylori? Lancet. 1997;350:31. doi: 10.1016/S0140-6736(05)66240-X. [DOI] [PubMed] [Google Scholar]
- 81.Haesebrouck F., Pasmans F., Flahou B., Chiers K., Baele M., Meyns T., Ducatelle R. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin. Microbiol. Rev. 2009;22:202–223. doi: 10.1128/CMR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Proietti P.C., Bietta A., Brachelente C., Lepri E., Davidson I., Franciosini M.P. Detection of Helicobacter spp. in gastric, fecal and saliva samples from swine affected by gastric ulceration. J. Vet. Sci. 2010;11:221. doi: 10.4142/jvs.2010.11.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdi F.S., Jamshidi S., Moosakhani F., Sasani F. RETRACTED ARTICLE: Detection of Helicobacter spp. DNA in the colonic biopsies of stray dogs: Molecular and histopathological investigations. Diagn. Pathol. 2014;9:1–9. doi: 10.1186/1746-1596-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Hong S., Chung Y., Kang W.-G., Choi Y.-S., Kim O. Comparison of three diagnostic assays for the identification of Helicobacter spp. in laboratory dogs. Lab. Anim. Res. 2015;31:86–92. doi: 10.5625/lar.2015.31.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van den Bulck K., Decostere A., Baele M., Driessen A., Debongnie J.-C., Burette A., Haesebrouck F. Identification of non-Helicobacter pylori spiral organisms in gastric samples from humans, dogs, and cats. J. Clin. Microbiol. 2005;43:2256–2260. doi: 10.1128/JCM.43.5.2256-2260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McIsaac W.J., Leung G.M. Peptic ulcer disease and exposure to domestic pets. Am. J. Public Health. 1999;89:81–84. doi: 10.2105/AJPH.89.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mladenova I., Durazzo M., Pellicano R. Transmission of Helicobacter pylori: Are there evidences for a fecal-oral route? Minerva Medica. 2006;97:15. [PubMed] [Google Scholar]
- 88.Duan M., Li Y., Liu J., Zhang W., Dong Y., Han Z., Wan M., Lin M., Lin B., Kong Q., et al. Transmission routes and patterns of helicobacter pylori. Helicobacter. 2023;28:e12945. doi: 10.1111/hel.12945. [DOI] [PubMed] [Google Scholar]
- 89.Papież D., Konturek P., Bielanski W., Plonka M., Dobrzanska M., Kaminska A., Szczyrk U., Bochenek A., Wierzchos E. Prevalence of Helicobacter pylori infection in Polish shepherds and their families. Dig. Liver Dis. 2003;35:10–15. doi: 10.1016/S1590-8658(02)00004-X. [DOI] [PubMed] [Google Scholar]
- 90.Soloski M.J., Poulain M., Pes G.M. Does the trained immune system play an important role in the extreme longevity that is seen in the Sardinian blue zone? Front. Aging. 2022;3:1069415. doi: 10.3389/fragi.2022.1069415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hemmatinezhad B., Momtaz H., Rahimi E. VacA, cagA, iceA and oipA genotypes status and antimicrobial resistance properties of Helicobacter pylori isolated from various types of ready to eat foods. Ann. Clin. Microbiol. Antimicrob. 2016;15:2. doi: 10.1186/s12941-015-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaaban S.I., Talat D., Khatab S.A., Nossair M.A., Ayoub M.A., Ewida R.M., Diab M.S. An investigative study on the zoonotic potential of Helicobacter pylori. BMC Vet. Res. 2023;19:16. doi: 10.1186/s12917-023-03572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamada M., Elbehiry A., Marzouk E., Moussa I.M., Hessain A.M., Alhaji J.H., Heme H.A., Zahran R., Abdeen E. Helicobacter pylori in a poultry slaughterhouse: Prevalence, genotyping and antibiotic resistance pattern. Saudi J. Biol. Sci. 2018;25:1072–1078. doi: 10.1016/j.sjbs.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aziz R.K., Khalifa M.M., Sharaf R.R. Contaminated water as a source of Helicobacter pylori infection: A review. J. Adv. Res. 2015;6:539–547. doi: 10.1016/j.jare.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quaglia N.C., Dambrosio A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018;24:3472. doi: 10.3748/wjg.v24.i31.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma C., Zhou F., Lu D., Xu S., Luo J., Gan H., Gao D., Yao Z., He W., Kurup P.U., et al. Quantification and cultivation of Helicobacter pylori (H. pylori) from various urban water environments: A comprehensive analysis of precondition methods and sample characteristics. Environ. Int. 2024;187:108683. doi: 10.1016/j.envint.2024.108683. [DOI] [PubMed] [Google Scholar]
- 97.Cellini L. Helicobacter pylori: A chameleon-like approach to life. World J. Gastroenterol. WJG. 2014;20:5575. doi: 10.3748/wjg.v20.i19.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein P.D., Opekun A., Smith E., Graham D., Gaillour A. Group GPW. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet. 1991;337:1503–1506. doi: 10.1016/0140-6736(91)93196-G. [DOI] [PubMed] [Google Scholar]
- 99.Amirhooshang A., Ramin A., Ehsan A., Mansour R., Shahram B. High frequency of Helicobacter pylori DNA in drinking water in Kermanshah, Iran, during June–November 2012. J. Water Health. 2014;12:504–512. doi: 10.2166/wh.2013.150. [DOI] [PubMed] [Google Scholar]
- 100.Ranjbar R., Khamesipour F., Jonaidi-Jafari N., Rahimi E. Helicobacter pylori in bottled mineral water: Genotyping and antimicrobial resistance properties. BMC Microbiol. 2016;16:40. doi: 10.1186/s12866-016-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vesga F.-J., Venegas C., Martinez V.F., Sánchez-Alfonso A.C., Trespalacios A.A. Origin of fecal contamination in lettuce and strawberries: From microbial indicators, molecular markers, and H. pylori. Heliyon. 2024;10:e36526. doi: 10.1016/j.heliyon.2024.e36526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mashak Z., Jafariaskari S., Alavi I., Sakhaei Shahreza M., Safarpoor Dehkordi F. Phenotypic and genotypic assessment of antibiotic resistance and genotyping of vacA, cagA, iceA, oipA, cagE, and babA2 alleles of Helicobacter pylori bacteria isolated from raw meat. Infect. Drug Resist. 2020;13:257–272. doi: 10.2147/IDR.S233612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ng H.-Y., Leung W.K., Cheung K.-S. Antibiotic resistance, susceptibility testing and stewardship in Helicobacter pylori infection. Int. J. Mol. Sci. 2023;24:11708. doi: 10.3390/ijms241411708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Francesco V., Zullo A., Manta R., Satriano A., Fiorini G., Pavoni M., Vaira D. Culture-based antibiotic susceptibility testing for Helicobacter pylori infection: A systematic review. Ann. Gastroenterol. 2022;35:127. doi: 10.20524/aog.2022.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shiotani A., Roy P., Lu H., Graham D.Y. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Ther. Adv. Gastroenterol. 2021;14:17562848211064080. doi: 10.1177/17562848211064080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee Y.-C., Dore M.P., Graham D.Y. Diagnosis and treatment of Helicobacter pylori infection. Annu. Rev. Med. 2022;73:183–195. doi: 10.1146/annurev-med-042220-020814. [DOI] [PubMed] [Google Scholar]
