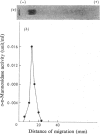
Abstract
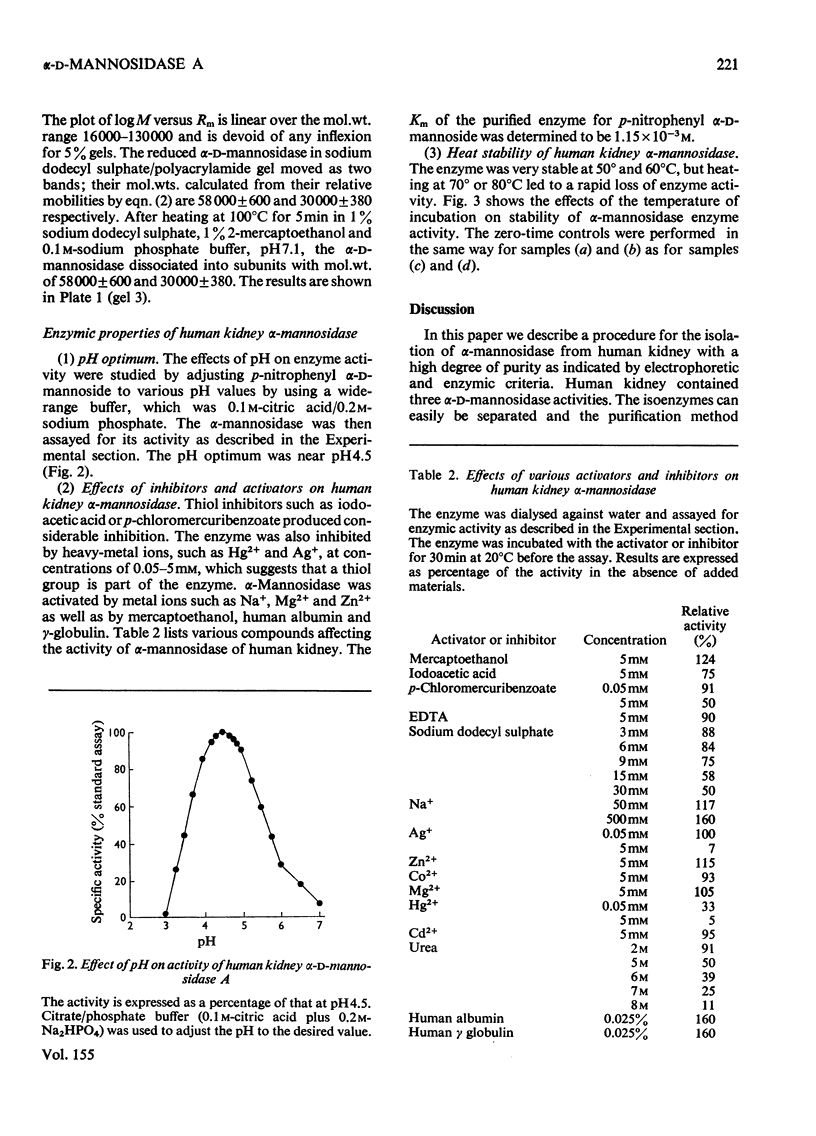
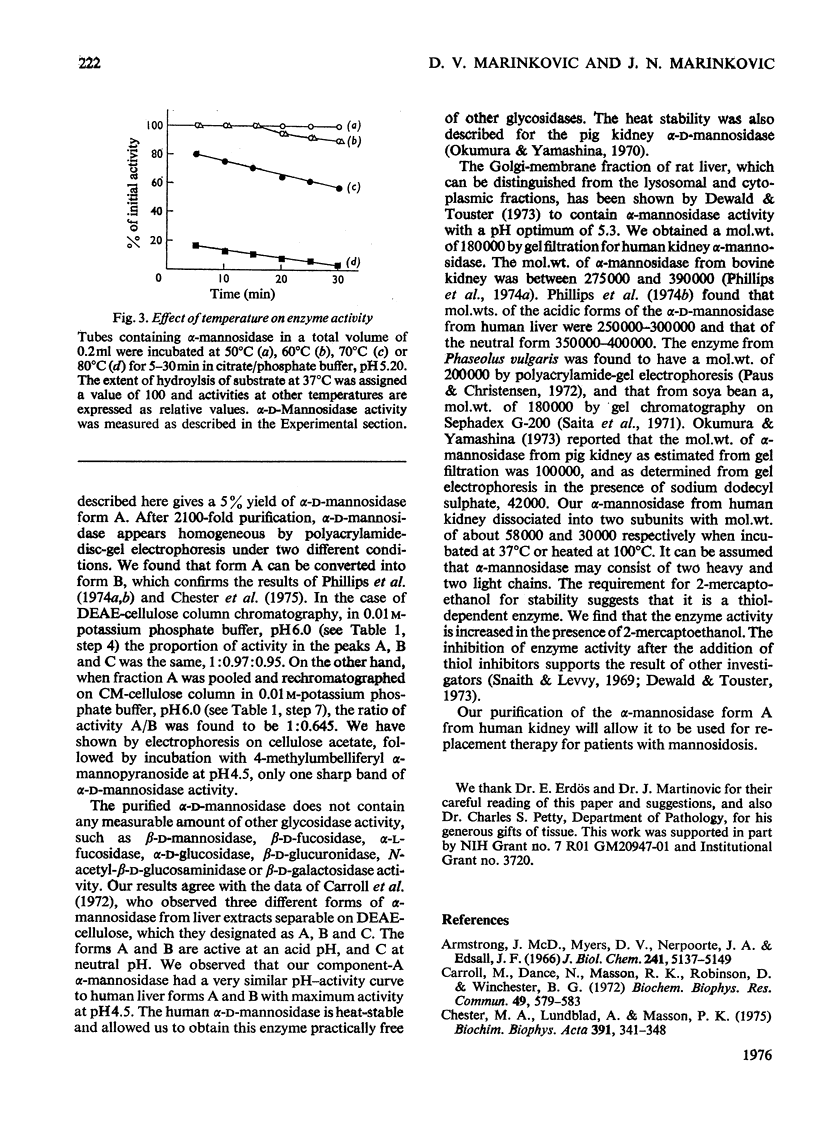
Alpha-D-Mannosidase activity exists in three forms that can be separated by DEAE-cellulose chromatography, alpha-D-Mannosidase was isolated from human kidney in a homogeneous state, and was purified 2100-fold, with p-nitrophenyl alpha-D-mannoside as substrate. The purified alpha-D-mannosidase was practically free from all other glycosidases tested. The Km of the synthetic substrate with the enzyme was 1 X 10(-3) M and the pH optimum 4.5. It was inhibited by heavy metals, sodium dodecyl sulphate, urea and compounds that react with the thiol groups, and was activated by Zn2+, Na+, 2-mercaptoethanol, human albumin and gamma-globulin. The mol. wt. of the enzyme was estimated to be 180 000 +/- 4500. After pretreatment with 2-mercaptoethanol and sodium dodecyl sulphate, alpha-D-mannosidase dissociated into subunits of mol. wts. of 58 000 +/- 600 and 30 000 +/- 380 respectively. Subunits of the same molecular weights were also obtained after the enzyme was heated at 100 degrees C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- CONCHIE J., FINDLAY J., LEVVY G. A. Mammalian glycosidases; distribution in the body. Biochem J. 1959 Feb;71(2):318–325. doi: 10.1042/bj0710318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Dance N., Masson P. K., Robinson D., Winchester B. G. Human mannosidosis--the enzyme defect. Biochem Biophys Res Commun. 