Abstract
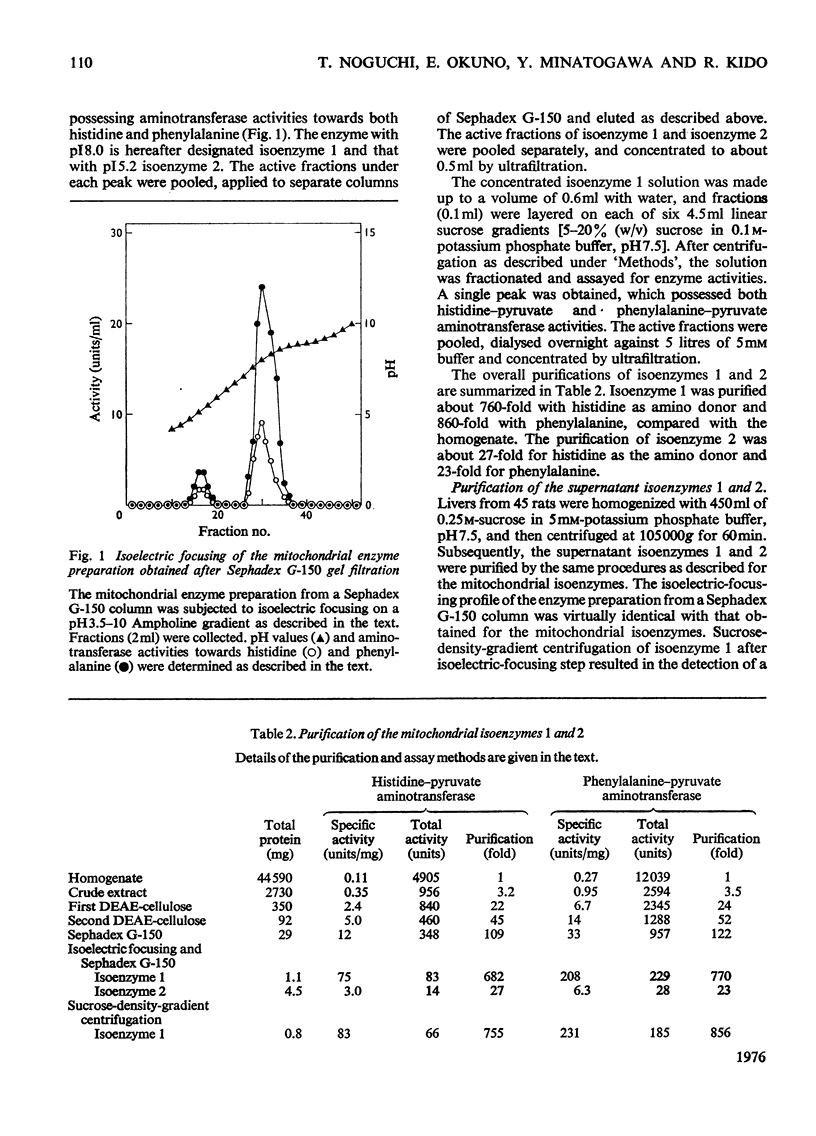
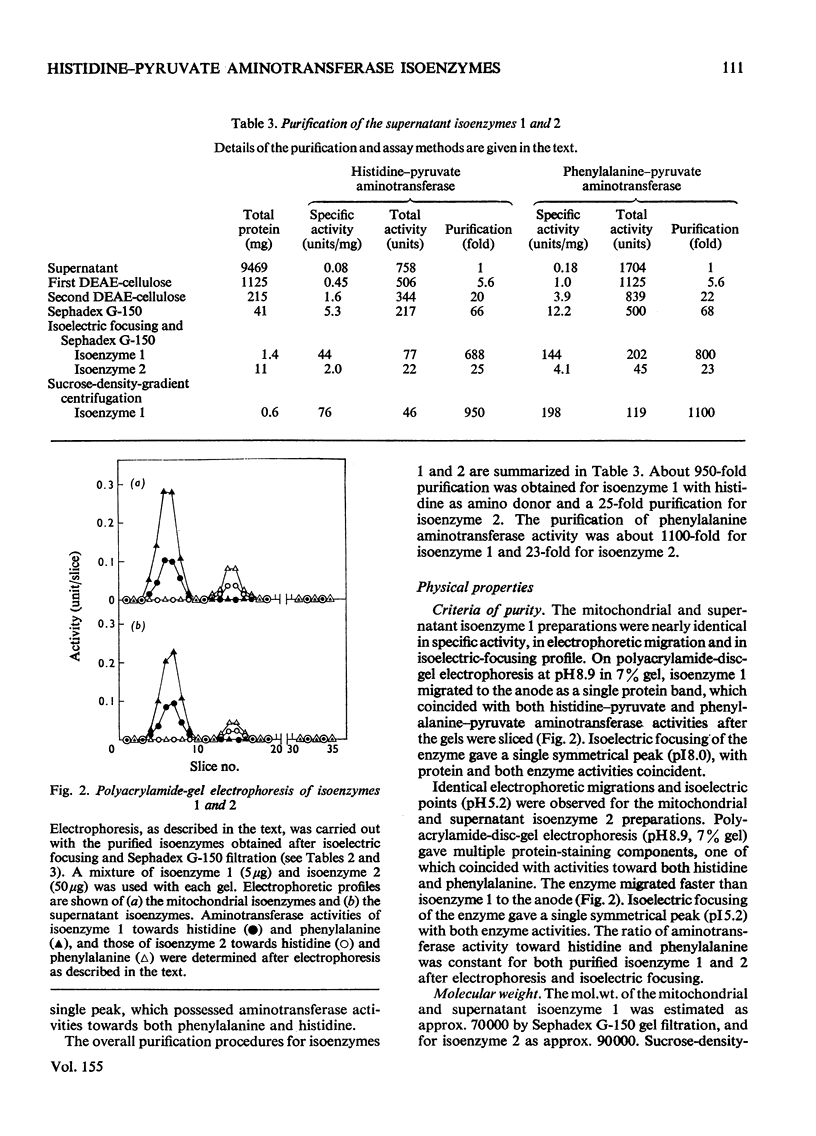
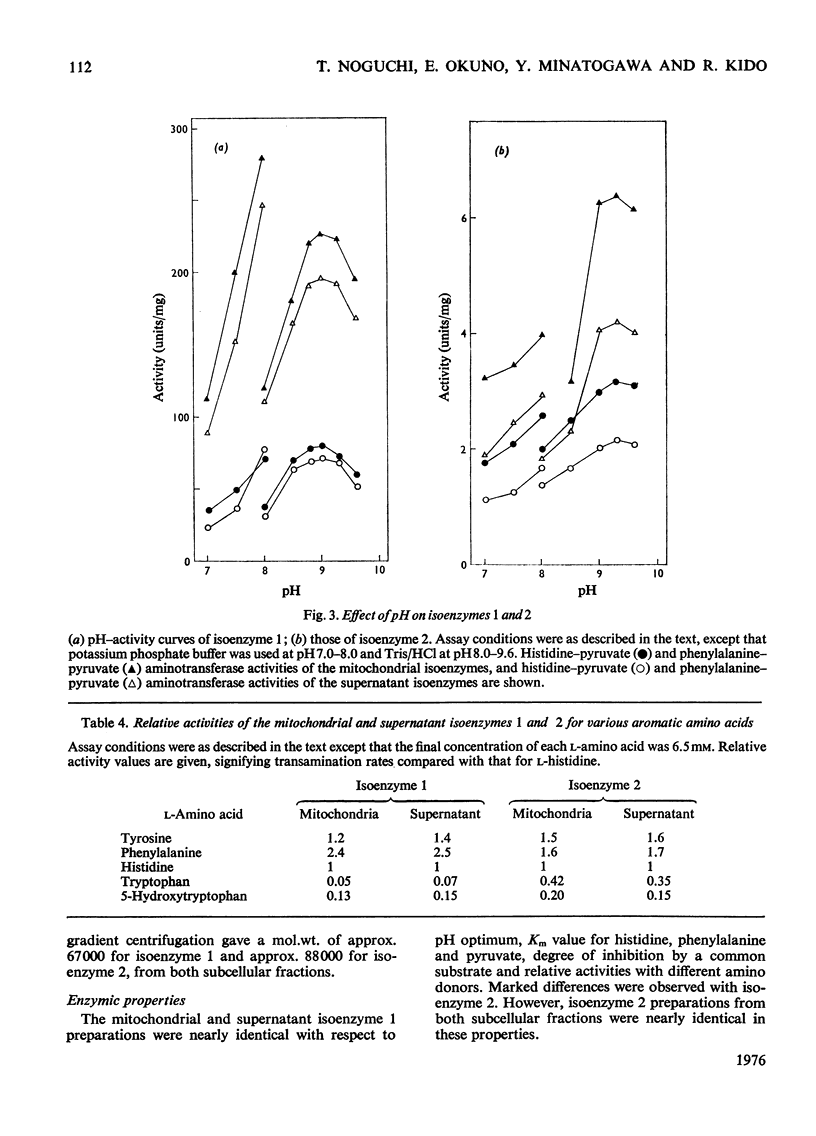
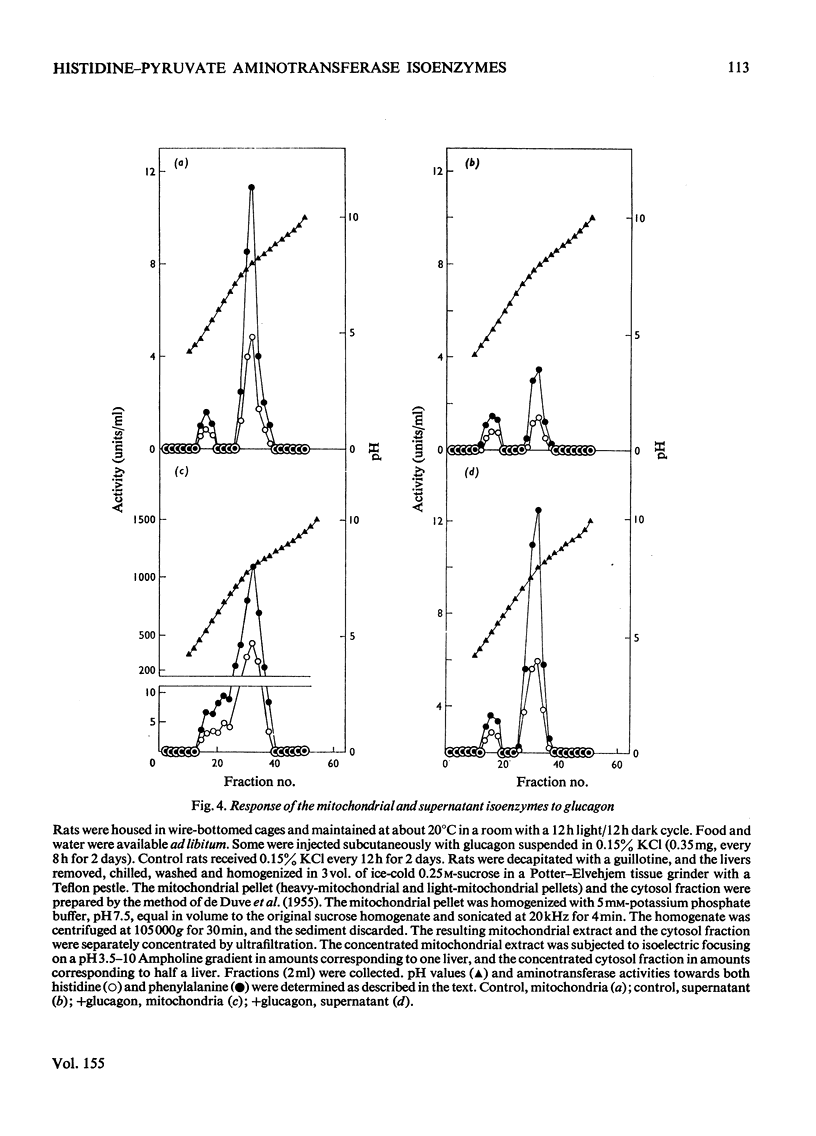
1. Histidine-pyruvate aminotransferase (isoenzyme 1) was purified to homogeneity from the mitochondrial and supernatant fractions of rat liver, as judged by polyacrylamide-gel electrophoresis and isolectric focusing. Both enzyme preparations were remarkably similar in physical and enzymic properties. Isoenzyme 1 had pI8.0 and a pH optimum of 9.0. The enzyme was active with pyruvate as amino acceptor but not with 2-oxoglutarate, and utilized various aromatic amino acids as amino donors in the following order of activity: phenylalanine greater than tyrosine greater than histidine. Very little activity was found with tryptophan and 5-hydroxytryptophan. The apparent Km values were about 2.6mM for histidine and 2.7 mM for phenylalanine. Km values for pyruvate were about 5.2mM with phenylalanine as amino donor and 1.1mM with histidine. The aminotransferase activity of the enzyme towards phenylalanine was inhibited by the addition of histidine. The mol.wt. determined by gel filtration and sucrose-density-gradient centrifugation was approx. 