- 107.Umar Z., Tang J.-W., Marshall B.J., Tay A.C.Y., Wang L. Rapid diagnosis and precision treatment of Helicobacter pylori infection in clinical settings. Crit. Rev. Microbiol. 2024:1–30. doi: 10.1080/1040841X.2024.2364194. [DOI] [PubMed] [Google Scholar]
- 108.White B., Winte M., DeSipio J., Phadtare S. Clinical factors implicated in antibiotic resistance in Helicobacter pylori patients. Microorganisms. 2022;10:322. doi: 10.3390/microorganisms10020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thung I., Aramin H., Vavinskaya V., Gupta S., Park J., Crowe S., Valasek M. The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016;43:514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zama D., Bossù G., Leardini D., Muratore E., Biagi E., Prete A., Pession A., Masetti R. Insights into the role of intestinal microbiota in hematopoietic stem-cell transplantation. Ther. Adv. Hematol. 2020;11:2040620719896961. doi: 10.1177/2040620719896961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marques A.T., Vítor J.M., Santos A., Oleastro M., Vale F.F. Trends in Helicobacter pylori resistance to clarithromycin: From phenotypic to genomic approaches. Microb. Genom. 2020;6:e000344. doi: 10.1099/mgen.0.000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alarcón-Millán J., Fernández-Tilapa G., Cortés-Malagón E.M., Castañón-Sánchez C.A., De Sampedro-Reyes J., Carmen I.C.-D., Betancourt-Linares R., Román-Román A. Clarithromycin resistance and prevalence of Helicobacter pylori virulent genotypes in patients from Southern México with chronic gastritis. Infect. Genet. Evol. 2016;44:190–198. doi: 10.1016/j.meegid.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 113.Boyanova L., Gergova G., Kandilarov N., Boyanova L., Yordanov D., Gergova R., Markovska R. Geographic distribution of antibiotic resistance of Helicobacter pylori: A study in Bulgaria. Acta Microbiol. Immunol. Hung. 2023;70:79–83. doi: 10.1556/030.2023.01940. [DOI] [PubMed] [Google Scholar]
- 114.Lin Y., Shao Y., Yan J., Ye G. Antibiotic resistance in Helicobacter pylori: From potential biomolecular mechanisms to clinical practice. J. Clin. Lab. Anal. 2023;37:e24885. doi: 10.1002/jcla.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ierardi E., Giorgio F., Losurdo G., Di Leo A., Principi M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J. Gastroenterol. WJG. 2013;19:8168. doi: 10.3748/wjg.v19.i45.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flores-Treviño S., Mendoza-Olazarán S., Bocanegra-Ibarias P., Maldonado-Garza H.J., Garza-González E. Helicobacter pylori drug resistance: Therapy changes and challenges. Expert Rev. Gastroenterol. Hepatol. 2018;12:819–827. doi: 10.1080/17474124.2018.1496017. [DOI] [PubMed] [Google Scholar]
- 117.Ghotaslou R., Leylabadlo H.E., Asl Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015;5:164. doi: 10.5662/wjm.v5.i3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alba C., Blanco A., Alarcón T. Antibiotic resistance in Helicobacter pylori. Curr. Opin. Infect. Dis. 2017;30:489–497. doi: 10.1097/QCO.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 119.Boyanova L., Hadzhiyski P., Gergova R., Markovska R. Evolution of Helicobacter pylori resistance to antibiotics: A topic of increasing concern. Antibiotics. 2023;12:332. doi: 10.3390/antibiotics12020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Srisuphanunt M., Wilairatana P., Kooltheat N., Duangchan T., Katzenmeier G., Rose J.B. Molecular mechanisms of antibiotic resistance and novel treatment strategies for Helicobacter pylori infections. Trop. Med. Infect. Dis. 2023;8:163. doi: 10.3390/tropicalmed8030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adachi K., Kishi K., Sakamoto U., Ishimura N., Ishihara S. Degree of Gastric Mucosal Atrophy Correlated Well with Gastric Cancer Occurrence in Patients with Helicobacter pylori-eradicated Status. Intern. Med. 2023;62:1389–1394. doi: 10.2169/internalmedicine.0506-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rokkas T., Graham D.Y. How widespread and convenient H. pylori susceptibility testing will result in pharmacological opportunities. Expert Rev. Gastroenterol. Hepatol. 2023;17:1–7. doi: 10.1080/17474124.2023.2162502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y., Zhang Y., Du S. Antibiotic resistance in Helicobacter pylori among children and adolescents in East Asia: A systematic review and meta-analysis. Chin. Med. J. 2024;10:1097. doi: 10.1097/CM9.0000000000002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balas R.B., Meliț L.E., Mărginean C.O. Current worldwide trends in pediatric Helicobacter pylori antimicrobial resistance. Children. 2023;10:403. doi: 10.3390/children10020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bujanda L., Nyssen O.P., Ramos J., Bordin D.S., Tepes B., Perez-Aisa A., Gisbert J.P. Effectiveness of Helicobacter pylori treatments according to antibiotic resistance. Off. J. Am. Coll. Gastroenterol. ACG. 2024;119:646–654. doi: 10.14309/ajg.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 126.Ho J.J., Navarro M., Sawyer K., Elfanagely Y., Moss S.F. Helicobacter pylori antibiotic resistance in the United States between 2011 and 2021: A systematic review and meta-analysis. Off. J. Am. Coll. Gastroenterol. ACG. 2022;117:1221–1230. doi: 10.14309/ajg.0000000000001828. [DOI] [PubMed] [Google Scholar]
- 127.Chen P., Chen M., Peng C., Yan J., Shen X., Zhang W., Yuan Y., Gan G., Luo X., Zhu W., et al. In vitro anti-bactrical activity and its preliminary mechanism of action of the non-medicinal parts of Sanguisorba officinalis L. against Helicobacter pylori infection. J. Ethnopharmacol. 2024;318:116981. doi: 10.1016/j.jep.2023.116981. [DOI] [PubMed] [Google Scholar]
