Abstract
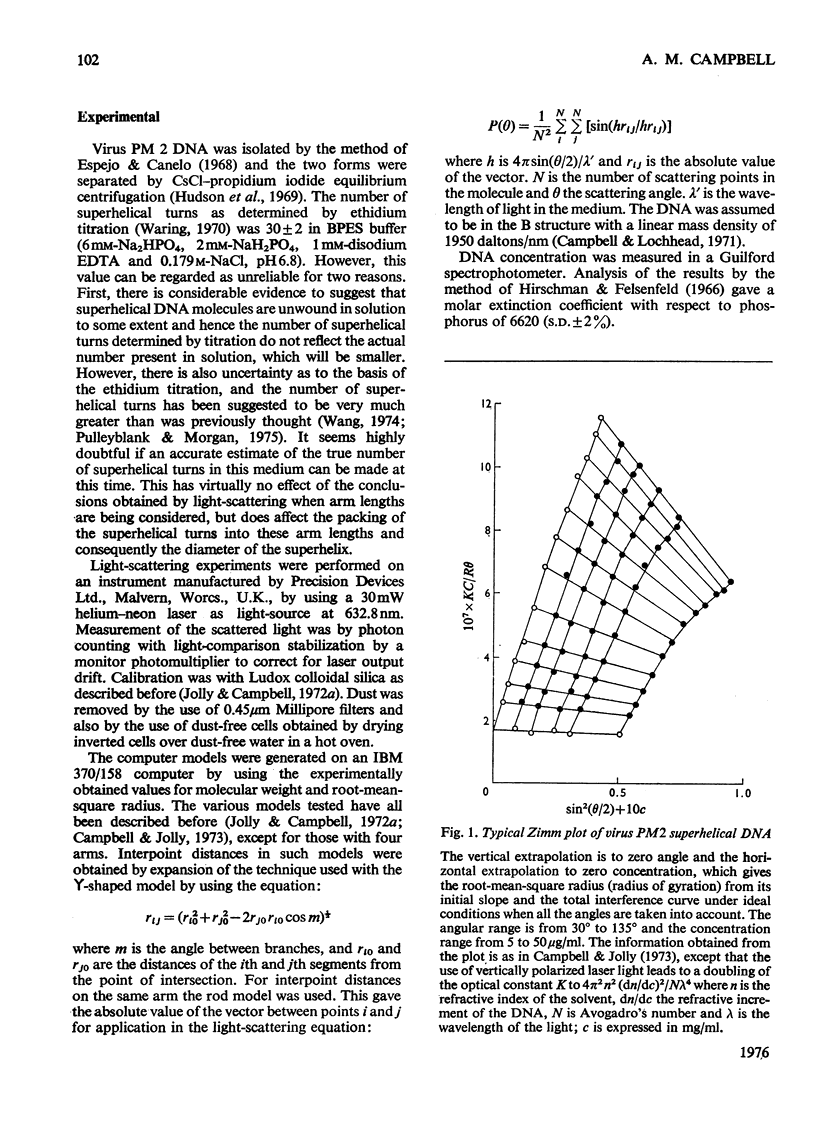
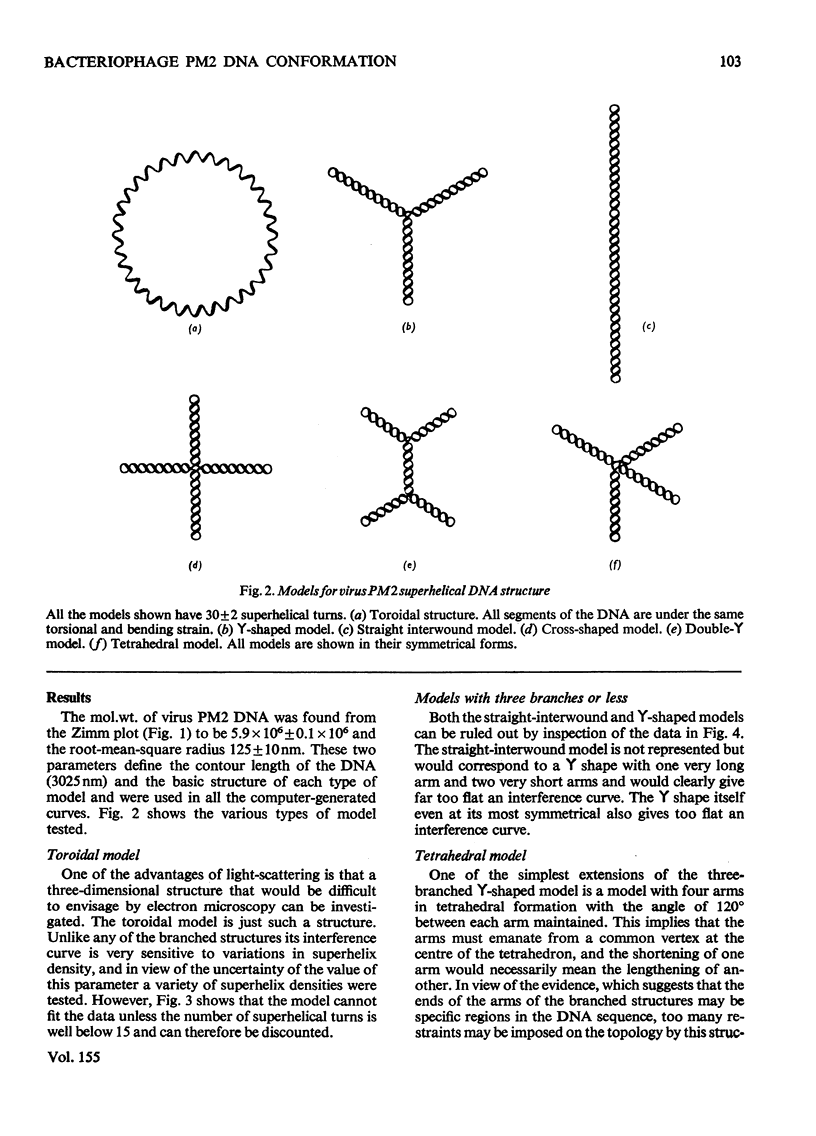
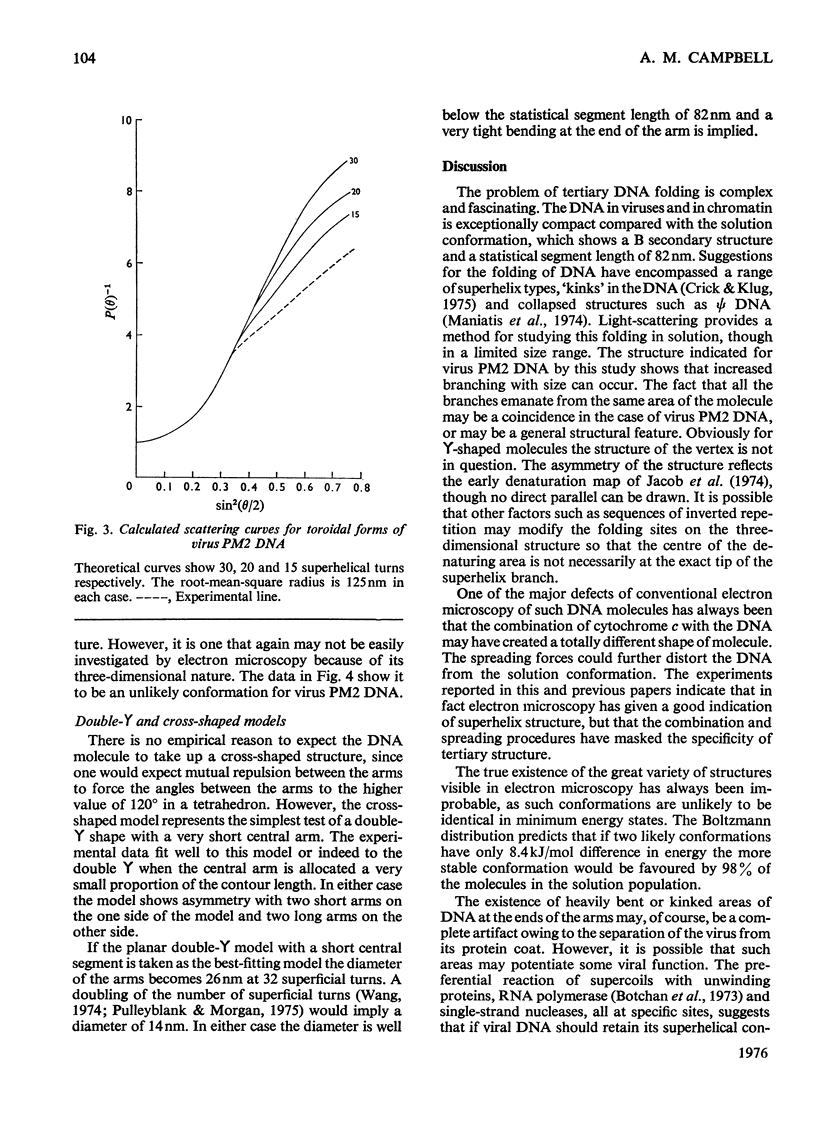
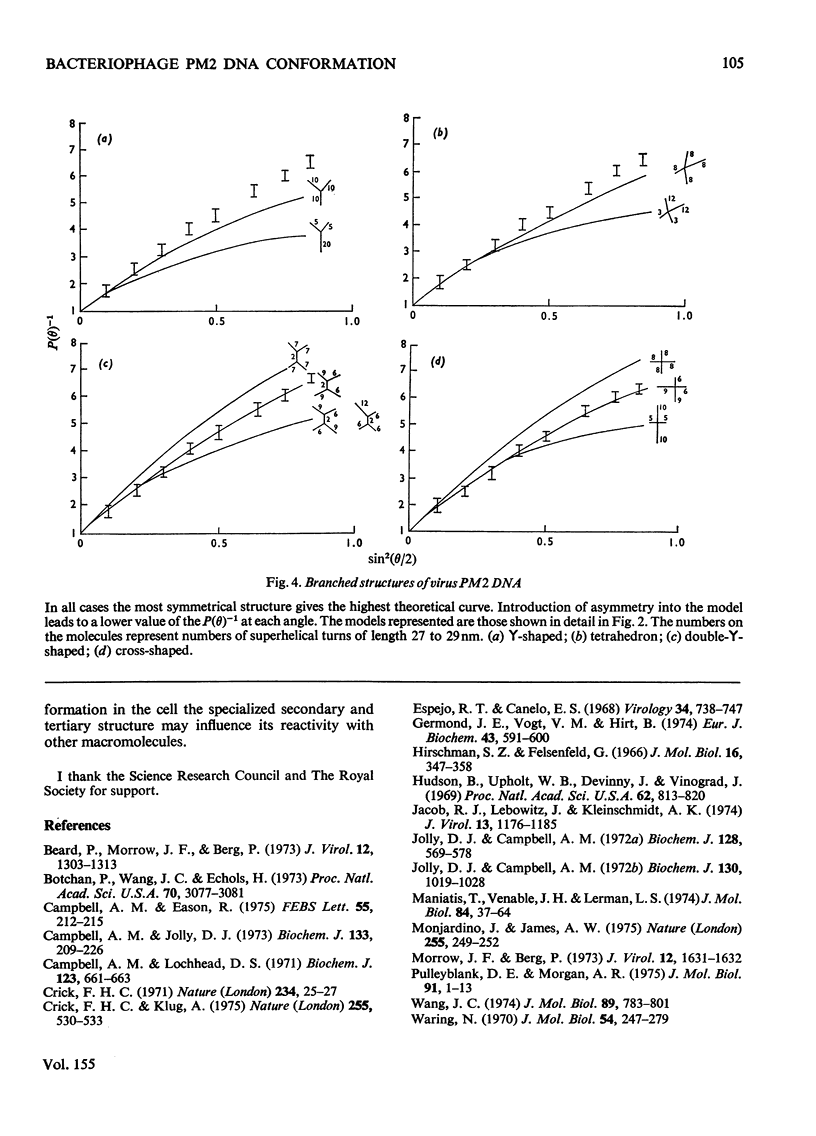
Laser light-scattering studies of bacteriophage PM2 DNA showed the molecule to have mol.wt. 5.9 X 10(6) and root-mean -square radius 125 nm at an ionic strength of 0.2 mol/litre. Computer-generated curves compatible with these data were compared with the experimental interference curve for several structural models of the molecules. The data fit best to an asymmetric four-armed planar molecule in which all four arms emerge from or close to the one area of the molecule. This contrasts with the smaller DNA molecules investigated, which have shown a three-armed molecule, whose symmetry varies with primary structure.