Abstract
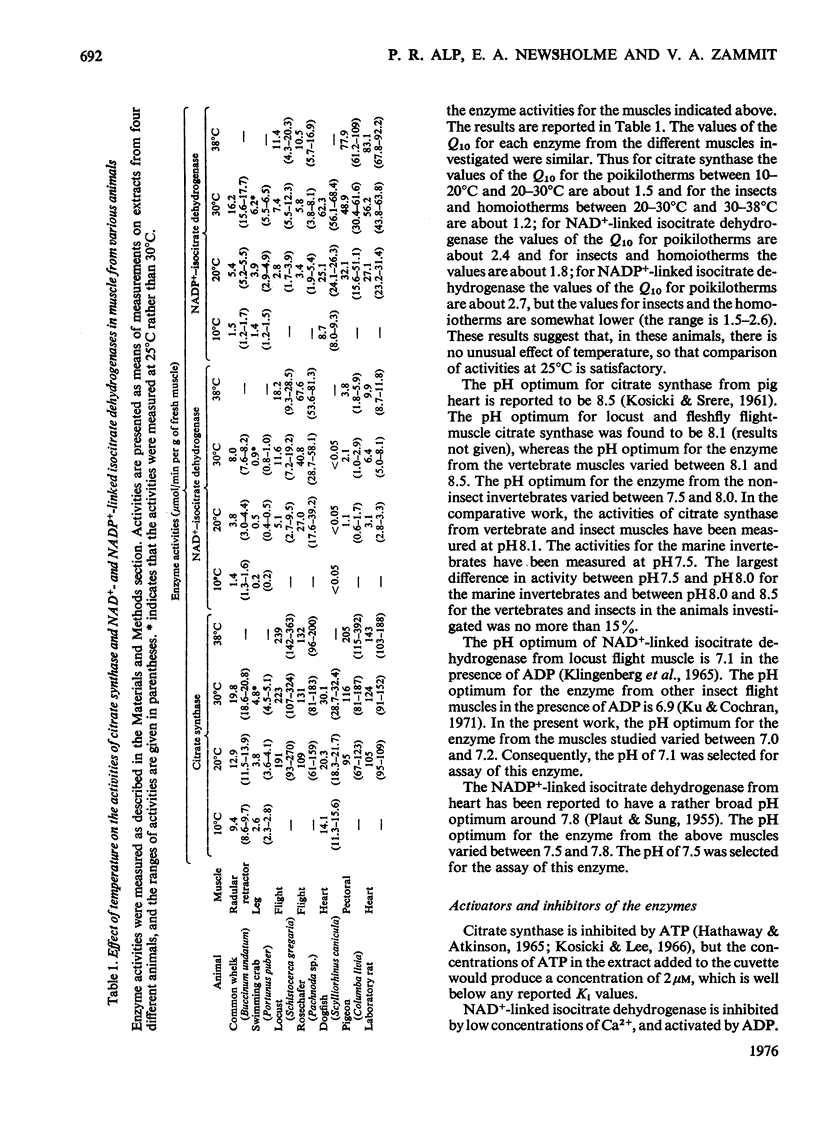
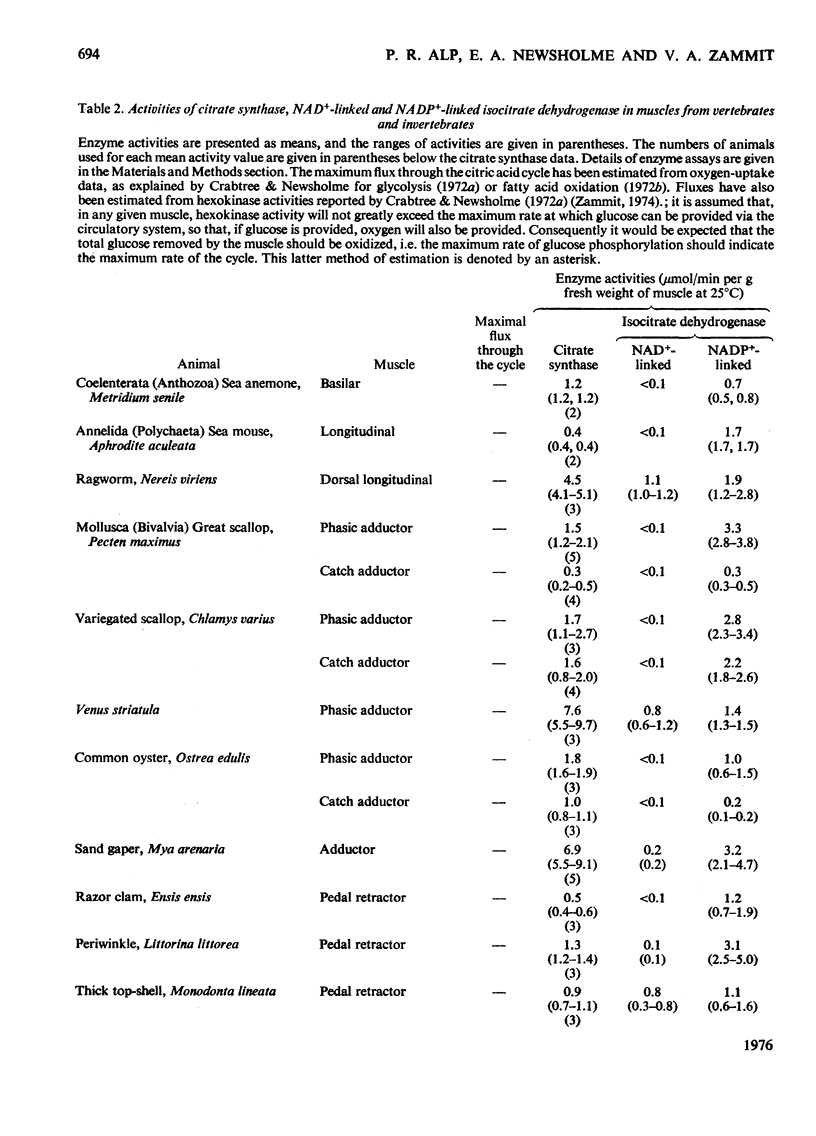
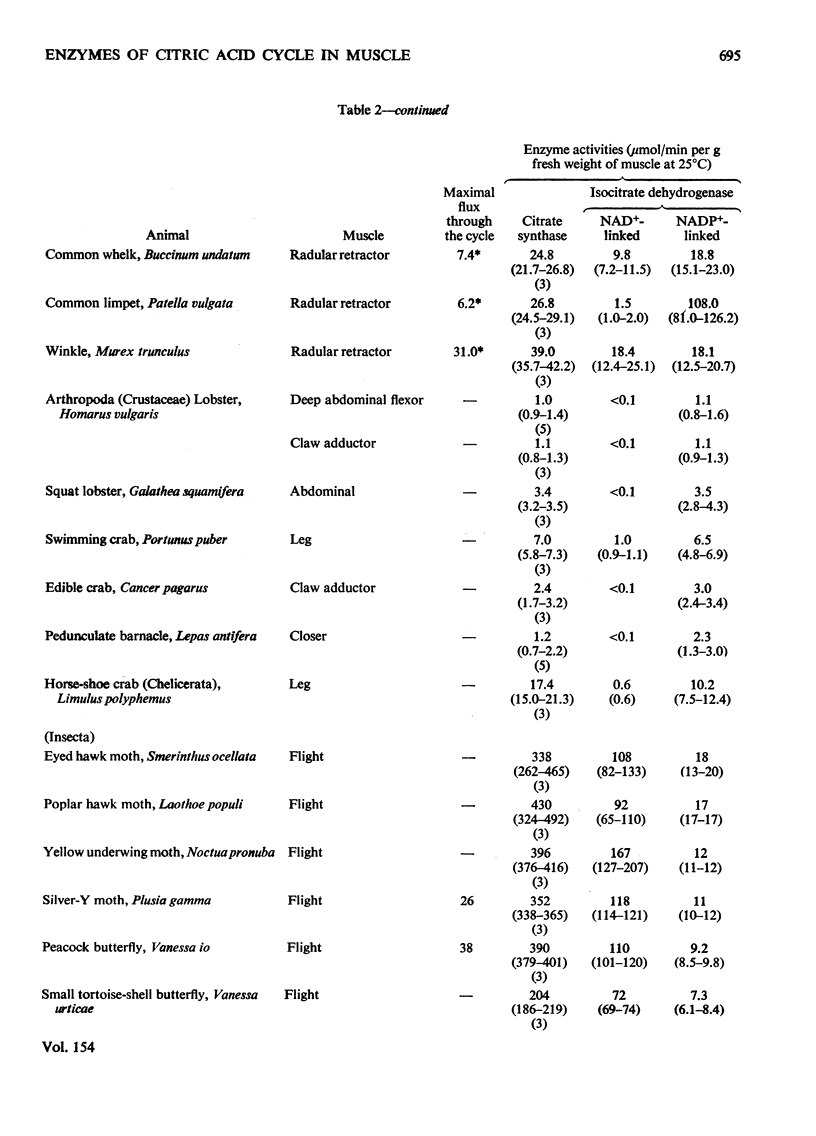
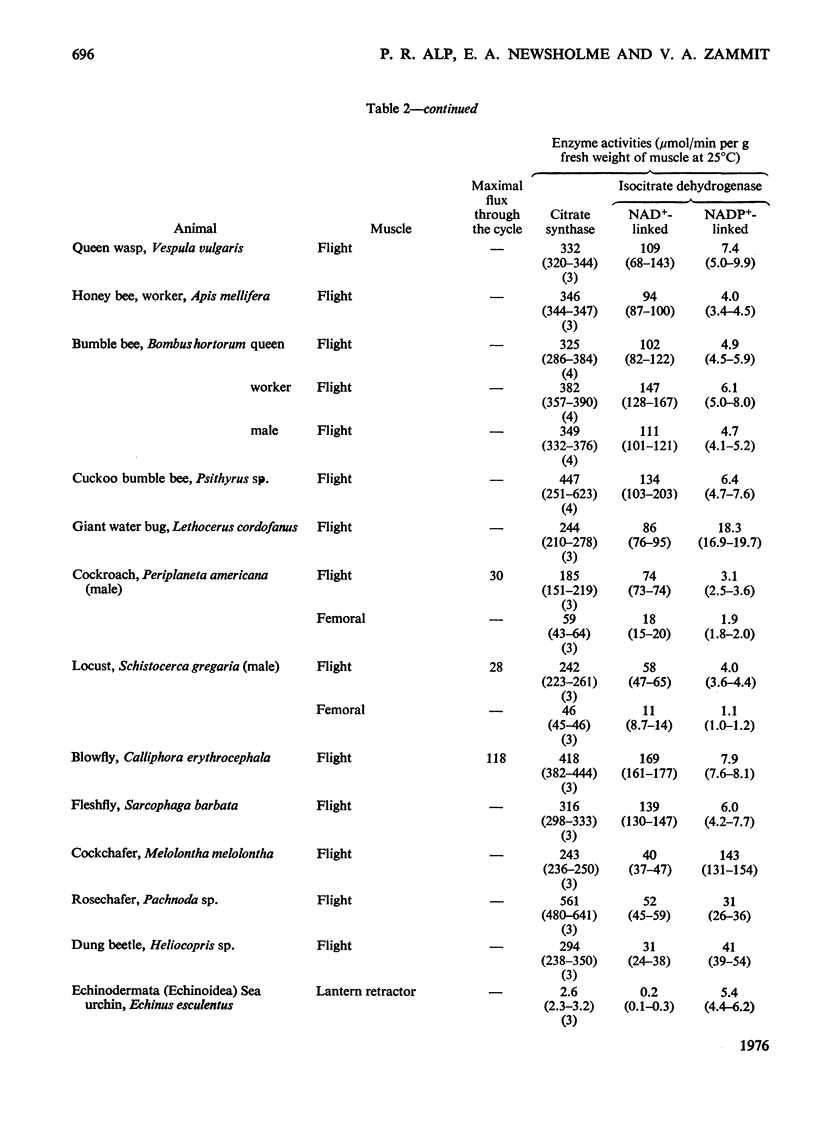
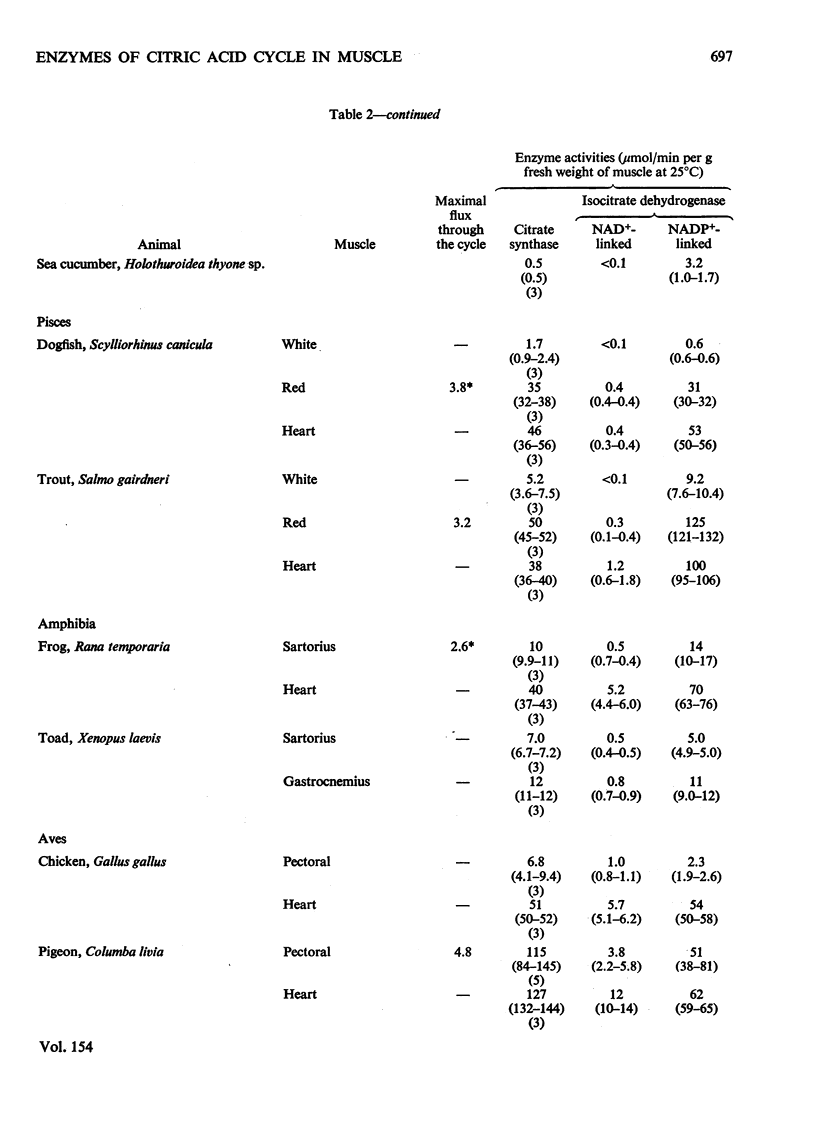
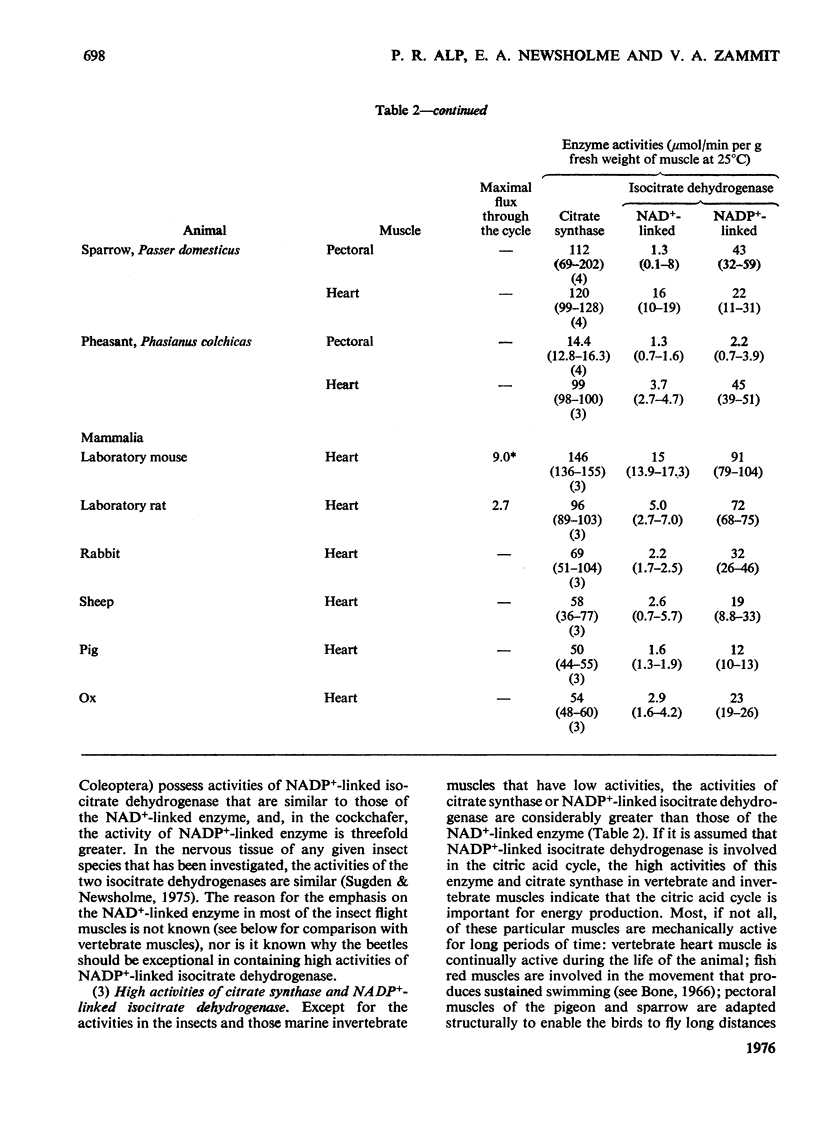
1. The activities of citrate synthase, NAD+-linked and NADP+-linked isocitrate dehydrogenase were measured in muscles from a large number of animals, in order to provide some indication of the importance of the citric acid cycle in these muscles. According to the differences in enzyme activities, the muscles can be divided into three classes. First, in a number of both vertebrate and invertebrate muscles, the activities of all three enzymes are very low. It is suggested that either the muscles use energy at a very low rate or they rely largely on anaerobic glycolysis for higher rates of energy formation. Second, most insect flight muscles contain high activities of citrate synthase and NAD+-linked