- 128.Hu Y., Zhang M., Lu B., Dai J. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter. 2016;21:349–363. doi: 10.1111/hel.12299. [DOI] [PubMed] [Google Scholar]
- 129.Cui R., Song Z., Suo B., Tian X., Xue Y., Meng L., Zhou L. Correlation analysis among genotype resistance, phenotype resistance and eradication effect of Helicobacter pylori. Infect. Drug Resist. 2021;14:1747–1756. doi: 10.2147/IDR.S305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chandan V., Logan S.M., Harrison B.A., Vinogradov E., Aubry A., Stupak J., Li J., Altman E. Characterization of a waaF mutant of Helicobacter pylori strain 26695 provides evidence that an extended lipopolysaccharide structure has a limited role in the invasion of gastric cancer cells. Biochem. Cell Biol. 2007;85:582–590. doi: 10.1139/O07-056. [DOI] [PubMed] [Google Scholar]
- 131.Lin J., Zhang X., Wen Y., Chen H., She F. A newly discovered drug resistance gene rfaF in Helicobacter pylori. Infect. Drug Resist. 2019;12:3507–3514. doi: 10.2147/IDR.S231152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashemi S.J., Sheikh A.F., Goodarzi H., Yadyad M.J., Seyedian S.S., Aslani S., Assarzadegan M.-A. Genetic basis for metronidazole and clarithromycin resistance in Helicobacter pylori strains isolated from patients with gastroduodenal disorders. Infect. Drug Resist. 2019;12:535–543. doi: 10.2147/IDR.S192942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hou C., Yin F., Wang S., Zhao A., Li Y., Liu Y. Helicobacter pylori biofilm-related drug resistance and new developments in its anti-biofilm agents. Infect. Drug Resist. 2022;15:1561–1571. doi: 10.2147/IDR.S357473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flemming H.-C., van Hullebusch E.D., Little B.J., Neu T.R., Nielsen P.H., Seviour T., Stoodley P., Wingender J., Wuertz S. Microbial extracellular polymeric substances in the environment, technology and medicine. Nat. Rev. Microbiol. 2024:1–19. doi: 10.1038/s41579-024-01098-y. [DOI] [PubMed] [Google Scholar]
- 135.Li X., Chen D., Xie S. Current progress and prospects of organic nanoparticles against bacterial biofilm. Adv. Colloid Interface Sci. 2021;294:102475. doi: 10.1016/j.cis.2021.102475. [DOI] [PubMed] [Google Scholar]
- 136.Krzyżek P., Grande R. Transformation of Helicobacter pylori into coccoid forms as a challenge for research determining activity of antimicrobial substances. Pathogens. 2020;9:184. doi: 10.3390/pathogens9030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Inamasu Y., Ogawa M., Saito M., Harada M., Fukuda K. Helicobacter pylori results in lysis and death after exposure to water. Helicobacter. 2022;27:e12921. doi: 10.1111/hel.12921. [DOI] [PubMed] [Google Scholar]
- 138.Chaput C., Ecobichon C., Cayet N., E Girardin S., Werts C., Guadagnini S., Prévost M.-C., Mengin-Lecreulx D., Labigne A., Boneca I.G. Role of AmiA in the morphological transition of Helicobacter pylori and in immune escape. PLoS Pathog. 2006;2:e97. doi: 10.1371/journal.ppat.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang K.-X., Wang X.-F. Cloning and sequencing of cagA gene fragment of Helicobacter pylori with coccoid form. World J. Gastroenterol. WJG. 2004;10:3511. doi: 10.3748/wjg.v10.i23.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bessa L.J., Grande R., Iorio D.D., Giulio M.D., Campli E.D., Cellini L. Helicobacter pylori free-living and biofilm modes of growth: Behavior in response to different culture media. Apmis. 2013;121:549–560. doi: 10.1111/apm.12020. [DOI] [PubMed] [Google Scholar]
- 141.Gong Y., Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Crit. Rev. Microbiol. 2018;44:371–392. doi: 10.1080/1040841X.2017.1418285. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y., Wang S., Yang F., Chi W., Ding L., Liu T., Zhu F., Ji D., Zhou J., Fang Y., et al. Antimicrobial resistance patterns and genetic elements associated with the antibiotic resistance of Helicobacter pylori strains from Shanghai. Gut Pathog. 2022;14:14. doi: 10.1186/s13099-022-00488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raj D.S., Kesavan D.K., Muthusamy N., Umamaheswari S. Efflux pumps potential drug targets to circumvent drug Resistance–Multi drug efflux pumps of Helicobacter pylori. Mater. Today Proc. 2021;45:2976–2981. doi: 10.1016/j.matpr.2020.11.955. [DOI] [Google Scholar]
- 144.Yonezawa H., Osaki T., Hojo F., Kamiya S. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithromycin. Microb. Pathog. 2019;132:100–108. doi: 10.1016/j.micpath.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 145.Krzyżek P., Migdał P., Grande R., Gościniak G. Biofilm formation of Helicobacter pylori in both static and microfluidic conditions is associated with resistance to clarithromycin. Front. Cell. Infect. Microbiol. 2022;12:868905. doi: 10.3389/fcimb.2022.868905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Regeimbal J.M., Jacobs A.C., Corey B.W., Henry M.S., Thompson M.G., Pavlicek R.L., Quinones J., Hannah R.M., Ghebremedhin M., Crane N.J., et al. Bifunctional enzyme SpoT is involved in biofilm formation of Helicobacter pylori with multidrug resistance by upregulating efflux pump Hp1174 (gluP) Antimicrob. Agents Chemother. 2018;62:5806–5816. doi: 10.1128/AAC.00957-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hasanuzzaman, Bang C.S., Gong E.J. Antibiotic resistance of Helicobacter pylori: Mechanisms and clinical implications. J. Korean Med. Sci. 2024;39:e44. doi: 10.3346/jkms.2024.39.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bujanda L., Nyssen O.P., Vaira D., Saracino I.M., Fiorini G., Lerang F., Georgopoulos S., Tepes B., Heluwaert F., Gasbarrini A., et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg) Antibiotics. 2021;10:1058. doi: 10.3390/antibiotics10091058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tshibangu-Kabamba E., Yamaoka Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021;18:613–629. doi: 10.1038/s41575-021-00449-x. [DOI] [PubMed] [Google Scholar]
- 150.Lee J.H., Ahn J.Y., Choi K.D., Jung H., Kim J.M., Baik G.H., Kim B., Park J.C., Jung H., Cho S.J., et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 2019;24:e12592. doi: 10.1111/hel.12592. [DOI] [PubMed] [Google Scholar]
- 151.Zagari R.M., Rabitti S., Eusebi L.H., Bazzoli F. Treatment of Helicobacter pylori infection: A clinical practice update. Eur. J. Clin. Investig. 2018;48:e12857. doi: 10.1111/eci.12857. [DOI] [PubMed] [Google Scholar]
