Abstract
Background
The burden of Aedes aegypti-transmitted viruses such as dengue, chikungunya, and Zika are increasing globally, fueled by urbanization and climate change, with some of the highest current rates of transmission in Asia. Local factors in the built environment have the potential to exacerbate or mitigate transmission.
Methods
In 24 informal urban settlements in Makassar, Indonesia and Suva, Fiji, we tested children under 5 years old for evidence of prior infection with dengue, chikungunya, and Zika viruses by IgG serology. We used a catalytic model using seroprevalence and mean age to estimate annual incidence of dengue in each country. We also conducted detailed questionnaires to evaluate environmental risk factors for a positive serology result. Dengue risk factors were evaluated for children by univariate and multivariable logistic regression accounting for settlement as a fixed effect. Trash and flooding were additionally evaluated as dengue risk factors at the settlement level by univariate linear regression.
Results
In Fiji and Indonesia respectively, 46% and 33% of children under 5 years old were seropositive for dengue, 3% and 3% for chikungunya, and 9% and 2% for Zika. In Indonesia, children living in a household where trash is routinely collected and removed were significantly less likely to be dengue seropositive in both unadjusted and adjusted models [adjusted model: OR 0.3 (95% CI: 0.1–0.8)]. In Indonesia, settlements with a higher proportion of households reporting flooding also had lower dengue rates (slope = 0.44; p-value: <0.05).
Conclusions
Household trash collection and community flood management are important targets for interventions to mitigate the increasing risk of Aedes aegypti-transmitted viruses.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10315-1.
Keywords: Aedes, Trash, Waste, Arboviruses, Vector borne diseases, Dengue, Built environment
Background
Warming temperatures and extreme weather events are expanding the range and availability of suitable habitats for Aedes aegypti, the primary mosquito vector for dengue, chikungunya, and Zika viruses [1–6]. Dengue virus is the most common arbovirus globally, has increased exponentially over the last several decades [7], and causes an acute febrile illness with clinical presentations ranging from asymptomatic to life-threatening hemorrhage and shock [8]. While less prevalent than dengue, chikungunya and Zika viruses have also emerged as global problems over recent decades and can cause long term morbidity [8–12].
As the climate continues to change, generating reliable estimates of infection rates for Ae. aegypti transmitted viral infections is essential to monitoring changing transmission dynamics and generating models to forecast future risk. Current estimates of recent disease transmission are limited by a reliance on acute febrile surveillance and cross-sectional serology studies in the general population. Acute febrile surveillance underreports the true burden of disease, only tracking cases that come to the hospital and receive a correct diagnosis. Dengue, chikungunya, and Zika infections all have non-specific clinical presentations that are easily misdiagnosed as other entities; and presentations range from asymptomatic infection to severe disease [13–17]. Underreporting of symptomatic cases is exacerbated because diagnostic tests for infections are not readily available in many medical systems and have a limited window for detection. The vast majority of dengue, chikungunya, and Zika infections therefore go unrecognized [1, 18, 19]. Furthermore, surveillance reporting requirements and resources vary between locations and over time [1, 13], making direct comparisons of surveillance data difficult. An alternative to acute febrile surveillance is estimating disease burden with cross-sectional serology studies. A positive immunoglobulin G (IgG) serology for dengue, chikungunya, or Zika virus indicates any past infection, not just recent infection or symptomatic infection [15–17, 20]. Seroprevalence studies can therefore capture all infections in a population, including cases that are asymptomatic, have mild or unusual presentations, or are managed without diagnostic confirmation. Serology testing has limitations: serology is unable to distinguish between a single versus multiple prior infections, it does not indicate when the infection occurred, and antibody levels can wane over time [20]. However, in places with a high incidence of disease, serology studies performed in young children can overcome these limitations and provide insights into recent disease incidence.
While climate change may be fueling the spread of Ae. aegypti-transmitted viruses, local environmental factors can also affect an individual’s risk of exposure. Ae. aegypti mosquitoes breed in small containers of fresh water, including water storage containers, trash, discarded tires, and gutters on houses that fill with rainwater [21–33]. Local conditions, including temperature, humidity, and air circulation, also affect where the adult mosquitoes reside [5, 34, 35]. The role of the built environment, such as housing construction [32, 35–37] and landscaping [35], in modulating dengue risk is beginning to be recognized. As climate change exacerbates Ae. aegypti-transmitted viruses, there is a critical need for better understanding modifiable features of the built environment that attenuate transmission risk and can be targets for local interventions.
Informal urban settlements in Indonesia and Fiji have known high rates of dengue infection [1, 38–40]. The hot, humid climate is highly suitable for the Ae. aegypti lifecycle and virus incubation [41–43]. Fluctuations between drought and flooding in the region [44, 45] and inadequate water infrastructure [46] in the settlements results in pooling of rainwater during heavy rains and storage of water during dry periods, both providing breeding grounds for Ae. aegypti mosquitoes. Inadequate trash management can additionally provide containers for mosquito oviposition [21, 35].
The objectives of this study are to measure seroprevalence and estimate the incidence of dengue, chikungunya, and Zika infections in young children living in informal urban settlements in Indonesia and Fiji. This study also aims to evaluate local environmental risk factors for Ae. aegypti – transmitted arbovirus infections.
Methods
Study population
The Revitalizing Informal Settlements and their Environment (RISE) study was conducted in 24 informal urban settlements in Makassar, Indonesia and Suva, Fiji with enrollment and study procedures previously described [47]. This study involved questionnaires and biological sampling of children between the ages of 6 months to 5 years old living in the RISE sites in 2018 and 2019 who were enrolled in the study and whose parents consented for their participation.
Questionnaires
Baseline questionnaires were used to assess household and individual demographic information and environmental exposures that were hypothesized to be predictive of dengue, chikungunya, and Zika seropositivity (Supplementary Table).