1972 Oct 17;49(2):579–583. doi: 10.1016/0006-291x(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Chester M. A., Lundblad A., Masson P. K. The relationship between different forms of human alpha-mannosidase. Biochim Biophys Acta. 1975 Jun 24;391(2):341–348. doi: 10.1016/0005-2744(75)90258-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dewald B., Touster O. A new alpha-D-mannosidase occurring in Golgi membranes. J Biol Chem. 1973 Oct 25;248(20):7223–7233. [PubMed] [Google Scholar]
- Fluharty A. L., Lassila E. L., Porter M. T., Kihara H. The electrophoretic separation of human -galactosidases on cellulose acetate. Biochem Med. 1971 Apr;5(2):158–164. doi: 10.1016/0006-2944(71)90083-4. [DOI] [PubMed] [Google Scholar]
- Hultberg B. Properties of alpha-mannosidase in mannosidosis. Scand J Clin Lab Invest. 1970 Sep;26(2):155–159. doi: 10.3109/00365517009049228. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li Y. T. Presence of alpha-D-mannosidic linkage in glycoproteins. Liberation of d-mannose from various glycoproteins by alpha-mannosidase isolated from jack bean meal. J Biol Chem. 1966 Feb 25;241(4):1010–1012. [PubMed] [Google Scholar]
- Marinkovic D. V., Marinkovic J. N. A cyanogen bromide fragment of beta-galactosidase from Escherichia coli with alpha-donor activity in complementation of the enzyme from mutant M15. Biochem J. 1976 May 1;155(2):209–216. doi: 10.1042/bj1550209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T., Yamashina I. Further purification and characterization of -mannosidase from hog kidney. J Biochem. 1973 Jan;73(1):131–138. [PubMed] [Google Scholar]
- Okumura T., Yamashina I. Purification of alpha-mannosidase from hog kidney and its action on glycopeptides. J Biochem. 1970 Oct;68(4):561–571. doi: 10.1093/oxfordjournals.jbchem.a129386. [DOI] [PubMed] [Google Scholar]