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan P., Wang J. C., Echols H. Effect of circularity and superhelicity on transcription from bacteriophagelambda DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3077–3081. doi: 10.1073/pnas.70.11.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. M., Jolly D. J. Light-scattering studies on supercoil unwinding. Biochem J. 1973 Jun;133(2):209–226. doi: 10.1042/bj1330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. M., Lochhead D. S. Optical rotatory dispersion and circular dichroism of superhelical deoxyribonucleic acid. Biochem J. 1971 Jul;123(4):661–663. doi: 10.1042/bj1230661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Eason R. Effects of DNA primary structure on tertiary structure. FEBS Lett. 1975 Jul 15;55(1):212–215. doi: 10.1016/0014-5793(75)80994-x. [DOI] [PubMed] [Google Scholar]
- Crick F. H., Klug A. Kinky helix. Nature. 1975 Jun 12;255(5509):530–533. doi: 10.1038/255530a0. [DOI] [PubMed] [Google Scholar]
- Crick F. General model for the chromosomes of higher organisms. Nature. 1971 Nov 5;234(5323):25–27. doi: 10.1038/234025a0. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Hirschman S. Z., Felsenfeld G. Determination of DNA composition and concentration by spectral analysis. J Mol Biol. 1966 Apr;16(2):347–358. doi: 10.1016/s0022-2836(66)80178-x. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. J., Lebowitz J., Kleinschmidt A. K. Locating interrupted hydrogen bonding in the secondary structure of PM2 circular DNA by comparative denaturation mapping. J Virol. 1974 Jun;13(6):1176–1185. doi: 10.1128/jvi.13.6.1176-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Campbell A. M. Light-scattering studies on deoxyribonucleic acid flexibility. The solution properties of a small circular deoxyribonucleic acid molecule. Biochem J. 1972 Dec;130(4):1019–1028. doi: 10.1042/bj1301019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Campbell A. M. The three-dimensional structure of supercoiled deoxyribonucleic acid in solution. Evidence obtained from the angular distribution of scattered light. Biochem J. 1972 Jul;128(3):569–578. doi: 10.1042/bj1280569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Venable J. H., Jr, Lerman L. S. The structure of psi DNA. J Mol Biol. 1974 Mar 25;84(1):37–64. doi: 10.1016/0022-2836(74)90211-3. [DOI] [PubMed] [Google Scholar]
- Monjardino J., James A. W. Denaturation of polyoma DNA by phage T4 gene 32 protein. Nature. 1975 May 15;255(5505):249–252. doi: 10.1038/255249a0. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Location of the T4 gene 32 protein binding site on simian virus 40 DNA. J Virol. 1973 Dec;12(6):1631–1632. doi: 10.1128/jvi.12.6.1631-1632.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]