- 152.Aumpan N., Mahachai V., Vilaichone R.K. Management of Helicobacter pylori infection. JGH Open. 2023;7:3–15. doi: 10.1002/jgh3.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Malfertheiner P., Megraud F., Rokkas T., Gisbert J.P., Liou J.-M., Schulz C., El-Omar E.M. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut. 2022;71:1724–1762. doi: 10.1136/gutjnl-2022-327745. [DOI] [Google Scholar]
- 154.Dore M.P., Lu H., Graham D.Y. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65:870–878. doi: 10.1136/gutjnl-2015-311019. [DOI] [PubMed] [Google Scholar]
- 155.Botija G., Galicia G., Martínez B., Cuadrado C., Soria M., Fernández S., Seghnp H. Efficacy of Bismuth Therapy in Eradicating Helicobacter pylori in Children—Data From the RENIHp Registry. Helicobacter. 2024;29:e13142. doi: 10.1111/hel.13142. [DOI] [PubMed] [Google Scholar]
- 156.Ko S.W., Kim Y.-J., Chung W.C., Lee S.J. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: Systemic review and meta-analysis. Helicobacter. 2019;24:e12565. doi: 10.1111/hel.12565. [DOI] [PubMed] [Google Scholar]
- 157.Nijevitch A.A., Sataev V.U., Akhmadeyeva E.N., Arsamastsev A.G. Nifuratel-containing initial anti-Helicobacter pylori triple therapy in children. Helicobacter. 2007;12:132–135. doi: 10.1111/j.1523-5378.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 158.Dascălu R.I., Bolocan A., Păduaru D.N., Constantinescu A., Mitache M.M., Stoica A.D., Andronic O. Multidrug resistance in Helicobacter pylori infection. Front. Microbiol. 2023;14:1128497. doi: 10.3389/fmicb.2023.1128497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mestrovic A., Perkovic N., Tonkic A., Sundov Z., Kumric M., Bozic J. Personalized approach in eradication of Helicobacter pylori infection. Antibiotics. 2022;12:7. doi: 10.3390/antibiotics12010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nyssen O.P., Espada M., Gisbert J.P. Empirical vs. susceptibility-guided treatment of Helicobacter pylori infection: A systematic review and meta-analysis. Front. Microbiol. 2022;13:913436. doi: 10.3389/fmicb.2022.913436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gingold-Belfer R., Niv Y., Schmilovitz-Weiss H., Levi Z., Boltin D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021;36:2649–2658. doi: 10.1111/jgh.15575. [DOI] [PubMed] [Google Scholar]
- 162.Malfertheiner P., Moss S.F., Daniele P., Pelletier C., Jacob R., Tremblay G., Hubscher E., Leifke E., Chey W.D. Potassium-competitive acid blocker and proton pump inhibitor–based regimens for first-line Helicobacter pylori eradication: A network meta-analysis. Gastro Hep Adv. 2022;1:824–834. doi: 10.1016/j.gastha.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kanu J.E., Soldera J. Treatment of Helicobacter pylori with potassium competitive acid blockers: A systematic review and meta-analysis. World J. Gastroenterol. 2024;30:1213. doi: 10.3748/wjg.v30.i9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aljaberi H., Ansari N.K., Xiong M., Peng H., He B., Wang S. Current Understanding of the Transmission, Diagnosis, and Treatment of H. pylori Infection: A Comprehensive Review. Int. J. Med. Pharm. Drug Res. 2023;7:2. doi: 10.22161/ijmpd.7.2.1. [DOI] [Google Scholar]
- 165.Furuta T., Graham D.Y. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol. Clin. 2010;39:465–480. doi: 10.1016/j.gtc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 166.Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: Pharmacokinetic and pharmacodynamic considerations. Clin. Pharmacokinet. 2016;55:409–418. doi: 10.1007/s40262-015-0326-7. [DOI] [PubMed] [Google Scholar]
- 167.Elazazi N.A.D.A., Eltabbakh M., Hussein H.M., Mahmood Y.M., Elwakil R. Efficacy of Potassium-competitive Acid Blockers versus Proton Pump Inhibitors in First-and Second-line Eradication Regimens for Helicobacter pylori in Egyptian Patients. J. Transl. Gastroenterol. 2024;2:131–138. doi: 10.14218/JTG.2023.00087. [DOI] [Google Scholar]
- 168.Graham D.Y. Why the vonoprazan Helicobacter pylori therapies in the US-European trial produced unacceptable cure rates. Dig. Dis. Sci. 2023;68:1691–1697. doi: 10.1007/s10620-023-07886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sue S., Maeda S. Is a potassium-competitive acid blocker truly superior to proton pump inhibitors in terms of Helicobacter pylori eradication? Gut Liver. 2021;15:799. doi: 10.5009/gnl20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mestre A., Narayanan R.S., Rivas D., John J., Abdulqader M.A., Khanna T., Gupta S. Role of Probiotics in the Management of Helicobacter pylori. Cureus. 2022;14:e26463. doi: 10.7759/cureus.26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lionetti E., Indrio F., Pavone L., Borrelli G., Cavallo L., Francavilla R. Role of probiotics in pediatric patients with Helicobacter pylori infection: A comprehensive review of the literature. Helicobacter. 2010;15:79–87. doi: 10.1111/j.1523-5378.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 172.Lesbros-Pantoflickova D., Corthèsy-Theulaz I., Blum A.L. Helicobacter pylori and Probiotics1. J. Nutr. 2007;137:812S–818S. doi: 10.1093/jn/137.3.812S. [DOI] [PubMed] [Google Scholar]
- 173.Batdorj B., Trinetta V., Dalgalarrondo M., Prévost H., Dousset X., Ivanova I., Haertlé T., Chobert J.-M. Isolation, taxonomic identification and hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis T31, isolated from Mongolian yoghurt: Inhibitory activity on food-borne pathogens. J. Appl. Microbiol. 2007;103:584–593. doi: 10.1111/j.1365-2672.2007.03279.x. [DOI] [PubMed] [Google Scholar]