70000. The mitochondrial and supernatant isoenzyme 1 activities increased approximately 25-fold and 3.2-fold respectively in rats repeatedly injected with glucagon for 2 days. 2. An additional histidine-pyruvate aminotransferase (isoenzyme 2) was partially purified from both the mitochondrial and supernatant fractions of rat liver. Nearly identical properties were observed with both preparations. Isoenzyme 2 had pI5.2 and a pH optimum of 9.3. The enzyme was specific for pyruvate and did not function with 2-oxoglutarate. The order of effectiveness of amino donors was tyrosine = phenylalanine greater than histidine greater than tryptophan greater than 5-hydroxytryptophan. The apparent Km values for histidine and phenylalanine were about 0.51 and 1.8 mM respectively. Km values for pyruvate were about 3.5mM with phenylalanine and 4.7mM with histidine as amino donors. Histidine inhibited phenylalanine aminotransferase activity of the enzyme. Gel filtration and sucrose-density-gradient centrifugation yielded a mol.wt. of approx. 90000. Neither the mitochondrial nor the supernatant isoenzyme 2 activity was elevated by glucagon injection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. B., Civen M. Control of rat liver aromatic amino acid transaminases by glucagon and insulin. Endocrinology. 1969 Feb;84(2):381–385. doi: 10.1210/endo-84-2-381. [DOI] [PubMed] [Google Scholar]
- Civen M., Trimmer B. M., Brown C. B. The induction of hepatic tyrosine alpha-ketoglutarate and phenylalanine pyruvate transaminases by glucagon. Life Sci. 1967 Jun 15;6(12):1331–1338. doi: 10.1016/0024-3205(67)90029-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. W., Baker J. C., Snoddy H. D. Elevation of hepatic phenylalanine: pyruvate aminotransferase by dibutyryl cyclic AMP in rats. Biochem Med. 1974 Mar;9(3):301–308. doi: 10.1016/0006-2944(74)90064-7. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Snoddy H. D., Wolen R. L., Coburn S. P., Sirlin E. M. Effect of glucagon and p-chlorophenylalanine on hepatic enzymes that metabolize phenylalanine. Adv Enzyme Regul. 1972;10:153–167. doi: 10.1016/0065-2571(72)90012-x. [DOI] [PubMed] [Google Scholar]
