Abstract
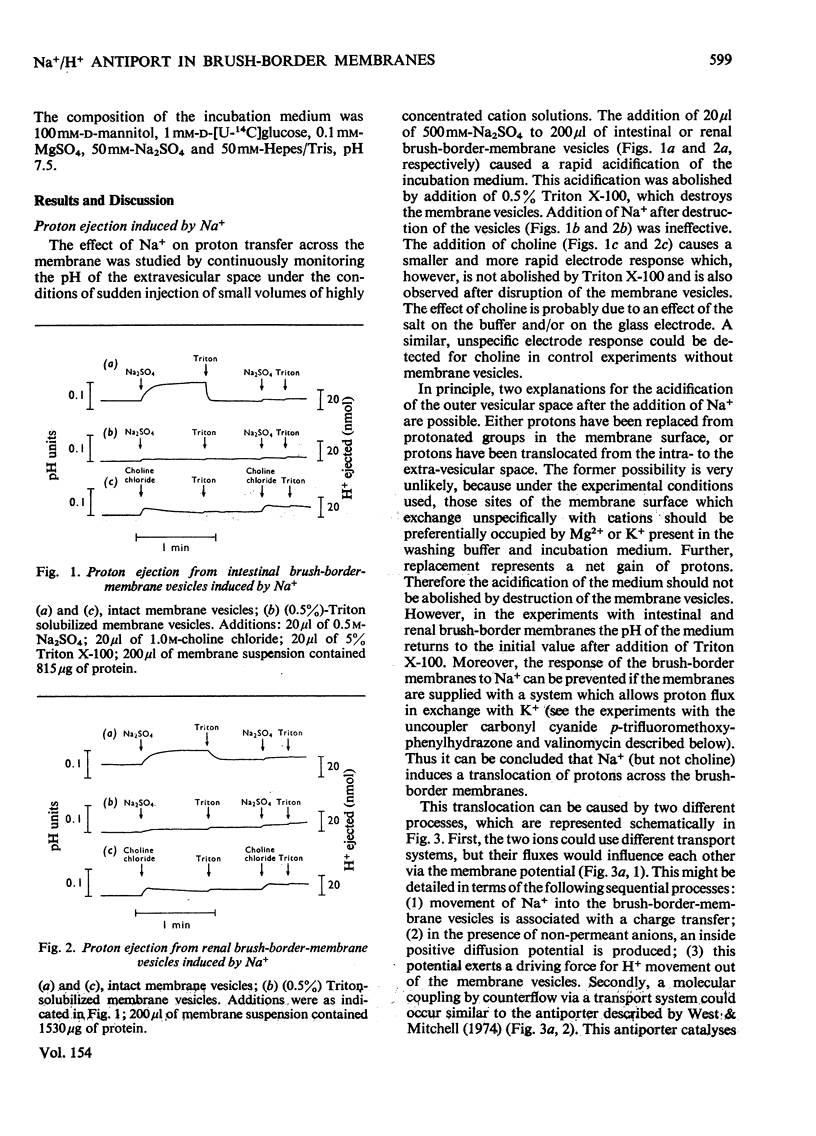
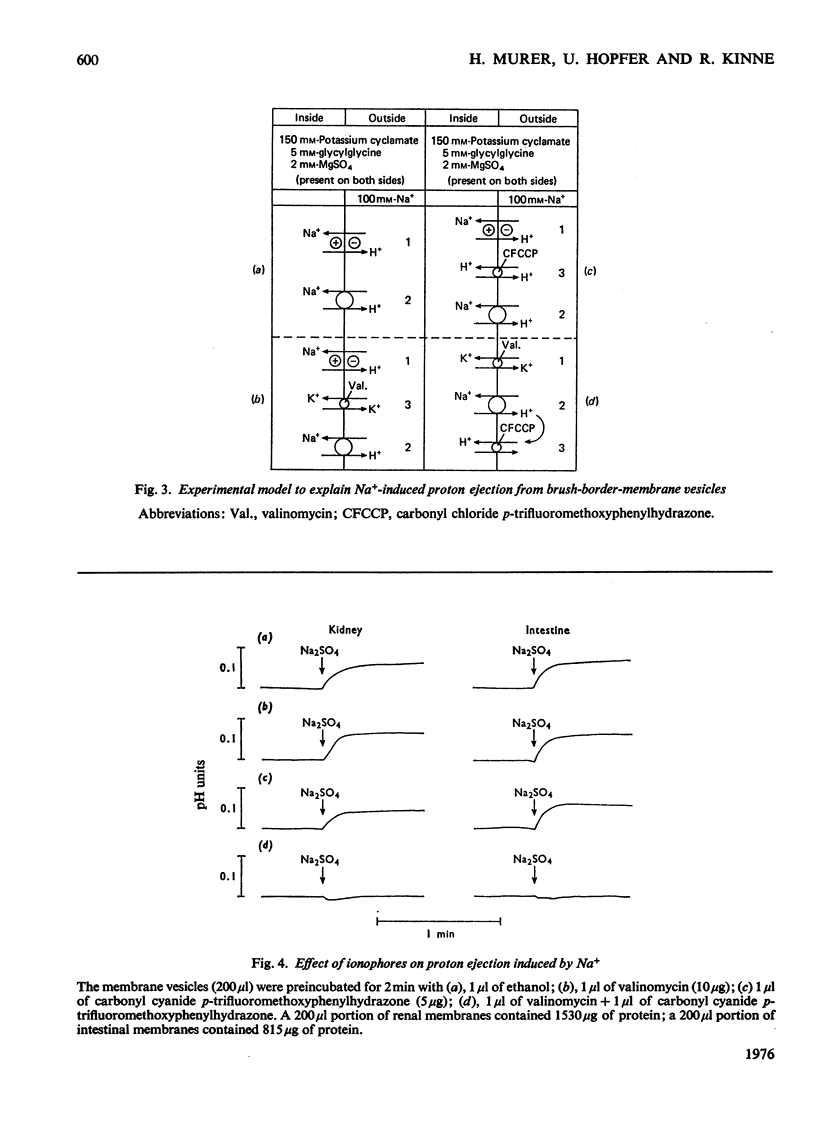
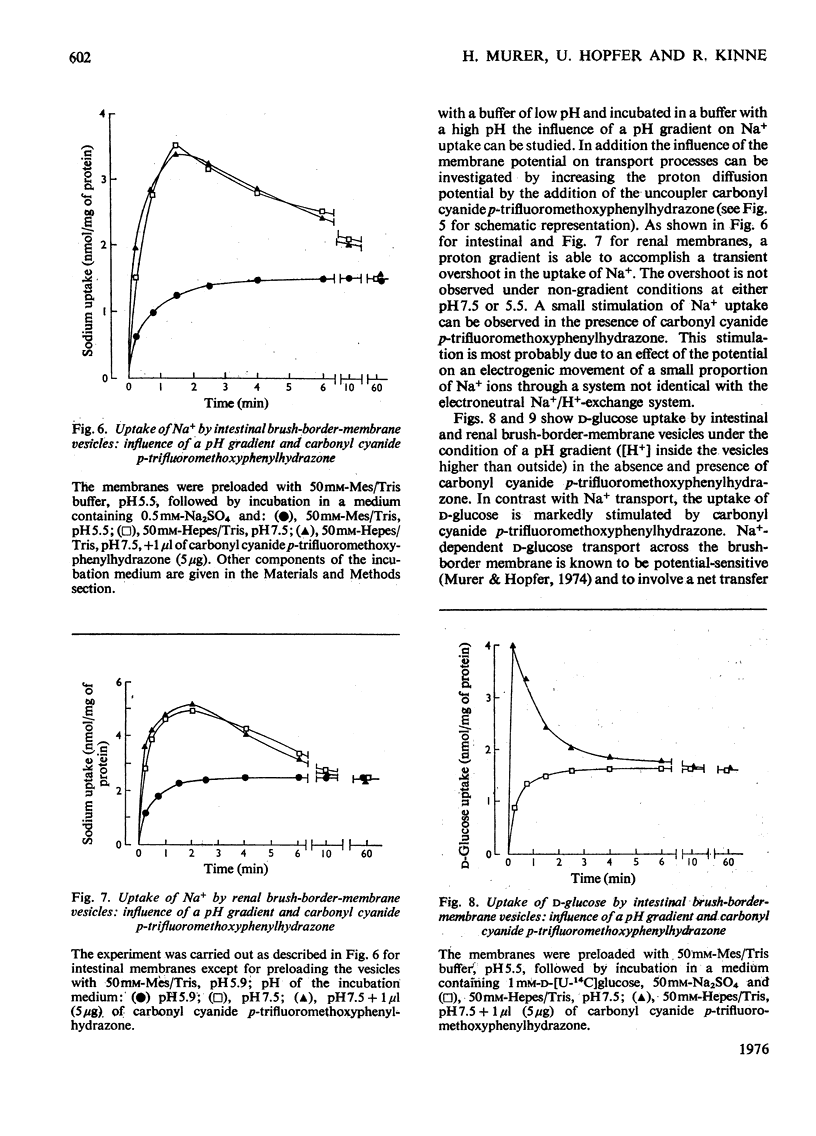
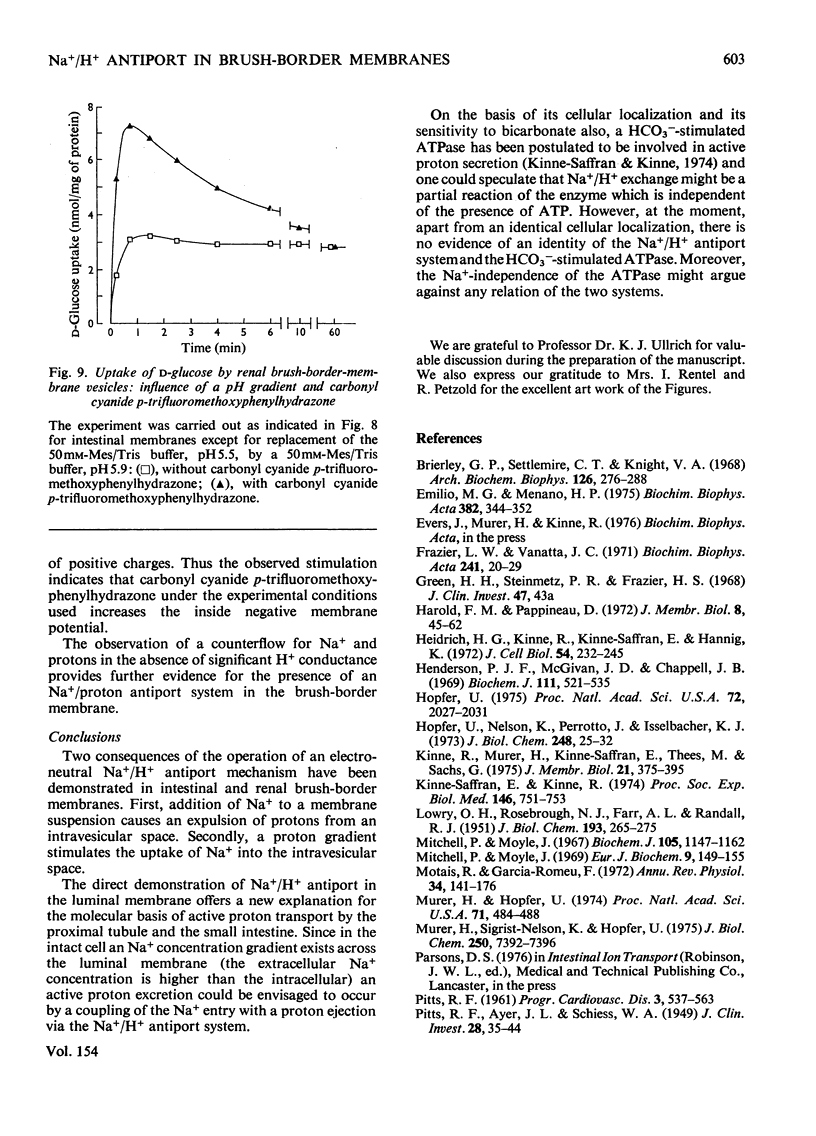
Studies on proton and Na+ transport by isolated intestinal and renal brush-border-membrane vesicles were carried out to test for the presence of an Na+/H+-exchange system. Proton transport was evaluated as proton transfer from the intravesicular space to the incubation medium by monitoring pH changes in the membrane suspension induced by sudden addition of cations. Na+ transport was determined as Na+ uptake into the vesicles by filtration technique. A sudden addition of sodium salts (but not choline) to the membrane suspension provokes an acidification of the incubation medium which is abolished by the addition of 0.5% Triton X-100. Pretreatment of the membranes with Triton X-100 prevents the acidification. The acidification is also not observed if the [K+] and proton conductance of the membranes have been increased by the simultaneous addition of valinomycin and carbonyl cyanide p-trifluoromethoxyphenylhydrazone to the K+-rich incubation medium. Either valinomycin or carbonyl cyanide p-trifluoromethoxyphenylhydrazone when added alone do not alter the response of