Serology testing
We performed dengue, chikungunya, and Zika virus serology testing on serum samples collected from children under 5 years old enrolled in the RISE study who underwent sampling in 2018 in Indonesia and in 2019 in both Indonesia and Fiji. Serum samples were stored in Sarstedt screw cap tubes at −80 °C for four years prior to serology testing. Serology testing was performed in duplicate using Abcam IgG ELISA’s kits to evaluate for evidence of prior exposure to dengue, chikungunya, and Zika viruses. Duplicate positive, negative, and cut-off controls were used on each plate. Following Abcam kit protocols, antibody titers were converted into standard units based on average cut-off values and all samples with titers greater than 10 standard units were considered positive. Seropositivity rates are reported for each arbovirus and for the proportion of children with evidence of multiple prior infections.
Incidence estimates
Catalytic models estimate the force of primary infection, or incidence rate, using seroprevalence data in diseases where seroprevalence is a marker of any past infection and indicates lifelong immunity [48, 49]. Dengue infection, particularly in young children, meets these criteria and catalytic models have previously been used to estimate dengue incidence [50]. Using a catalytic model, we estimated dengue incidence in each country assuming a constant force of infection over time whereby incidence = 1–(1–seroprevalence)^(1/ mean years of exposure).
Risk factor analysis
Baseline demographic and environmental risk factors for dengue infection in the children enrolled in the RISE study were evaluated by a univariate logistic regression model and a multivariable logistic regression accounting for settlement as a fixed effect and individual characteristics thought to be plausible risk factors for dengue as random effects. For the two breastfeeding questions in the household questionnaire, “breastfeeding currently” was retained in the multivariable model since it was significant in the univariate model in Fiji; however, a sensitivity analysis was also performed which replaced “breastfed in the past 3 months” in the model, which did not change the findings.
As a further exploration of the potential impact of household variables that might have an impact on the surrounding settlement arbovirus exposure risk – namely flooding and trash collection - we also conducted univariate linear regression at the settlement level to evaluate whether the proportion of households reporting flooding and trash collection were predictive of dengue seropositivity across the settlement. This settlement analysis was restricted to settlements with at least 10 children tested and multivariable regression was not conducted given the small sample size.
All risk factor analyses were conducted to evaluate risk of dengue infection. Given the overall low chikungunya and Zika seroprevalence and the fact that the majority of individuals with evidence of either of these two infections were seropositive for dengue, risk factor assessment was not conducted for these other viruses. All analyses were performed in R version 2023.06.1.
Ethics
Ethics review and approval was provided by participating universities and local IRBs, including: Monash University Human Research Ethics Committee (Melbourne, Australia; project ID 35903), Ministry of Research, Technology and Higher Education Ethics Committee of Medical Research at the Faculty of Medicine, Universitas Hasanuddin (Makassar, Indonesia; protocol UH18020110), and Fiji National University College Human Health Research Ethics Committee (CHREC ID 137.19). Written informed consent was obtained from the parents/guardians of all child participants.
Results
Demographic characteristics
A total of 191 children in Fiji and 181 children in Indonesia were included in the study. Overall, the mean age of children at the time of serum sample collection was 3.3 years old and male children comprised 61% of the study population. Breastfeeding, household trash collection, having a household member who grows plants, and porous building materials for household construction were more commonly reported amongst participants in Fiji than Indonesia. In contrast, flooding in or around the house was more commonly reported in Indonesia (Table 1).
Table 1.
Baseline characteristics of children in Fiji and Indonesia
| Fiji n (%) (N = 191) |
Indonesia n (%) (N = 181) |
|
|---|---|---|
| Child characteristics | ||
| Male | 107/191 (56%) | 119/181 (66%) |
| Age: mean (sd) | 3.2 (1.2) | 3.5 (1.0) |
| Breastfed in the past 3 months | 50/185 (27%) | 20/181 (11%) |
| Breastfeeding currently | 35/185 (19%) | 17/181 (9%) |
| Ethnicity & religion of household respondent | ||
| Ethnicity | ||
| I-Taukei | 168/189 (89%) | |
| Indo Fijian | 11/189 (6%) | |
| Other Fijian or Mixed | 10/189 (5%) | |
| Makassar | 108/163 (66%) | |
| Bugis | 11/163 (7%) | |
| Toraja | 8/163 (5%) | |
| Other Indonesian or Mixed | 36/163 (22%) | |
| Religion | ||
| Christian | 76/189 (40%) | |
| Lotu Vakarisito | 101/189 (53%) | |
| Other or Mixed (Fiji) | 12/189 (6%) | |
| Islam | 152/163 (93%) | |
| Other or Mixed (Indonesia) | 11/163 (7%) | |
| Household characteristics | ||
| Reports many mosquito bitesa | 156/191 (82%) | 151/160 (94%) |
| Household trash collectionb | 161/191 (84%) | 103/160 (64%) |
| Grows plantsc | 126/191 (66%) | 79/160 (49%) |
| Experiences flooding in or outside the house | 52/191 (27%) | 86/181 (48%) |
| Flooring made of porous materiald | 182/191 (95%) | 53/160 (33%) |
| Walls made of porous materiald | 84/191 (44%) | 37/160 (23%) |
| Stores water | 145/191 (76%) | 146/160 (91%) |
In Fiji, there were 2 children with missing data on ethnicity and religion and 6 children with missing data on breastfeeding status. In Indonesia, there were 21 children with missing data on various household characteristics
Abbreviations: sd standard deviation
aHousehold respondent reported that in the past 6 weeks they experienced mosquitoes biting inside the house at least several times a week
bTrash is always collected and taken away or taken to a neighborhood collection point
cA household member grows plants in the house, garden, or settlement croplands
dPorous housing material included wood, bamboo, woven mat, dirt, and tent material; in contrast, non-porous materials included cement, ceramic tiles, bricks, laminate, granite, and metal
Seropositivity
Dengue IgG seropositivity was high in both countries, with 46% of children in Fiji and 33% of children in Indonesia demonstrating evidence of prior dengue exposure. Zika seropositivity was higher in Fiji than Indonesia. Chikungunya seropositivity was low but detectable in both countries. A total of 19 children (10%) in Fiji and 7 children (3%) in Indonesia were seropositive for more than one arbovirus (Table 2).
Table 2.