isocitrate dehydrogenase, but the activities of the NADP+-linked enzyme are very low. The high activities indicate the dependence of insect flight on energy generated via the citric acid cycle. The flight muscles of the beetles investigated contain high activities of both isocitrate dehydrogenases. Third, other muscles of both vertebrates and invertebrates contain high activities of citrate synthase and NADP+-liniked isocitrate dehydrogenase. Many, if not all, of these muscles are capable of sustained periods of mechanical activity (e.g. heart muscle, pectoral muscles of some birds). Consequently, to support this activity fuel must be supplied continually to the muscle via the circulatory system which, in most animals, also transports oxygen so that energy can be generated by complete oxidation of the fuel. It is suggested that the low activities of NAD+-linked isocitrate dehydrogenase in these muscles may be involved in oxidation of isocitrate in the cycle when the muscles are at rest. 2. A comparison of the maximal activities of the enzymes with the maximal flux through the cycle suggests that, in insect flight muscle, NAD+-linked isocitrate dehydrogenase catalyses a non-equilibrium reaction and citrate synthease catalyses a near-equilibrium reaction. In other muscles, the enzyme-activity data suggest that both citrate synthase and the isocitrate dehydrogenase reactions are near-equilibrium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHEN R. F., PLAUT G. W. ACTIVATION AND INHIBITION OF DPN-LINKED ISOCITRATE DEHYDROGENASE OF HEART BY CERTAIN NUCLEOTIDES. Biochemistry. 1963 Sep-Oct;2:1023–1032. doi: 10.1021/bi00905a020. [DOI] [PubMed] [Google Scholar]
- Cox G. F., Davies D. D. Nicotinamide-adenine dinucleotide-specific isocitrate dehydrogenase from pea mitochondria. Purification and properties. Biochem J. 1967 Nov;105(2):729–734. doi: 10.1042/bj1050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of