- 174.Mohtasham M., Joukar F., Maroufizadeh S., Mojtahedi K., Asgharnezhad M., Mansour-Ghanaei F. Lactobacillus ruteri compared with placebo as an adjuvant in quadruple therapy for Helicobacter pylori eradication: A randomized, double-blind, controlled trial. Arab. J. Gastroenterol. 2023;24:40–44. doi: 10.1016/j.ajg.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 175.Wang Y., Wang X., Cao X.-Y., Zhu H.-L., Miao L. Comparative effectiveness of different probiotics supplements for triple Helicobacter pylori eradication: A network meta-analysis. Front. Cell. Infect. Microbiol. 2023;13:1120789. doi: 10.3389/fcimb.2023.1120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goderska K., Agudo Pena S., Alarcon T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018;102:1–7. doi: 10.1007/s00253-017-8535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lü M., Yu S., Deng J., Yan Q., Yang C., Xia G., Zhou X. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: A meta-analysis of randomized controlled trials. PLoS ONE. 2016;11:e0163743. doi: 10.1371/journal.pone.0163743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dahiya D., Nigam P.S. Antibiotic-therapy-induced gut dysbiosis affecting gut microbiota—Brain axis and cognition: Restoration by intake of probiotics and synbiotics. Int. J. Mol. Sci. 2023;24:3074. doi: 10.3390/ijms24043074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Éliás A.J., Barna V., Patoni C., Demeter D., Veres D.S., Bunduc S., Erőss B., Hegyi P., Földvári-Nagy L., Lenti K. Probiotic supplementation during antibiotic treatment is unjustified in maintaining the gut microbiome diversity: A systematic review and meta-analysis. BMC Med. 2023;21:262. doi: 10.1186/s12916-023-02961-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yuan Z., Xiao S., Li S., Suo B., Wang Y., Meng L., Zhou L. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter. 2021;26:e12848. doi: 10.1111/hel.12848. [DOI] [PubMed] [Google Scholar]
- 181.Mazziotta C., Tognon M., Martini F., Torreggiani E., Rotondo J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. 2023;12:184. doi: 10.3390/cells12010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hevia A., Delgado S., Sánchez B., Margolles A. Molecular players involved in the interaction between beneficial bacteria and the immune system. Front. Microbiol. 2015;6:1285. doi: 10.3389/fmicb.2015.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boger M.C.L., van Bueren A.L., Dijkhuizen L. Cross-feeding among probiotic bacterial strains on prebiotic inulin involves the extracellular exo-inulinase of Lactobacillus paracasei strain W20. Appl. Environ. Microbiol. 2018;84:e01539-18. doi: 10.1128/AEM.01539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Turroni F., Serafini F., Foroni E., Duranti S., Motherway M.O., Taverniti V., Mangifesta M., Milani C., Viappiani A., Roversi T., et al. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium–host interactions. Proc. Natl. Acad. Sci. USA. 2013;110:11151–11156. doi: 10.1073/pnas.1303897110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen L., Xu W., Lee A., He J., Huang B., Zheng W., Su T., Lai S., Long Y., Chu H., et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine. 2018;35:87–96. doi: 10.1016/j.ebiom.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Clardy J., Fischbach M.A., Currie C.R. The natural history of antibiotics. Current Biol. 2009;19:R437–R441. doi: 10.1016/j.cub.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Melander R.J., Basak A.K., Melander C. Natural products as inspiration for the development of bacterial antibiofilm agents. Nat. Prod. Rep. 2020;37:1454–1477. doi: 10.1039/D0NP00022A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carradori S., Di Giacomo N., Lobefalo M., Luisi G., Campestre C., Sisto F. Biofilm and quorum sensing inhibitors: The road so far. Expert Opin. Ther. Pat. 2020;30:917–930. doi: 10.1080/13543776.2020.1830059. [DOI] [PubMed] [Google Scholar]
- 189.Maccelli A., Carradori S., Puca V., Sisto F., Lanuti P., Crestoni M.E., Lasalvia A., Muraro R., Bysell H., Di Sotto A., et al. Correlation between the antimicrobial activity and metabolic profiles of cell free supernatants and membrane vesicles produced by Lactobacillus reuteri DSM 17938. Microorganisms. 2020;8:1653. doi: 10.3390/microorganisms8111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jia J., Zhang C., Liu Y., Huang Y., Bai Y., Hang X., Bi H. Armeniaspirol A: A novel anti-Helicobacter pylori agent. Microb. Biotechnol. 2022;15:442–454. doi: 10.1111/1751-7915.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Di Lodovico S., Napoli E., Di Campli E., Di Fermo P., Gentile D., Ruberto G., Nostro A., Marini E., Cellini L., Di Giulio M. Pistacia vera L. Pistacia vera L. oleoresin and levofloxacin is a synergistic combination against resistant Helicobacter pylori strains. Sci. Rep. 2019;9:4646. doi: 10.1038/s41598-019-40991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cataldi V., Di Bartolomeo S., Di Campli E., Nostro A., Cellini L., Di Giulio M. In vitro activity of Aloe vera inner gel against microorganisms grown in planktonic and sessile phases. Int. J. Immunopathol. Pharmacol. 2015;28:595–602. doi: 10.1177/0394632015600594. [DOI] [PubMed] [Google Scholar]
- 193.Tenório M.C.D.S., Graciliano N.G., Moura F.A., Oliveira A.C.M.D., Goulart M.O.F. N-acetylcysteine (NAC): Impacts on human health. Antioxidants. 2021;10:967. doi: 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Amaral E.P., Conceição E.L., Costa D.L., Rocha M.S., Marinho J.M., Cordeiro-Santos M., Andrade B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016;16:251. doi: 10.1186/s12866-016-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jang S., Bak E.-J., Cha J.-H. N-acetylcysteine prevents the development of gastritis induced by Helicobacter pylori infection. J. Microbiol. 2017;55:396–402. doi: 10.1007/s12275-017-7089-9. [DOI] [PubMed] [Google Scholar]
- 196.Fontes L.E.S., Martimbianco A.L.C., Zanin C., Riera R. N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2019;2 doi: 10.1002/14651858.CD012357.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Feridouni F., Gerist F., Malekzadeh J. The Effect of N-Acetylcysteine on the Treatment of Persistent Helicobacter pylori Infection. SN Compr. Clin. Med. 2021;3:2497–2503. doi: 10.1007/s42399-021-01040-w. [DOI] [Google Scholar]
- 198.Cammarota G., Branca G., Ardito F., Sanguinetti M., Ianiro G., Cianci R., Torelli R., Masala G., Gasbarrini A., Fadda G., et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: A clinical trial. Clin. Gastroenterol. Hepatol. 2010;8:817–820.e3. doi: 10.1016/j.cgh.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 199.Chen X., Li P., Shen Y., Zou Y., Yuan G., Hu H. Rhamnolipid-involved antibiotics combinations improve the eradication of Helicobacter pylori biofilm in vitro: A comparison with conventional triple therapy. Microb. Pathog. 2019;131:112–119. doi: 10.1016/j.micpath.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 200.Ng Y.J., Chan S.S., Khoo K.S., Munawaroh H.S.H., Lim H.R., Chew K.W., Ling T.C., Saravanan A., Ma Z., Show P.L. Recent advances and discoveries of microbial-based glycolipids: Prospective alternative for remediation activities. Biotechnol. Adv. 2023;68:108198. doi: 10.1016/j.biotechadv.2023.108198. [DOI] [PubMed] [Google Scholar]