IgG seropositivity of Aedes-transmitted arboviruses in children under 5 years old
| Arbovirus | Fiji (N = 191) |
Indonesia (N = 181) |
|---|---|---|
| All results | ||
| Dengue | 88 (46.1%) | 59 (32.6%) |
| Chikungunya | 5 (2.6%) | 5 (2.8%) |
| Zika | 18 (9.4%) | 3 (1.7%) |
| Multiple infections | ||
| Dengue & Chikungunya | 4 (2.1%) | 5 (2.8%) |
| Dengue & Zika | 14 (7.3%) | 2 (1.1%) |
| Dengue & Chikungunya & Zika | 1 (0.5%) | 0 (0%) |
“All results” includes all children who tested positive on IgG ELISA for each arbovirus, regardless of results of the other serology tests. “Multiple infections” refers to the number of children who tested positive on multiple serology tests, indicating infection with multiple arboviruses during their lifetime, although not necessarily co-infection at the same time
Abbreviations: IgG Immunoglobulin G
Dengue seropositivity overall increased with age, with the exception of the 0.5 to < 1 year olds who showed a relatively high seropositivity but had very low numbers of participants. By the age of 4 to 5 years old, 71% and 51% of the children in Fiji and Indonesia respectively had been infected with dengue (Table 3). Using a catalytic model, we estimated an annual incidence rate of 18% in Fiji and 11% in Indonesia. Although seroprevalence rates of chikungunya and Zika were too low to model incidence, seropositivity was found in multiple age groups.
Table 3.
Dengue seropositivity by age
| Age (years) | Fiji N = 191 |
Indonesia N = 181 |
|---|---|---|
| 0.5 to < 1* | 1/10 (10%) | 1/3 (33%) |
| 1 to < 2 | 2/28 (7%) | 1/17 (6%) |
| 2 to < 3 | 10/37 (27%) | 9/36 (25%) |
| 3 to < 4 | 33/57 (58%) | 14/58 (24%) |
| 4 to < 5 | 42/59 (71%) | 34/67 (51%) |
Dengue risk increases with age in both countries, consistent with continuous increasing exposure over time. This is consistent with endemic transmission rather than a single epidemic
*Seroprevalence estimates may be artificially elevated for this age category because this only includes children > 6 months, not across the entire range. Estimates in this group are also less precise due to small sample size in this group
Demographic and environmental risk factors for dengue
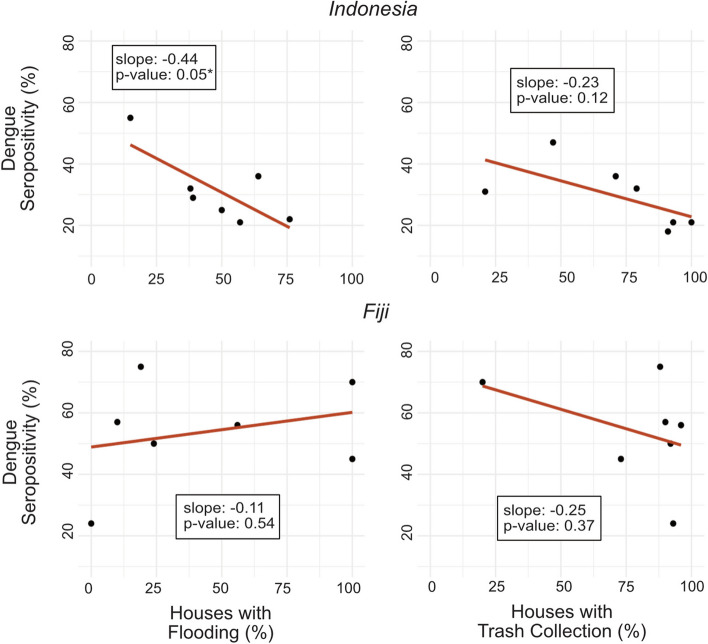
Age was a significant predictor of dengue serostatus amongst children in Fiji and Indonesia in both unadjusted and adjusted models [Adjusted models - Fiji: OR 4.0 (95% CI: 2.5–6.3); Indonesia: OR 2.2 (95% CI: 1.4–3.6)]. In Indonesia, children living in a household with trash collection were significantly less likely to be dengue seropositive in both unadjusted and adjusted models [Adjusted model OR 0.3 (95% CI: 0.1–0.8)]. Living in a house made of porous flooring material was also protective against dengue exposure amongst children in Indonesia, although this was only significant in the unadjusted model [OR 0.4 (95% CI: 0.2–0.9)]. In Fiji, children who were currently breastfeeding at the time of serum sampling were less likely to be seropositive for dengue in the unadjusted model [OR 0.3 (95% CI: 0.1–0.7)], but this did not remain statistically significant in the adjusted model accounting for age (Table 4). Household flooding was not a significant predictor of individual dengue seropositivity in Indonesia or Fiji; however, in Indonesia, settlements with a higher proportion of households reporting flooding had lower rates of dengue (Fig. 1).
Table 4.