lipases and carnitine palmitoyltransferase in muscles from vertebrates and invertebrates. Biochem J. 1972 Dec;130(3):697–705. doi: 10.1042/bj1300697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEBELL H., KLINGENBERG M. DPN-SPEZIFISCHE ISOCITRAT-DEHYDROGENASE DER MITOCHONDRIEN. I. KINETISCHE EIGENSSCHAFTEN, VORKOMMEN UND FUNKTION DER DPN-SPEZIFISCHEN ISOCITRAT-DEHYDROGENASE. Biochem Z. 1964 Sep 28;340:441–464. [PubMed] [Google Scholar]
- Hathaway J. A., Atkinson D. E. Kinetics of regulatory enzymes: effect of adenosine triphosphate on yeast citrate synthase. Biochem Biophys Res Commun. 1965 Sep 8;20(5):661–665. doi: 10.1016/0006-291x(65)90452-3. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG M., GOEBELL H., WENSKE G. DPN-SPECIFIC ISOCITRATE-DEHYDROGENASE OF MITOCHONDRIA. II. PH-DEPENDENCE OF THE KINETICS AND THE MECHANISM OF ACTIVATION. Biochem Z. 1965 Feb 8;341:199–223. [PubMed] [Google Scholar]
- KOSICKI G. W., SRERE P. A. Kinetic studies on the citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2560–2565. [PubMed] [Google Scholar]
- Kosicki G. W., Lee L. P. Effect of divalent metal ions on nucleotide inhibition of pig heart citrate synthase. J Biol Chem. 1966 Aug 10;241(15):3571–3574. [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg J. C. Smooth muscle tone. Physiol Rev. 1971 Jan;51(1):201–248. doi: 10.1152/physrev.1971.51.1.201. [DOI] [PubMed] [Google Scholar]
- Smith Colleen M., Williamson John R. Inhibition of citrate synthase by succinyl-CoA and other metabolites. FEBS Lett. 1971 Oct 15;18(1):35–38. doi: 10.1016/0014-5793(71)80400-3. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Newsholme E. A. Activities of citrate synthase, NAD+-linked and NADP+-linked isocitrate dehydrogenases, glutamate dehydrogenase, aspartate aminotransferase and alanine aminotransferase in nervous tissues from vertebrates and invertebrates. Biochem J. 1975 Jul;150(1):105–111. doi: 10.1042/bj1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Newsholme E. A. Activities of hexokinase, phosphofructokinase, 3-oxo acid coenzyme A-transferase and acetoacetyl-coenzyme A thiolase in nervous tissue from vertebrates and invertebrates. Biochem J. 1973 May;134(1):97–101. doi: 10.1042/bj1340097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan H., Newsholme E. A. The effects of Ca(2+) and ADP on the activity of NAD-linked isocitrate dehydrogenase of muscle. FEBS Lett. 1969 Oct 21;5(2):124–126. doi: 10.1016/0014-5793(69)80311-x. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Newsholme E. A. Effects of calcium ions and adenosine diphosphate on the activities of NAD+-linked isocitrate dehydrogenase from the radular muscles of the whelk and flight muscles of insects. Biochem J. 1976 Mar 15;154(3):677–687. doi: 10.1042/bj1540677. [DOI] [PMC free article] [PubMed] [Google Scholar]