- 201.Niaz B., Saeed F., Ahmed A., Imran M., Maan A.A., Khan M.K.I., Tufail T., Anjum F.M., Hussain S., Suleria H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019;22:1626–1641. doi: 10.1080/10942912.2019.1666137. [DOI] [Google Scholar]
- 202.González-Chávez S.A., Arévalo-Gallegos S., Rascón-Cruz Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents. 2009;33:301.e1–301.e8. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 203.Cao X., Ren Y., Lu Q., Wang K., Wu Y., Wang Y., Zhang Y., Cui X.-S., Yang Z., Chen Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023;9:1018336. doi: 10.3389/fnut.2022.1018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yamazaki N., Yamauchi K., Kawase K., Hayasawa H., Nakao K., Imoto I. Antibacterial effects of lactoferrin and a pepsin-generated lactoferrin peptide against Helicobacter pylori in vitro. J. Infect. Chemother. 1997;3:85–89. doi: 10.1007/BF02490180. [DOI] [Google Scholar]
- 205.Wada T., Aiba Y., Shimizu K., Takagi A., Miwa T., Koga Y. The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand. J. Gastroenterol. 1999;34:238–243. doi: 10.1080/00365529950173627. [DOI] [PubMed] [Google Scholar]
- 206.Ciccaglione A.F., Di Giulio M., Di Lodovico S., Di Campli E., Cellini L., Marzio L. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: An in vitro and in vivo study. J. Antimicrob. Chemother. 2019;74:1069–1077. doi: 10.1093/jac/dky510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dial E.J., Hall L.R., Serna H., Fox J.G., Lichtenberger L.M. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. Dig. Dis. Sci. 1998;43:2750–2756. doi: 10.1023/A:1026675916421. [DOI] [PubMed] [Google Scholar]
- 208.Sachdeva A., Nagpal J. Meta-analysis: Efficacy of bovine lactoferrin in Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2009;29:720–730. doi: 10.1111/j.1365-2036.2009.03934.x. [DOI] [PubMed] [Google Scholar]
- 209.Lu J., Haley K.P., Francis J.D., Guevara M.A., Doster R.S., Craft K.M., Moore R.E., Chambers S.A., Delgado A.G., Piazuelo M.B., et al. The innate immune glycoprotein lactoferrin represses the Helicobacter pylori cag type IV secretion system. Chembiochem. 2021;22:2783–2790. doi: 10.1002/cbic.202100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ferreira T., Moreira C., Cária N., Victoriano G., Silva W., Jr., Magalhães J. Phytotherapy: An introduction to its history, use and application. Rev. Bras. Plantas Med. 2014;16:290–298. doi: 10.1590/S1516-05722014000200019. [DOI] [Google Scholar]
- 211.Vale F.F., Oleastro M. Overview of the phytomedicine approaches against Helicobacter pylori. World J. Gastroenterol. WJG. 2014;20:5594. doi: 10.3748/wjg.v20.i19.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sathianarayanan S., Ammanath A.V., Biswas R., Sukumaran S., Venkidasamy B. A new approach against Helicobacter pylori using plants and its constituents: A review study. Microb. Pathog. 2022;168:105594. doi: 10.1016/j.micpath.2022.105594. [DOI] [PubMed] [Google Scholar]
- 213.Bent S. Herbal medicine in the United States: Review of efficacy, safety, and regulation: Grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 2008;23:854–859. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vítor J.M., Vale F.F. Alternative therapies for Helicobacter pylori: Probiotics and phytomedicine. FEMS Immunol. Med. Microbiol. 2011;63:153–164. doi: 10.1111/j.1574-695X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 215.Ju L.Z., Ke F., Yadav P.K. Herbal medicine in the treatment of ulcerative colitis. Saudi J. Gastroenterol. 2012;18:3–10. doi: 10.4103/1319-3767.91726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li X.-H., Xu J.-Y., Wang X., Liao L.-J., Huang L., Huang Y.-Q., Zhang Z.-F. BanXiaXieXin decoction treating gastritis mice with drug-resistant Helicobacter pylori and its mechanism. World J. Gastroenterol. 2023;29:2818. doi: 10.3748/wjg.v29.i18.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.El-Moaty H.I.A., Soliman N.A., Hamad R.S., Ismail E.H., Sabry D.Y., Khalil M.M. Comparative therapeutic effects of Pituranthos tortuosus aqueous extract and phyto-synthesized gold nanoparticles on Helicobacter pylori, diabetic and cancer proliferation. S. Afr. J. Bot. 2021;139:167–174. doi: 10.1016/j.sajb.2021.02.009. [DOI] [Google Scholar]
- 218.Guerra-Valle M., Orellana-Palma P., Petzold G. Plant-based polyphenols: Anti-Helicobacter pylori effect and improvement of gut microbiota. Antioxidants. 2022;11:109. doi: 10.3390/antiox11010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fahmy N.M., Al-Sayed E., Michel H.E., El-Shazly M., Singab A.N.B. Gastroprotective effects of Erythrina speciosa (Fabaceae) leaves cultivated in Egypt against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2020;248:112297. doi: 10.1016/j.jep.2019.112297. [DOI] [PubMed] [Google Scholar]
- 220.Zardast M., Namakin K., Kaho J.E., Hashemi S.S. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J. Phytomedicine. 2016;6:495. [PMC free article] [PubMed] [Google Scholar]
- 221.Ayoub I.M., Abdel-Aziz M.M., Elhady S.S., Bagalagel A.A., Malatani R.T., Elkady W.M. Valorization of Pimenta racemosa essential oils and extracts: GC-MS and LC-MS phytochemical profiling and evaluation of Helicobacter pylori inhibitory activity. Molecules. 2022;27:7965. doi: 10.3390/molecules27227965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shmuely H., Domniz N., Yahav J. Non-pharmacological treatment of Helicobacter pylori. World J. Gastrointest. Pharmacol. Ther. 2016;7:171. doi: 10.4292/wjgpt.v7.i2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Deng R., Chen X., Zhao S., Zhang Q., Shi Y. The effects and mechanisms of natural products on Helicobacter pylori eradication. Front. Cell. Infect. Microbiol. 2024;14:1360852. doi: 10.3389/fcimb.2024.1360852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Deng G., Wu Y., Song Z., Li S., Du M., Deng J., Xu Q., Deng L., Bahlol H.S., Han H. Tea polyphenol liposomes overcome gastric mucus to treat Helicobacter pylori infection and enhance the intestinal microenvironment. ACS Appl. Mater. Interfaces. 2022;14:13001–13012. doi: 10.1021/acsami.1c23342. [DOI] [PubMed] [Google Scholar]