Demographic and environmental risk factors for dengue exposure amongst children in Fiji and Indonesia
| Risk Factors | Fiji | Indonesia | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative (N = 103) | Positive (N = 88) | Unadjusted Model OR (95% CI) |
Adjusted Model OR (95% CI) |
Negative (N = 122) | Positive (N = 59) | Unadjusted Model OR (95% CI) |
Adjusted Model OR (95% CI) |
|
| Male gender | 57 (55%) | 50 (57%) | 1.1 (0.6–1.9) | 0.9 (0.4–1.9) | 83 (68%) | 36 (61%) | 0.7 (0.4–1.4) | 0.9 (0.4–2.1) |
| Age (years) | 2.6 (1.2) | 3.8 (0.8) | 2.9 (2.1–4.1) * | 4.0 (2.5–6.3)* | 3.3 (1.0) | 3.9 (0.9) | 2.0 (1.4–2.9) * | 2.2 (1.4–3.6)* |
| Breastfed in the past 3 months | 33/102 (32%) | 17/83 (21%) | 0.5 (0.3–1.0) | 2.7 (1–7.8) | 17 (14%) | 3 (5%) | 0.3 (0.1–1) | 0.7 (0.1–3.9) |
| Breastfeeding currently | 27/102 (26%) | 8/83 (10%) | 0.3 (0.1–0.7) * | 1.5 (0.5–4.8) | 14 (11%) | 3 (5%) | 0.4 (0.1–1.3) | 0.9 (0.2–4.9) |
| Reports many mosquito bitesa | 82 (80%) | 74 (84%) | 1.4 (0.6–2.9) | 0.6 (0.2–1.7) | 104/111 (94%) | 47/49 (96%) | 1.6 (0.4–10.9) | 3.3 (0.5–21.5) |
| Household trash collectionb | 86 (83%) | 75 (85%) | 1.1 (0.5–2.5) | 2.2 (0.7–7) | 79/111 (71%) | 24/49 (49%) | 0.4 (0.2–0.8) * | 0.3 (0.1–0.8)* |
| Grows plantsc | 65 (63%) | 61 (69%) | 1.3 (0.7–2.4) | 1.3 (0.6–3.2) | 54/111 (49%) | 25/49 (51%) | 1.1 (0.6–2.2) | 1.1 (0.5–2.4) |
| Experiences flooding in or outside the house | 27 (26%) | 25 (28%) | 1.1 (0.6–2.1) | 0.5 (0.2–1.4) | 59 (48%) | 27 (46%) | 0.9 (0.5–1.7) | 1.6 (0.7–3.7) |
| Flooring made of porous materiald | 97 (94%) | 85 (97%) | 1.8 (0.4–8.5) | 2.5 (0.4–15.3) | 43/111 (39%) | 10/49 (20%) | 0.4 (0.2–0.9) * | 0.4 (0.2–1.2) |
| Walls made of porous materiald | 47 (46%) | 37 (42%) | 0.9 (0.5–1.5) | 0.8 (0.3–1.7) | 28/111 (25%) | 9/49 (18%) | 0.7 (0.3–1.5) | 1.0 (0.4–2.7) |
| Stores water | 78 (76%) | 67 (76%) | 1.0 (0.5–2.0) | 0.9 (0.4–2.3) | 101/111 (92%) | 45/49 (90%) | 0.8 (0.3–2.7) | 0.5 (0.1–2.3) |
Denominators provided for all variables with missing data
In the adjusted models, we ran a second model for breastfeeding currently that excluded breastfed in the past 3 months and a second model for flooring and walls made of porous material that excluded the individual variables for flooring and walls
Abbreviations: OR odds ratio, CI confidence interval
aHousehold respondent reported that in the past 6 weeks they experienced mosquitoes biting inside the house at least several times a week
bTrash is always collected and taken away or taken to a neighborhood collection point
cA household member grows plants in the house, garden, or settlement croplands
dPorous housing material included wood, bamboo, woven mat, dirt, and tent material; in contrast, non-porous materials included cement, ceramic tiles, bricks, laminate, granite, and metal
*Indicates statistically significant result with a threshold of p < 0.05
Fig. 1.
Settlement level dengue seropositivity versus flooding and trash collection rates
*Indicates statistically significant result with p-value<0.05
Settlements in Indonesia with a higher percentage of houses that reported flooding in or around their house had significantly lower dengue seropositivity rates, suggestive that flooding could reduce breeding habitats.
Settlements in both Fiji and Indonesia with a higher percentage of houses reporting trash collection seem to have lower dengue seropositivity rates, consistent with the individual level analysis, but this was not found to be significant at the settlement level. In Fiji, trash collection was found to be high across most settlements, making any potential correlation to dengue risk difficult to ascertain.
Discussion
Our study found a high prevalence of dengue in Fiji and Indonesia, with over half of children in each country having had an infection by the age of five years old. Although chikungunya and Zika exposure was lower, a seroprevalence of 2–9% in such young children suggests ongoing transmission of these other Ae. aegypti – transmitted viruses in these countries as well.
Our dengue seroprevalence estimates are similar to those found in a 2014 study of urban children across Indonesia [40], and in South Sulawesi in particular [51], corroborating the high rates of dengue in Indonesia. Although dengue circulation in Fiji is well established with many known outbreaks over the years, dengue seroprevalence and incidence data across Fiji is limited [52, 53]. Our study is one of the few studies providing such estimates and showed high circulation of dengue in Suva, the capital city located in the Central District of the Island of Vitu Levi. Furthermore, by measuring dengue serology, which does not rely on clinical case finding, our study provides incidence estimates that capture the large proportion of cases that go unrecognized by acute febrile surveillance studies. Our study also showed a steady increase in seropositivity for each year of age in children under 5 years old in both countries, indicating high levels of non-epidemic transmission.
Other studies in Indonesia and Fiji looking at demographic or environmental risk factors have not identified significant risk factors for dengue seropositive test results [40, 52]. Our study focused on environmental features of the built environment that would be expected to increase dengue exposure and looked at the youngest children as they acquire their first dengue infections, thus giving a potentially unique window into risk factors in places with very high levels of risk.
Trash being collected and removed from the household in our cohort in Indonesia was a significant protective factor against dengue infection in the individual level analysis. In the secondary analysis at the settlement level, we observe a similar correlation between settlement trash collection and settlement dengue risk. Although the settlement level analysis was not found to be statistically significantly, we suspect this is due to inadequate statistical power with this small sample size or that household practices are a stronger driver of risk given the relatively short flight range of Ae. aegypti. We did not find a significant association between trash collection and dengue in the Fiji cohort in either the individual or community level analyses. In Fiji, the higher overall dengue risk may overwhelm such individual environmental risk; furthermore, trash collection was very common (83%) in Fiji, diminishing our power to detect a difference in dengue exposure in this cohort. Ae. aegypti breed in small containers of water [21, 22, 26, 28] and have a relatively short range of about 100 meters [54]. Our findings in Indonesia corroborate other studies demonstrating Ae. aegypti breeding in trash filled with rainwater [21, 35, 55–57] and implicate household trash disposal practices as a dengue exposure risk. Our study adds to the existing literature not only by showing this direct association between household trash disposal practices and individual disease risk but also by highlighting the risk posed by inadequate trash collection in informal settlements. Because they are not legally recognized neighborhoods, informal settlements are often excluded from government trash collection programs, particularly in Makassar, Indonesia. Our study indicates that household trash removal could decrease disease exposure and endorses the inclusion of informal settlements in regular government trash collection programs.