the membranes to the addition of Na+. Na+ uptake by brush-border microvilli is enhanced in the presence of a proton gradient directed from the intravesicular space to the incubation medium. Under these conditions a transient accumulation of Na+ inside the vesicles is observed. It is concluded that intestinal and renal brush-border membranes contain a NA+/H+ antiport system which catalyses an electroneutral exchange of Na+ against protons and consequently can produce a proton gradient in the presence of a concentration difference for Na+. This system might be involved in the active proton secretion of the small intestine and the proximal tubule of the kidney.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brierley G. P., Settlemire C. T., Knight V. A. Ion transport by heart mitochondria. XI. The spontaneous and induced permeability of heart mitochondria to cations. Arch Biochem Biophys. 1968 Jul;126(1):276–288. doi: 10.1016/0003-9861(68)90584-5. [DOI] [PubMed] [Google Scholar]
- Emilio M. G., Menano H. P. The excretion of hydrogen ion by the isolated amphibian skin: effects of antidiuretic hormone and amiloride. Biochim Biophys Acta. 1975 Mar 25;382(3):344–352. doi: 10.1016/0005-2736(75)90276-x. [DOI] [PubMed] [Google Scholar]
- Frazier L. W., Vanatta J. C. Excretion of H + and NH 4 + by the urinary bladder of the acidotic toad and the effect of short-circuit current on the excretion. Biochim Biophys Acta. 1971 Jul 6;241(1):20–29. doi: 10.1016/0005-2736(71)90299-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U. Diabetes mellitus: changes in the transport properties of isolated intestinal microvillous membranes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2027–2031. doi: 10.1073/pnas.72.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kinne-Saffran E., Kinne R. Presence of bicarbonate stimulated ATPase in the brush border microvillus membranes of the proximal tubule. Proc Soc Exp Biol Med. 1974 Jul;146(3):751–753. doi: 10.3181/00379727-146-38186. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Motais R., Garcia-Romeu F. Transport mechanisms in the teleostean gill and amphibian skin. Annu Rev Physiol. 1972;34:141–176. doi: 10.1146/annurev.ph.34.030172.001041. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U. Demonstration of electrogenic Na+-dependent D-glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):484–488. doi: 10.1073/pnas.71.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Sigrist-Nelson K., Hopfer U. On the mechanism of sugar and amino acid interaction in intestinal transport. J Biol Chem. 1975 Sep 25;250(18):7392–7396. [PubMed] [Google Scholar]
- PITTS R. F. A comparison of the modes of action of certain diuretic agents. Prog Cardiovasc Dis. 1961 May;3:537–562. doi: 10.1016/s0033-0620(61)80024-8. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Sigrist-Nelson K., Murer H., Hopfer U. Active alanine transport in isolated brush border membranes. J Biol Chem. 1975 Jul 25;250(14):5674–5680. [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J., Radtke H. W., Rumrich G. The role of bicarbonate and other buffers on isotonic fluid absorption in the proximal convolution of the rat kidney. Pflugers Arch. 1971;330(2):149–161. doi: 10.1007/BF00643031. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Baumann K. Renal proximal tubular buffer-(glycodiazine) transport. Inhomogeneity of local transport rate, dependence on sodium, effect of inhibitors and chronic adaptation. Pflugers Arch. 1975 Jun 26;357(3-4):149–163. doi: 10.1007/BF00585971. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Hydrogen ion transport by isolated rabbit gallbladder. Am J Physiol. 1969 Jul;217(1):310–316. doi: 10.1152/ajplegacy.1969.217.1.310. [DOI] [PubMed] [Google Scholar]