- JACOBY G. A., LADU B. N. STUDIES ON THE SPECIFICITY OF TYROSINE-ALPHA-KETOGLUTARATE TRANSAMINASE. J Biol Chem. 1964 Feb;239:419–424. [PubMed] [Google Scholar]
- LIN E. C., PITT B. M., CIVEN M., KNOX W. E. The assay of aromatic amino acid transaminations and keto acid oxidation by the enol borate-tautomerase method. J Biol Chem. 1958 Sep;233(3):668–673. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Morris M. L., Lee S. C., Harper A. E. A comparison of the responses of mitochondrial and cytosol histidine-pyruvate aminotransferases to nutritional and hormonal treatments. J Biol Chem. 1973 Feb 25;248(4):1459–1465. [PubMed] [Google Scholar]
- SPOLTER H., BALDRIDGE R. C. MULTIPLE FORMS OF HISTIDINE-PYRUVATE TRANSAMINASE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 19;90:287–290. doi: 10.1016/0304-4165(64)90191-6. [DOI] [PubMed] [Google Scholar]
- Tottmar S. O., Pettersson H., Kiessling K. H. The subcellular distribution and properties of aldehyde dehydrogenases in rat liver. Biochem J. 1973 Dec;135(4):577–586. doi: 10.1042/bj1350577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]