- 225.Liu Q., Tang J., Chen S., Hu S., Shen C., Xiang J., Chen N., Wang J., Ma X., Zhang Y., et al. Berberine for gastric cancer prevention and treatment: Multi-step actions on the Correa’s cascade underlie its therapeutic effects. Pharmacol. Res. 2022;184:106440. doi: 10.1016/j.phrs.2022.106440. [DOI] [PubMed] [Google Scholar]
- 226.Bao Z., Wu G., Du J., Ye Y., Zheng Y., Wang Y., Ji R. The comparative efficacy and safety of 9 traditional Chinese medicines combined with standard quadruple therapy for Helicobacter pylori-associated gastritis: A systematic review and network meta-analysis. Ann. Transl. Med. 2022;10:1349. doi: 10.21037/atm-22-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li Y., Li X., Tan Z. An overview of traditional Chinese medicine therapy for Helicobacter pylori–related gastritis. Helicobacter. 2021;26:e12799. doi: 10.1111/hel.12799. [DOI] [PubMed] [Google Scholar]
- 228.Im B.N., Shin H., Lim B., Lee J., Kim K.S., Park J.M., Na K. Helicobacter pylori-targeting multiligand photosensitizer for effective antibacterial endoscopic photodynamic therapy. Biomaterials. 2021;271:120745. doi: 10.1016/j.biomaterials.2021.120745. [DOI] [PubMed] [Google Scholar]
- 229.Dąbrowski J.M. Advances in Inorganic Chemistry. Volume 70. Elsevier; Amsterdam, The Netherlands: 2017. Reactive oxygen species in photodynamic therapy: Mechanisms of their generation and potentiation; pp. 343–394. [Google Scholar]
- 230.Ghorbani J., Rahban D., Aghamiri S., Teymouri A., Bahador A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018;27:293–302. doi: 10.5978/islsm.27_18-RA-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ribeiro M., Gomes I., Saavedra M., Simões M. Photodynamic therapy and combinatory treatments for the control of biofilm-associated infections. Lett. Appl. Microbiol. 2022;75:548–564. doi: 10.1111/lam.13762. [DOI] [PubMed] [Google Scholar]
- 232.Sousa C., Ferreira R., Azevedo N.F., Oleastro M., Azeredo J., Figueiredo C., Melo L.D.R. Helicobacter pylori infection: From standard to alternative treatment strategies. Crit. Rev. Microbiol. 2022;48:376–396. doi: 10.1080/1040841X.2021.1975643. [DOI] [PubMed] [Google Scholar]
- 233.Xu P.-Y., Kankala R.K., Wang S.-B., Chen A.-Z. Sonodynamic therapy-based nanoplatforms for combating bacterial infections. Ultrason. Sonochemistry. 2023;100:106617. doi: 10.1016/j.ultsonch.2023.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Luo Q., Liu C., Zhang A., Zhang D. Research progress in photodynamic therapy for Helicobacter pylori infection. Helicobacter. 2024;29:e13068. doi: 10.1111/hel.13068. [DOI] [PubMed] [Google Scholar]
- 235.Lim B., Kim K.S., Ahn J.Y., Na K. Overcoming antibiotic resistance caused by genetic mutations of Helicobacter pylori with mucin adhesive polymer-based therapeutics. Biomaterials. 2024;308:122541. doi: 10.1016/j.biomaterials.2024.122541. [DOI] [PubMed] [Google Scholar]
- 236.Dunne C., Dolan B., Clyne M. Factors that mediate colonization of the human stomach by Helicobacter pylori. World J. Gastroenterol. WJG. 2014;20:5610. doi: 10.3748/wjg.v20.i19.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Huang Y., Wang Q.-L., Cheng D.-D., Xu W.-T., Lu N.-H. Adhesion and invasion of gastric mucosa epithelial cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016;6:159. doi: 10.3389/fcimb.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Simon P.M., Goode P.L., Mobasseri A., Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect. Immun. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Martins Antunes de Melo W.C., Celiešiūtė-Germanienė R., Šimonis P., Stirkė A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence. 2021;12:2247–2272. doi: 10.1080/21505594.2021.1960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.De Melo W.C.M.A., Avci P., De Oliveira M.N., Gupta A., Vecchio D., Sadasivam M., Chandran R., Huang Y.Y., Yin R., Perussi L.R., et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert Rev. Anti-Infect. Ther. 2013;11:669–693. doi: 10.1586/14787210.2013.811861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hu X., Huang Y.-Y., Wang Y., Wang X., Hamblin M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018;9:1299. doi: 10.3389/fmicb.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dosselli R., Millioni R., Puricelli L., Tessari P., Arrigoni G., Franchin C., Segalla A., Teardo E., Reddi E. Molecular targets of antimicrobial photodynamic therapy identified by a proteomic approach. J. Proteom. 2012;77:329–343. doi: 10.1016/j.jprot.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 243.Lopes D., Melo T., Santos N., Rosa L., Alves E., Gomes M.C., Cunha Â., Neves M.G., Faustino M.A., Domingues M.R.M., et al. Evaluation of the interplay among the charge of porphyrinic photosensitizers, lipid oxidation and photoinactivation efficiency in Escherichia coli. J. Photochem. Photobiol. B Biol. 2014;141:145–153. doi: 10.1016/j.jphotobiol.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 244.Lam M., Jou P.C., Lattif A.A., Lee Y., Malbasa C.L., Mukherjee P.K., Oleinick N.L., Ghannoum M.A., Cooper K.D., Baron E.D. Photodynamic therapy with Pc 4 induces apoptosis of Candida albicans. Photochem. Photobiol. 2011;87:904–909. doi: 10.1111/j.1751-1097.2011.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Beirão S., Fernandes S., Coelho J., Faustino M.A.F., Tomé J.P.C., Neves M.G.P.M.S., Tomé A.C., Almeida A., Cunha A. Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochem. Photobiol. 2014;90:1387–1396. doi: 10.1111/php.12331. [DOI] [PubMed] [Google Scholar]
- 246.Konopka K., Goslinski T. Photodynamic therapy in dentistry. J. Dent. Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 247.Jiang Z., Fu L., Wei C., Fu Q., Pan S. Antibacterial micro/nanomotors: Advancing biofilm research to support medical applications. J. Nanobiotechnol. 2023;21:388. doi: 10.1186/s12951-023-02162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Qiao Y., Ma Y., Tong Y., Liu W., Wang S., Zheng Y., Men C., Yu J., Pan J., Wan D., et al. Phototherapy and Mechanism Exploration of Biofilm and Multidrug-Resistant Helicobacter pylori by Bacteria-Targeted NIR Photosensitizer. Small. 2023;19:2205248. doi: 10.1002/smll.202205248. [DOI] [PubMed] [Google Scholar]
- 249.Wei J., Peng N., Liang Y., Li K., Li Y. Phage therapy: Consider the past, embrace the future. Appl. Sci. 2020;10:7654. doi: 10.3390/app10217654. [DOI] [Google Scholar]