The impact of flooding on dengue risk is complex and remains poorly understood. While dengue outbreaks following large floods frequently make news headlines [58–60], it is unclear whether these outbreaks are due to the floods themselves, heavy rainfall regardless of flooding, or an extended duration of pooled water as floodwaters recede. Other studies have proposed that while heavy rainfall fills up potential Ae. aegypti breeding grounds with water, severe flooding may in fact flush out those small containers holding water and Ae. aegypti larva, or wash away trash containers from the area, thereby decreasing risk [43, 61]. Our analysis at the individual level did not show flooding to significantly increase or decrease the risk of dengue infection in the child cohorts in either country, perhaps reflecting this complexity. Interestingly though, when we considered flooding to be a settlement risk and evaluated the relationship between the degree of settlement flood exposure and settlement dengue prevalence, in Indonesia we found that settlements with more households experiencing flooding had fewer children with positive dengue serologies. Our findings support the hypothesis that flooding may flush out Ae. aegypti larva or reduce trash that support those larva [43], although given our small sample size, these findings should be interpreted cautiously. As extreme flooding increases with climate change, further studies are needed to better understand how flood microclimates affect Ae. aegypti proliferation and consequent disease risk.
Finally, our study hints at the possibility that housing construction made of porous materials could be protective against dengue exposure. We had initially hypothesized that porous housing materials might let mosquitoes into the house which would increase risk. However porous housing construction could allow for increased ventilation and lower indoor temperatures, thereby decreasing the attractiveness of the home to Ae. aegypti mosquitoes which favor hot, humid environments. Porous flooring material was only found to decrease dengue risk significantly in univariate analysis in the Indonesia cohort. Porous walls were associated with a decrease which was not statistically significant in either model. Other studies have suggested that housing construction features that affect air flow modulate dengue risk [32, 35]. More research is needed to further evaluate how porous housing materials may impact ventilation and indoor temperature and thus potentially lower mosquito burden.
Our study is limited by arbovirus serology assay accuracy, particularly in the setting of multiple circulating arboviruses. Dengue and Zika are both flaviviruses with consequent potential for cross-reactivity on serology studies. However, since these viruses are transmitted by the same mosquitoes, in places with co-circulation, risk for one virus equates to risk for the other and prior infection with both viruses in some children would be expected. Using validated commercial ELISA kits, we ran all samples in duplicate and found complete concordance between duplicate runs; additionally, positive versus negative results separated clearly when evaluating titer values. We identified 1 individual in Indonesia and 3 individuals in Fiji who tested positive for Zika and negative for dengue; and the ratio of Zika to dengue positive results in the two countries was dissimilar. Based on these findings, we infer that at least some, if not all, of our Zika positive results were true positives. Chikungunya virus serology also has the potential to cross-react with other alphaviruses such as Ross River virus. Ross River virus is known to circulate in the broader region but is largely considered to cause asymptomatic infections, so prevalence is not well documented. Given these potential limitations with the Zika and chikungunya serologies and the relatively low prevalence, we elected not to estimate incidence or include them in the risk factor analysis.
Japanese encephalitis virus (JEV) is another flavivirus which can cross-react with dengue and Zika serology; it is transmitted in Southeast Asia and the Pacific Islands by Culex mosquitoes, predominantly around rice paddies in rural areas where pig farming and waterfowl presence amplify transmission [62]. We suspect the impact of JEV cross-reactivity on our results would be negligible, given that JEV circulation, JEV vaccination, and pig farming are not known to be common in our study areas and that JEV is transmitted by a different vector. However, JEV is a potentially fatal vector-borne disease and further research is needed to characterize prevalence and distribution of JEV across Asia, particularly in the face of environmental changes.
Finally, there is the potential for false positive serology results due to transfer of maternal antibodies. For this reason, we restricted sampling to children over 6 months of age, past the point of placental antibody transfer and often past the time for exclusive breastfeeding. Additionally, we found that breastfeeding rates at the time of serum sampling was low in both places, that breastfeeding children tested positive and negative on serology, and that breastfeeding was not associated with an increased likelihood of seropositivity. Therefore, we believe our results were not significantly impacted by the possibility of maternal antibody transfer.
One limitation of our incidence estimates is that they are modeled based on seroprevalence and age, not measured by case counts. Unlike an acute febrile surveillance system, serology results cannot tell you when and where a case occurred. However, by testing very young children, we know that infection occurred during their relatively short lifespan and likely around their current residence. The other advantage of this approach to measuring incidence is that we can capture all infections, not only infections that resulted in symptomatic infection and diagnostic testing within a narrow detection window. One caveat though is that children in such a high incidence setting may have had multiple infections with different dengue serotypes, which would be missed by serology, resulting in an underestimate of incidence. However, this problem is more likely as children get older. Additionally, in places where childhood incidence is very high and infection results in lifelong immunity, the incidence in the adult population may be significantly lower than in the child population. Despite these limitations, estimations of recent dengue incidence in children more accurately reflects recent transmission dynamics and can be used to help monitor how disease transmission is changing.
Finally, our risk factor analysis had some limitations. In Fiji we did not identify any built or natural environment risk factors for dengue infection. This may have been due to inadequate statistical power, relatively high or low rates of certain risk factors across the entire study population, or simply the extremely high burden of disease that obscured individual risk factors. A previous study in Fiji during a 2013–2014 dengue outbreak similarly did not identify any demographic or environmental risk factors for infection [52]. In Indonesia where dengue burden was lower and there was greater heterogeneity of key risk factors, such as trash collection, we did find that these factors contributed to dengue risk. We elected not to do a combined analysis of the Indonesia and Fiji data given the differences in seropositivity and various risk factors between these two countries. Self-reporting of risk factors, lack of information about neighbor practices that could affect the local environment, and unmeasured housing construction features in neighborhoods based on flood risk (i.e. housing built off the ground in flood-prone areas) are additional limitations of the risk factor analysis. We accounted for unmeasured neighborhood risk factors in our multivariable model by including settlement as a random effect and conducted a secondary analysis of overall settlement flooding and trash collection rates.
