Abstract
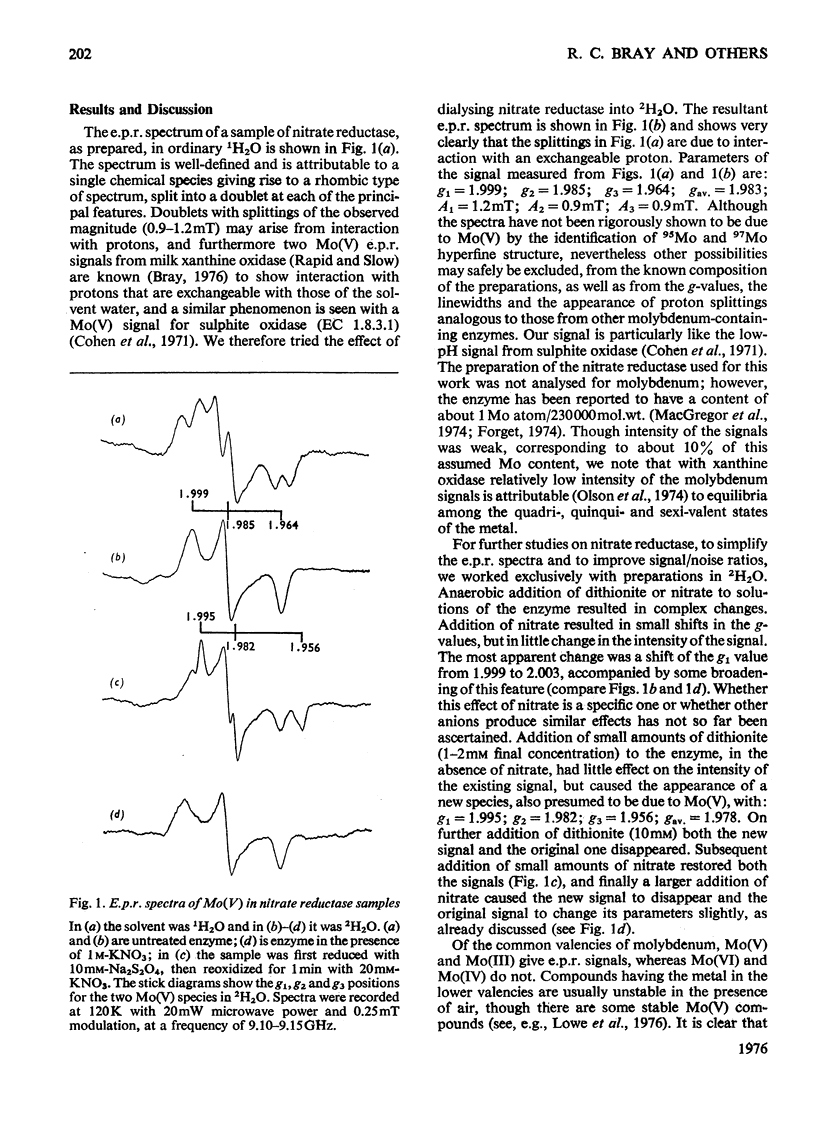
Studies on the respiratory nitrate reductase (EC 1.7.99.4) from Escherichia coli K12 by electron-paramagnetic-resonance spectroscopy indicate that its molybdenum centre is comparable with that in other molybdenum-containing enzymes. Two Mo(V) signals may be observed; one shows interaction of Mo(V) with a proton exchangeable with the solvent and has: A (1H) 0.9-1.2mT; g1 = 1.999; g2=1.985; g3 = 1.964; gav. = 1.983. Molybdenum of both signal-giving species may be reduced with dithionite and reoxidized with nitrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber M. J., Bray R. C., Lowe D. J., Coughlan M. P. Studies by electron-paramagnetic-resonance spectroscopy and stopped-flow spectrophotometry on the mechanism of action of turkey liver xanthine dehydrogenase. Biochem J. 1976 Feb 1;153(2):297–307. doi: 10.1042/bj1530297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Meriwether L. S. Electron spin resonance of xanthine oxidase substituted with molybdenum-95. Nature. 1966 Oct 29;212(5061):467–469. doi: 10.1038/212467a0. [DOI] [PubMed] [Google Scholar]
- Clegg R. A. Purification and some properties of nitrate reductase (EC 1.7.99.4) from Escherichia coli K12. Biochem J. 1976 Mar 1;153(3):533–541. doi: 10.1042/bj1530533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I., Rajagopalan K. V. Hepatic sulfite oxidase. A functional role for molybdenum. J Biol Chem. 1971 Jan 25;246(2):374–382. [PubMed] [Google Scholar]
- Dalton H., Lowe D. J., Pawlik T., Bray R. C. Studies by electron-paramagnetic-resonance spectroscopy on the mechanism of action of xanthine dehydrogenase from Veillonella alcalescens. Biochem J. 1976 Feb 1;153(2):287–295. doi: 10.1042/bj1530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- FEWSON C. A., NICHOLAS D. J. Nitrate reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:335–349. doi: 10.1016/0006-3002(61)90133-0. [DOI] [PubMed] [Google Scholar]
- Forget P., Dervartanian D. V. The bacterial nitrate reductases: EPR studies on nitrate reductase A from Micrococcus denitrificans. Biochim Biophys Acta. 1972 Feb 28;256(2):600–606. doi: 10.1016/0005-2728(72)90089-8. [DOI] [PubMed] [Google Scholar]
- Forget P. The bacterial nitrate reductases. Solubilization, purification and properties of the enzyme A of Escherichia coli K 12. Eur J Biochem. 1974 Mar 1;42(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Barber M. J., Pawlik R. T., Bray R. C. A new non-functional form of milk xanthine oxidase containing stable quinquivalent molybdenum. Biochem J. 1976 Apr 1;155(1):81–85. doi: 10.1042/bj1550081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- Meriwether L. S., Marzluff W. F., Hodgson W. G. Molybdenum-thiol complexes as models for molybdenum bound in enzymes. Nature. 1966 Oct 29;212(5061):465–467. doi: 10.1038/212465a0. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Molybdenum and nitrate reductase. II. Molybdenum as a constituent of nitrate reductase. J Biol Chem. 1954 Mar;207(1):353–360. [PubMed] [Google Scholar]
- Olson J. S., Ballou D. P., Palmer G., Massey V. The mechanism of action of xanthine oxidase. J Biol Chem. 1974 Jul 25;249(14):4363–4382. [PubMed] [Google Scholar]
- Stiefel E. I. Proposed molecular mechanism for the action of molybedenum in enzymes: coupled proton and electron transfer. Proc Natl Acad Sci U S A. 1973 Apr;70(4):988–992. doi: 10.1073/pnas.70.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]