- 250.Gordillo Altamirano F.L., Barr J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Strathdee S.A., Hatfull G.F., Mutalik V.K., Schooley R.T. Phage therapy: From biological mechanisms to future directions. Cell. 2023;186:17–31. doi: 10.1016/j.cell.2022.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sharma S., Chatterjee S., Datta S., Prasad R., Dubey D., Prasad R.K., Vairale M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017;62:17–55. doi: 10.1007/s12223-016-0471-x. [DOI] [PubMed] [Google Scholar]
- 253.Fernández L., Gutiérrez D., García P., Rodríguez A. The perfect bacteriophage for therapeutic applications—A quick guide. Antibiotics. 2019;8:126. doi: 10.3390/antibiotics8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Luo Q., Liu N., Pu S., Zhuang Z., Gong H., Zhang D. A review on the research progress on non-pharmacological therapy of Helicobacter pylori. Front. Microbiol. 2023;14:1134254. doi: 10.3389/fmicb.2023.1134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ferreira R., Sousa C., Gonçalves R.F.S., Pinheiro A.C., Oleastro M., Wagemans J., Lavigne R., Figueiredo C., Azeredo J., Melo L.D.R. Characterization and genomic analysis of a new phage infecting Helicobacter pylori. Int. J. Mol. Sci. 2022;23:7885. doi: 10.3390/ijms23147885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cuomo P., Papaianni M., Fulgione A., Guerra F., Capparelli R., Medaglia C. An innovative approach to control H. pylori-induced persistent inflammation and colonization. Microorganisms. 2020;8:1214. doi: 10.3390/microorganisms8081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Oliveira H., Thiagarajan V., Walmagh M., Sillankorva S., Lavigne R., Neves-Petersen M.T., Kluskens L.D., Azeredo J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE. 2014;9:e108376. doi: 10.1371/journal.pone.0108376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Oliveira H., Melo L.D.R., Santos S.B., Nóbrega F.L., Ferreira E.C., Cerca N., Azeredo J., Kluskens L.D. Molecular aspects and comparative genomics of bacteriophage endolysins. J. Virol. 2013;87:4558–4570. doi: 10.1128/JVI.03277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Oliveira H., São-José C., Azeredo J. Phage-derived peptidoglycan degrading enzymes: Challenges and future prospects for in vivo therapy. Viruses. 2018;10:292. doi: 10.3390/v10060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lood R., Winer B.Y., Pelzek A.J., Diez-Martinez R., Thandar M., Euler C.W., Fischetti V.A. Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 2015;59:1983–1991. doi: 10.1128/AAC.04641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Öcal S. Managing Helicobacter pylori infection: Transitioning from conventional to alternative treatment approaches. Eur. Res. J. 2024;10:136–143. doi: 10.18621/eurj.1320819. [DOI] [Google Scholar]
- 262.Malfertheiner P., Selgrad M., Wex T., Romi B., Borgogni E., Spensieri F., Zedda L., Ruggiero P., Pancotto L., Censini S., et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: A randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol. Hepatol. 2018;3:698–707. doi: 10.1016/S2468-1253(18)30125-0. [DOI] [PubMed] [Google Scholar]
- 263.Yunle K., Tong W., Jiyang L., Guojun W. Advances in Helicobacter pylori vaccine research: From candidate antigens to adjuvants—A review. Helicobacter. 2024;29:e13034. doi: 10.1111/hel.13034. [DOI] [PubMed] [Google Scholar]
- 264.Blanchard T.G., Czinn S.J. Identification of Helicobacter pylori and the evolution of an efficacious childhood vaccine to protect against gastritis and peptic ulcer disease. Pediatr. Res. 2017;81:170–176. doi: 10.1038/pr.2016.199. [DOI] [PubMed] [Google Scholar]
- 265.Banga Ndzouboukou J.L., Lei Q., Ullah N., Zhang Y., Hao L., Fan X. Helicobacter pylori adhesins: HpaA a potential antigen in experimental vaccines for H. pylori. Helicobacter. 2021;26:e12758. doi: 10.1111/hel.12758. [DOI] [PubMed] [Google Scholar]
- 266.Dos Santos Viana I., Cordeiro Santos M.L., Santos Marques H., Lima de Souza Goncalves V., Bittencourt de Brito B., Franca da Silva F.A., Freire de Melo F. Vaccine development against Helicobacter pylori: From ideal antigens to the current landscape. Expert Rev. Vaccines. 2021;20:989–999. doi: 10.1080/14760584.2021.1945450. [DOI] [PubMed] [Google Scholar]
- 267.Pan X., Ke H., Niu X., Li S., Lv J., Pan L. Protection against Helicobacter pylori infection in BALB/c mouse model by oral administration of multivalent epitope-based vaccine of cholera toxin B subunit-HUUC. Front. Immunol. 2018;9:1003. doi: 10.3389/fimmu.2018.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Guo L., Hong D., Wang S., Zhang F., Tang F., Wu T., Chu Y., Liu H., He M., Yang H., et al. Therapeutic protection against H. pylori infection in Mongolian gerbils by oral immunization with a tetravalent epitope-based vaccine with polysaccharide adjuvant. Front. Immunol. 2019;10:1185. doi: 10.3389/fimmu.2019.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wang S., Ma J., Ji Q., Liu Q. Evaluation of an attenuated Listeria monocytogenes as a vaccine vector to control Helicobacter pylori infection. Immunol. Lett. 2021;238:68–74. doi: 10.1016/j.imlet.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 270.Guo L., Yin R., Xu G., Gong X., Chang Z., Hong D., Liu K. Immunologic properties and therapeutic efficacy of a multivalent epitope-based vaccine against four Helicobacter pylori adhesins (urease, Lpp20, HpaA, and CagL) in Mongolian gerbils. Helicobacter. 2017;22:e12428. doi: 10.1111/hel.12428. [DOI] [PubMed] [Google Scholar]
- 271.Katsande P.M., Nguyen V.D., Nguyen T.L.P., Mills G., Bailey D.M.D., Christie G., Hong H.A., Cutting S.M. Prophylactic immunization to Helicobacter pylori infection using spore vectored vaccines. Helicobacter. 2023;28:e12997. doi: 10.1111/hel.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zeng M., Mao X.-H., Li J.-X., Tong W.-D., Wang B., Zhang Y.-J., Guo G., Zhao Z.-J., Li L., Wu D.-L., et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1457–1464. doi: 10.1016/S0140-6736(15)60310-5. [DOI] [PubMed] [Google Scholar]
- 273.Sutton P., Boag J.M. Status of vaccine research and development for Helicobacter pylori. Vaccine. 2019;37:7295–7299. doi: 10.1016/j.vaccine.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.