Further investigation is needed to evaluate how dengue risk is affected by nuanced differences in the built environment – including different types of construction that reduce flood water intrusion into the house, divert or absorb floodwater around a neighborhood, alter household air circulation or temperature, or practices that minimize standing water in trash, planters, tires, or other containers. Moreover, improved techniques to objectively measure environmental risk factors such as trash and flood exposure and housing design could improve our understanding of how these factors affect individual risk for arbovirus infection [63]. Despite these limitations, we do see a strong effect of household trash disposal practices on infection risk in Indonesia and indications that flooding and housing construction affects risk and warrants further study. Additional research evaluating how trash disposal practices, housing construction, or floodwater management can attenuate dengue risk would better inform urban planning and policies in areas with high or increasing dengue risk.
Conclusions
In summary, our study found very high rates of dengue in young children living in informal urban settlements in Makassar, Indonesia and Suva, Fiji as well as lower rates of Zika and chikungunya in this population suggesting ongoing low-level transmission of these other two Ae. aegypti-transmitted viruses. Household trash collection and community flooding appear to be protective factors against dengue exposure. Further work to evaluate these modifiable risk factors and test interventions designed to disrupt transmission pathways can help mitigate the increasing risk of Ae. aegypti-transmitted viruses globally.
Supplementary Information
Acknowledgements
We would like to acknowledge the field and laboratory staff in Indonesia and Fiji, in particular Maghfira Saifuddaolah and Silivia Rosova-Vilsoni, and the RISE Consortium (10.26180/ctjf-vf69).
Clinical trial number
Not applicable.
Abbreviations
- RISE
Revitalizing Informal Settlements and their Environment
- SD
standard deviation
- IgG
Immunoglobulin G
- OR
odds ratio
- CI
confidence interval
Authors' contributions
JIR conceived of the study design, led the laboratory work, verified and analyzed the data, and drafted the manuscript. JJO, AL, FB, RRT, and AT assisted with data acquisition. NT, NPEA, MA, and EB assisted with laboratory evaluations. KL, A, and IW supervised the project. All authors provided critical review of the manuscript.
Funding
This research was supported by NIH K32 AI168581 (JIR), ASTMH-Burroughs Wellcome Trust (JIR), Stanford Center for Innovation in Global Health Seed Grant (JIR). This research was part of the RISE program which is funded by the Wellcome Trust [grant 205222/Z/16/Z], the New Zealand Ministry of Foreign Affairs and Trade, the Australian Department of Foreign Affairs and Trade, the Government of Fiji, the Asian Development Bank and Monash University, and involves partnerships and in-kind contributions from the City of Makassar, the Cooperative Research Centre for Water Sensitive Cities (now Water Sensitive Cities Australia), Fiji National University, Hasanuddin University, Stanford University, Emory University, Melbourne University, Southeast Water, Melbourne Water, Live and Learn Environmental Education, UN-Habitat, UNU-IIGH, WaterAid International and Oxfam.
Data availability
At the end of the research trial, deidentified data will be stored on secure Monash infrastructure and made available, upon application, as approved by the ethics committees. Researchers interested in accessing data may contact the RISE Program (10.26180/ctjf-vf69).
Declarations
Ethics approval and consent to participate
Ethics review and approval was provided by participating universities and local IRBs, including: Monash University Human Research Ethics Committee (Melbourne, Australia; project ID 35903), Ministry of Research, Technology and Higher Education Ethics Committee of Medical Research at the Faculty of Medicine, Universitas Hasanuddin (Makassar, Indonesia; protocol UH18020110), and Fiji National University College Human Health Research Ethics Committee (CHREC ID 137.19). Written informed consent was obtained from the parents/guardians of all child participants.
Consent for publication
Not applicable.
Competing interests
None of the authors have any relevant conflict of interest or other financial disclosures relevant to the subject matter.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mordecai EA, Ryan SJ, Caldwell JM, Shah MM, LaBeaud AD. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Health. 2020;4:e416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SU, Ogden NH, Fazil AA, Gachon PH, Dueymes GU, Greer AL, et al. Current and Projected Distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S. Environ Health Perspect. 2020;128:057007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe R, Gasparrini A, Meerbeeck CJV, Lippi CA, Mahon R, Trotman AR, et al. Nonlinear and delayed impacts of climate on dengue risk in Barbados: a modelling study. PLOS Med. 2018;15:e1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11:e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber DG, Gulledge J. Extreme Weather and Climate Change: understanding the Link, managing the risk. White Pap Sci Impacts Program. 2011;12(5).
- 7.WHO. WHO | Global Strategy for dengue prevention and control, 2012–2020. 2012. https://www.who.int/denguecontrol/9789241504034/en/. Accessed 27 Oct 2019.
- 8.Labeaud AD, Bashir F, King CH. Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Popul Health Metr. 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MMO, Tauro LB, Kikuti M, Anjos RO, Santos VC, Gonçalves TSF, et al. Concomitant Transmission of Dengue, Chikungunya, and Zika Viruses in Brazil: clinical and epidemiological findings from surveillance for Acute Febrile illness. Clin Infect Dis. 2019;69:1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward C, Chapman J. Chikungunya in children: a clinical review. Pediatr Emerg Care. 2018;34:510–5. [DOI] [PubMed] [Google Scholar]
- 11.Grossi-Soyster EN, LaBeaud AD. Clinical aspects of Zika virus. Curr Opin Pediatr. 2017;29:102–6. [DOI] [PubMed] [Google Scholar]
- 12.Puntasecca CJ, King CH, LaBeaud AD. Measuring the global burden of chikungunya and Zika viruses: a systematic review. PLoS Negl Trop Dis. 2021;15:e0009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Santis O, Bouscaren N, Flahault A. Asymptomatic dengue infection rate: a systematic literature review. Heliyon. 2023;9:e20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asish PR, Dasgupta S, Rachel G, Bagepally BS, Kumar CPG. Global prevalence of asymptomatic dengue infections - a systematic review and meta-analysis. Int J Infect Dis. 2023;134:292–8. [DOI] [PubMed] [Google Scholar]
- 15.Chikungunya | CDC Yellow Book. 2024. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/chikungunya. Accessed 26 Oct 2024.
