Abstract
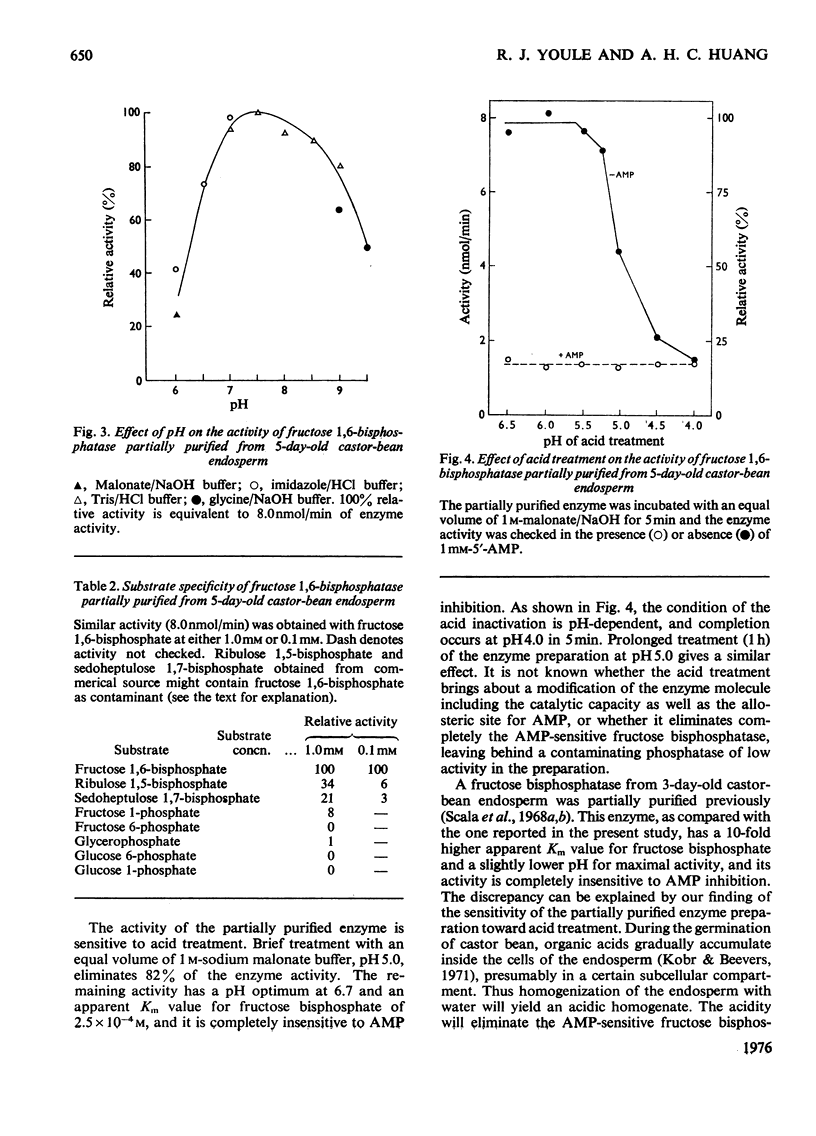
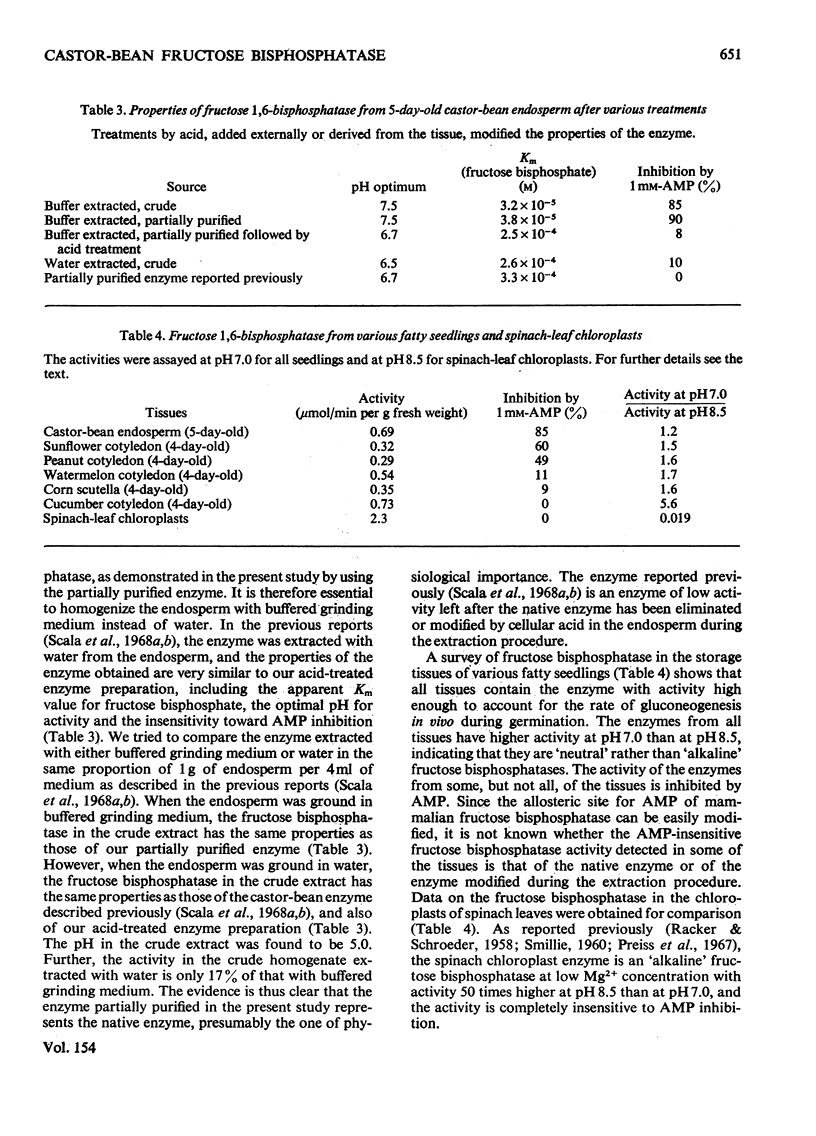
1. The activity of fructose 1,6-bisphosphatase (EC 3.1.3.11) in the fatty endosperm of castor bean (Ricinus communis) increases 25-fold during germination and then declines. The developmental pattern follows that of catalase, a marker enzyme for gluconeogenesis in this tissue. 2. The enzyme at its peak of development was partially purified, and its properties were studied. It has an optimal activity at neutral pH (7.0-8.0). The apparent Km value for fructose 1,6-bisphosphate is 3.8 X 10(-5) M. The activity is inhibited by AMP allosterically with an apparent Ki value of 2.2 X 10(-4) M. The enzyme hydrolyses fructose 1,6-bisphosphate and not ribulose 1,5-bisphosphate or sedoehptulose 1,7-bisphosphate. 3. Treatment of the partially purified enzyme with acid leads to an 80% decrease in activity. The remaining activity is insensitive to AMP and has optimal activity at pH 6.7 and a high apparent Km value (2.5 X 10(-4) M) for fructose 1.6-bisphosphate. Enzyme extracted from the tissue with water instead of buffer has a similar modification. The effect of acid explains the discrepancies between this report and previous ones on the properties of the enzyme in this tissue. 4. The storage tissues of various fatty seedlings all contain a 'neutral' fructose 1,6-bisphosphatase. The activities of the enzyme from some of the tissues are inhibited by AMP. 5. The properties of the enzyme in fatty seedlings and in green leaves are discussed in comparison with that in animal tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Enzymes of glycerol metabolism in the storage tissues of Fatty seedlings. Plant Physiol. 1975 Mar;55(3):555–558. doi: 10.1104/pp.55.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratowich N., Mendicino J. Role of enzyme interactions in the regulation of gluconeogenesis. Modification of the binding properties of fructose 1,6-diphosphatase by adenosine monophosphate, adenosine triphosphate, and fructose 1,6-diphosphate. J Biol Chem. 1974 Sep 10;249(17):5485–5494. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus C. J., Geller A. M., Byrne W. L. Studies on bovine hepatic fructose 1,6-diphosphatase. Substrate inhibition and the kinetic mechanism. J Biol Chem. 1973 Dec 25;248(24):8567–8573. [PubMed] [Google Scholar]