- 16.Dengue | CDC Yellow Book. 2024. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/dengue. Accessed 26 Oct 2024.
- 17.Zika | CDC Yellow Book. 2024. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/zika. Accessed 26 Oct 2024.
- 18.Haby MM, Pinart M, Elias V, Reveiz L. Prevalence of asymptomatic Zika virus infection: a systematic review. Bull World Health Organ. 2018;96:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer AD, Guerrero SM, Dean NE, Anderson KB, Stoddard ST, Perkins TA. Model-based estimates of chikungunya epidemiological parameters and outbreak risk from varied data types. Epidemics. 2023;45:100721. [DOI] [PubMed] [Google Scholar]
- 20.Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization; 2009. 4, LABORATORY DIAGNOSIS AND DIAGNOSTIC TESTS. Available from: https://www-ncbi-nlm-nihgov.laneproxy.stanford.edu/books/NBK143156/. [PubMed]
- 21.Krystosik A, Njoroge G, Odhiambo L, Forsyth JE, Mutuku F, LaBeaud AD. Solid wastes provide breeding sites, Burrows, and Food for Biological Disease vectors, and Urban Zoonotic reservoirs: a call to action for solutions-based research. Front Public Health. 2020;7:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getachew D, Tekie H, Gebre-Michael T, Balkew M, Mesfin A. Breeding sites of Aedes aegypti: potential dengue vectors in dire Dawa, East Ethiopia. Interdiscip Perspect Infect Dis. 2015;2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan A, Bisanzio D, Mutuku F, Ndenga B, Grossi-Soyster EN, Jembe Z, et al. Spatiotemporal overlapping of dengue, Chikungunya, and malaria infections in children in Kenya. BMC Infect Dis. 2023;23:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nosrat C, Altamirano J, Anyamba A, Caldwell JM, Damoah R, Mutuku F, et al. Impact of recent climate extremes on mosquito-borne disease transmission in Kenya. PLoS Negl Trop Dis. 2021;15:e0009182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsyth JE, Kempinsky A, Pitchik HO, Alberts CJ, Mutuku FM, Kibe L, et al. Larval source reduction with a purpose: Designing and evaluating a household- and school-based intervention in coastal Kenya. PLoS Negl Trop Dis. 2022;16:e0010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwakutwaa AS, Ngugi HN, Ndenga BA, Krystosik A, Ngari M, Abubakar LU, et al. Pupal productivity of larval habitats of Aedes aegypti in Msambweni, Kwale County, Kenya. Parasitol Res. 2023;122:801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsyth JE, Mutuku FM, Kibe L, Mwashee L, Bongo J, Egemba C, et al. Source reduction with a purpose: Mosquito ecology and community perspectives offer insights for improving household mosquito management in coastal Kenya. PLoS Negl Trop Dis. 2020;14:e0008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngugi HN, Mutuku FM, Ndenga BA, Musunzaji PS, Mbakaya JO, Aswani P, et al. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors. 2017;10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngugi HN, Nyathi S, Krystosik A, Ndenga B, Mbakaya JO, Aswani P, et al. Risk factors for Aedes aegypti household pupal persistence in longitudinal entomological household surveys in urban and rural Kenya. Parasit Vectors. 2020;13:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekhon DH, Minhas DS. A study of larval indices of Aedes and the risk for dengue outbreak. Sch Acad J Biosci. 2014;2(8):544–7.
- 31.Hayes JM, García-Rivera E, Flores-Reyna R, Suárez-Rangel G, Biggerstaff BJ, Rodríguez-Mata T, et al. Risk factors for infection during a severe dengue outbreak in El Salvador in 2000. Am J Trop Med Hyg. 2003;69:629–33. [PubMed] [Google Scholar]
- 32.Gustave J, Fouque F, Cassadou S, Leon L, Anicet G, Ramdini C, et al. Increasing role of Roof Gutters as Aedes aegypti (Diptera: Culicidae) breeding sites in Guadeloupe (French West Indies) and consequences on Dengue Transmission and Vector Control. J Trop Med. 2012;2012:249524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trewin BJ, Darbro JM, Zalucki MP, Jansen CC, Schellhorn NA, Devine GJ. Life on the margin: Rainwater tanks facilitate overwintering of the dengue vector, Aedes aegypti, in a sub-tropical climate. PLoS ONE. 2019;14:e0211167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukiato F, Wasserman RJ, Foo SC, Wilson RF, Cuthbert RN. The effects of temperature and shading on mortality and development rates of Aedes aegypti (Diptera: Culicidae). J Vector Ecol J Soc Vector Ecol. 2019;44:264–70. [DOI] [PubMed] [Google Scholar]
- 35.Lippi CA, Stewart-Ibarra AM, Endy TP, Abbott M, Cueva C, Heras F, et al. Exploring the utility of social-ecological and entomological risk factors for dengue infection as surveillance indicators in the dengue hyper-endemic city of Machala, Ecuador. PLoS Negl Trop Dis. 2021;15:e0009257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohn M. Occurrence of Aedes aegypti (L.) and Culex quinquefasciatus Say (Diptera, Culicidae) in houses of different constructions in Phnom Penh, Kampuchea. Folia Parasitol (Praha). 1991;38:75–8. [PubMed] [Google Scholar]
- 37.Manrique-Saide P, Herrera-Bojórquez J, Villegas-Chim J, Puerta-Guardo H, Ayora-Talavera G, Parra-Cardeña M, et al. Protective effect of house screening against indoor Aedes aegypti in Mérida, Mexico: a cluster randomised controlled trial. Trop Med Int Health TM IH. 2021;26:1677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker JL, Gadgil GU. East Asia and Pacific cities: Expanding opportunities for the Urban Poor. Washington, DC: World Bank; 2017. [Google Scholar]
- 39.Mavian C, Dulcey M, Munoz O, Salemi M, Vittor AY, Capua I. Islands as hotspots for emerging Mosquito-Borne viruses: a one-health perspective. Viruses. 2018;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prayitno A, Taurel A-F, Nealon J, Satari HI, Karyanti MR, Sekartini R, et al. Dengue seroprevalence and force of primary infection in a representative population of urban dwelling Indonesian children. PLoS Negl Trop Dis. 2017;11:e0005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, et al. Thermal biology of mosquito-borne disease. Ecol Lett. 2019;22:1690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hii YL, Zhu H, Ng N, Ng LC, Rocklöv J. Forecast of Dengue Incidence using temperature and rainfall. PLoS Negl Trop Dis. 2012;6:e1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell JM, LaBeaud AD, Lambin EF, Stewart-Ibarra AM, Ndenga BA, Mutuku FM, et al. Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents. Nat Commun. 2021;12:1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodysill JR, Russell JM, Vuille M, Dee S, Lunghino B, Bijaksana S. La Niña-driven flooding in the Indo-Pacific warm pool during the past millennium. Quat Sci Rev. 2019;225:106020. [Google Scholar]
- 45.Ummenhofer CC, D’Arrigo RD, Anchukaitis KJ, Buckley BM, Cook ER. Links between Indo-Pacific climate variability and drought in the Monsoon Asia Drought Atlas. Clim Dyn. 2013;40:1319–34. [Google Scholar]
- 46.Statistics Indonesia. Statistical yearbook of Indonesia 2014. Statistics Indonesia; Jakarta. 2014. https://media.neliti.com/media/publications/49018-ID-statistik-indonesia-2014.pdf.
- 47.Leder K, Openshaw JJ, Allotey P, Ansariadi A, Barker SF, Burge K, et al. Study design, rationale and methods of the Revitalising Informal settlements and their environments (RISE) study: a cluster randomised controlled trial to evaluate environmental and human health impacts of a water-sensitive intervention in informal settlements in Indonesia and Fiji. BMJ Open. 2021;11:e042850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitaker HJ, Farrington CP. Estimation of infectious disease parameters from serological survey data: the impact of regular epidemics. Stat Med. 2004;23:2429–43. [DOI] [PubMed] [Google Scholar]
- 49.Tedijanto C, Solomon AW, Martin DL, Nash SD, Keenan JD, Lietman TM, et al. Monitoring transmission intensity of trachoma with serology. Nat Commun. 2023;14:3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasmono RT, Taurel A-F, Prayitno A, Sitompul H, Yohan B, Hayati RF, et al. Dengue virus serotype distribution based on serological evidence in pediatric urban population in Indonesia. PLoS Negl Trop Dis. 2018;12:e0006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sasmono RT, Johar E, Yohan B, Ma’roef CN, Pronyk P, Hadinegoro SR, et al. Spatiotemporal heterogeneity of Zika Virus Transmission in Indonesia: Serosurveillance Data from a Pediatric Population. Am J Trop Med Hyg. 2021;104:2220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kucharski AJ, Kama M, Watson CH, Aubry M, Funk S, Henderson AD, et al. Using paired serology and surveillance data to quantify dengue transmission and control during a large outbreak in Fiji. eLife. 2018;7:e34848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthews RJ, Kaluthotage I, Russell TL, Knox TB, Horwood PF, Craig AT. Arboviral Disease outbreaks in the Pacific Islands Countries and Areas, 2014 to 2020: a systematic literature and document review. Pathogens. 2022;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore TC, Brown HE. Estimating Aedes aegypti (Diptera: Culicidae) Flight Distance: Meta-Data Analysis. J Med Entomol. 2022;59:1164–70. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee S, Aditya G, Saha GK. Household disposables as breeding habitats of dengue vectors: linking wastes and public health. Waste Manag. 2013;33:233–9. [DOI] [PubMed] [Google Scholar]
- 56.Banerjee S, Aditya G, Saha GK. Household Wastes as Larval habitats of Dengue vectors: comparison between Urban and Rural areas of Kolkata, India. PLoS One. 2015;10:e0138082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dieng H, Satho T, Meli NKKB, Abang F, Nolasco-Hipolito C, Hakim H, et al. Occurrence of sweet refuse at disposal sites: rainwater retention capacity and potential breeding opportunities for Aedes aegypti. Environ Sci Pollut Res Int. 2018;25:13833–43. [DOI] [PubMed] [Google Scholar]
- 58.Shaikh OA, Baig MT, Tahir S, Parekh A-DE, Nashwan AJ. Dengue outbreak following unprecedented flooding in Pakistan. Hyg Environ Health Adv. 2023;7:100076. [Google Scholar]
- 59.UNICEF Peru Flash Update No. 1 (Flooding and Dengue Outbreak) – 01 Dec 2023–01 March 2024 - Peru | ReliefWeb. 2024. https://reliefweb.int/report/peru/unicef-peru-flash-update-no-1-flooding-and-dengue-outbreak-01-dec-2023-01-march-2024. Accessed 16 Jul 2024.
- 60.Post-Flood Dengue Outbreak Puts UAE Migrant Workers at Heightened Risk. – FairSquare. https://fairsq.org/post-flood-dengue-outbreak-puts-uae-migrant-workers-at-heightened-risk/. Accessed 16 Jul 2024.
- 61.Coalson JE, Anderson EJ, Santos EM, Madera Garcia V, Romine JK, Dominguez B, et al. The Complex Epidemiological relationship between flooding events and human outbreaks of Mosquito-Borne diseases: a scoping review. Environ Health Perspect. 2021;129:96002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Japanese Encephalitis | CDC Yellow Book. 2024. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/japanese-encephalitis. Accessed 9 Nov 2024.
- 63.Rosser JI, Tarpenning MS, Bramante JT, Tamhane A, Chamberlin AJ, Mutuku PS, et al. Development of a trash classification system to map potential Aedes aegypti breeding grounds using unmanned aerial vehicle imaging. Environ Sci Pollut Res. 2024;31:41107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
At the end of the research trial, deidentified data will be stored on secure Monash infrastructure and made available, upon application, as approved by the ethics committees. Researchers interested in accessing data may contact the RISE Program (10.26180/ctjf-vf69).