- OGSTON A. G., PHELPS C. F. Exclusion of inulin from solutions of hyaluronic acid. Nature. 1960 Sep 17;187:1024–1024. doi: 10.1038/1871024a0. [DOI] [PubMed] [Google Scholar]
- Osmond C. B., Akazawa T., Beevers H. Localization and properties of ribulose diphosphate carboxylase from castor bean endosperm. Plant Physiol. 1975 Feb;55(2):226–230. doi: 10.1104/pp.55.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- RACKER E., SCHROEDER E. A. The reductive pentose phosphate cycle. II. Specific C-1 phosphatases for fructose 1,6-diphosphate and sedoheptulose 1,7-diphosphate. Arch Biochem Biophys. 1958 Apr;74(2):326–344. doi: 10.1016/0003-9861(58)90004-3. [DOI] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Preparation of cellular plant organelles from spinach leaves. Arch Biochem Biophys. 1970 Oct;140(2):398–407. doi: 10.1016/0003-9861(70)90081-0. [DOI] [PubMed] [Google Scholar]
- Scala J., Patrick C., Macbeth G. Castor bean FDPase from endosperm tissue: properties and partial purification. Life Sci. 1968 Apr 15;7(8):407–412. doi: 10.1016/0024-3205(68)90041-6. [DOI] [PubMed] [Google Scholar]
- Scala J., Patrick C., Macbeth G. FDPases of the castor bean endosperm and leaf: properties and partial purification. Arch Biochem Biophys. 1968 Sep 20;127(1):576–584. doi: 10.1016/0003-9861(68)90265-8. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Stachow C. S. Fructose 1,6-diphosphatase from Rhodopseudomonas palustris. I. Purification and properties. Arch Biochem Biophys. 1972 Sep;152(1):1–12. doi: 10.1016/0003-9861(72)90186-5. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Luby L. J. Studies on the effect of fructose diphosphatase on phosphofructokinase. J Biol Chem. 1974 Jul 25;249(14):4562–4570. [PubMed] [Google Scholar]