- Oshima G., Gecse A., Erdös E. G. Angiotensin I-converting enzyme of the kidney cortex. Biochim Biophys Acta. 1974 May 20;350(1):26–37. doi: 10.1016/0005-2744(74)90199-5. [DOI] [PubMed] [Google Scholar]
- Paus E., Christensen T. B. Alpha-mannosidase from Phaseolus vulgaris. Purification and characterization. Eur J Biochem. 1972 Feb 15;25(2):308–314. doi: 10.1111/j.1432-1033.1972.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Human liver alpha-D-mannosidase activity. Clin Chim Acta. 1974 Aug 30;55(1):11–19. doi: 10.1016/0009-8981(74)90328-3. [DOI] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G., Jolly R. D. Mannosidosis in Angus cattle. The enzymic defect. Biochem J. 1974 Feb;137(2):363–371. doi: 10.1042/bj1370363. [DOI] [PMC free article] [PubMed] [Google Scholar]
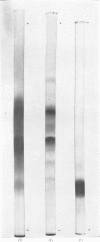
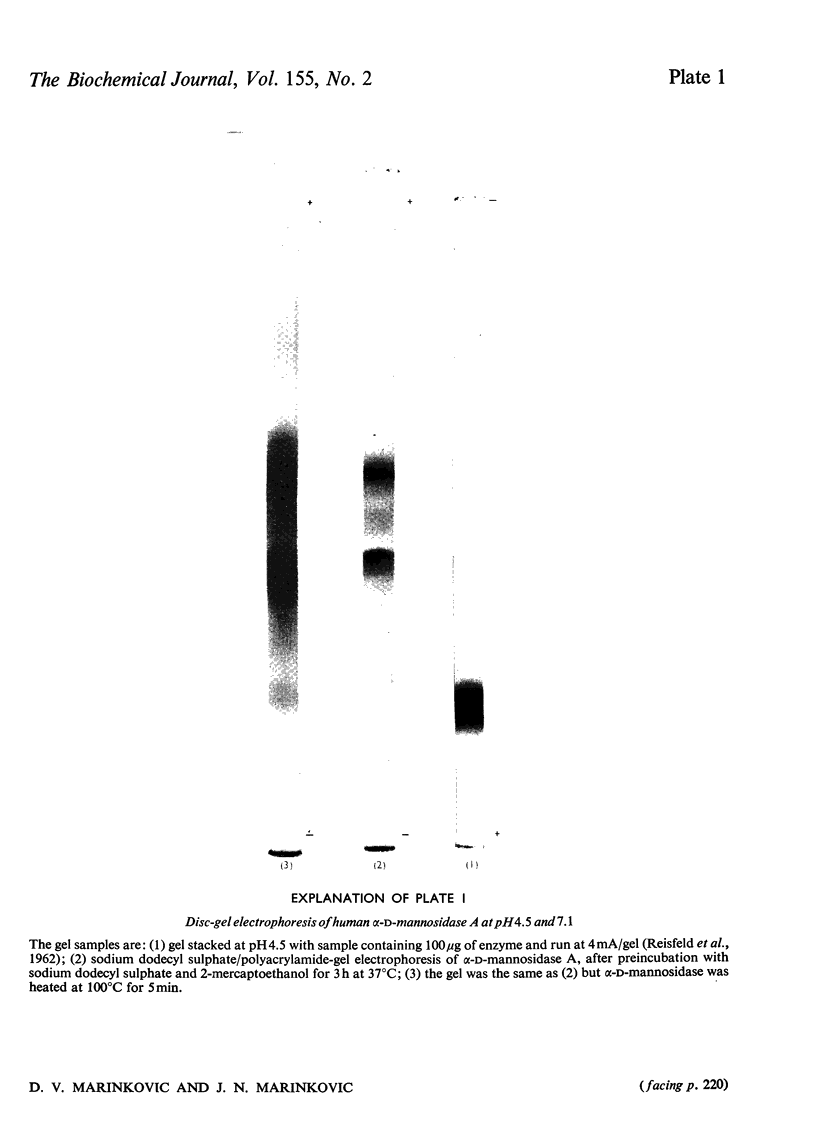
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Saita M., Ikenaka T., Matsushima Y. Isolation and characterization of -D-mannosidase from soy bean. J Biochem. 1971 Nov;70(5):827–833. doi: 10.1093/oxfordjournals.jbchem.a129700. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Snaith S. M., Levvy G. A. Alpha-mannosidase as a zinc-dependent enzyme. Nature. 1968 Apr 6;218(5136):91–92. doi: 10.1038/218091a0. [DOI] [PubMed] [Google Scholar]
- Snaith S. M., Levvy G. A. Purification and properties of alpha-D-mannosidase from jack-bean meal. Biochem J. 1968 Dec;110(4):663–670. doi: 10.1042/bj1100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith S. M., Levvy G. A. Purification and properties of alpha-D-mannosidase from rat epididymis. Biochem J. 1969 Aug;114(1):25–33. doi: 10.1042/bj1140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof F., Hers H. G. The abnormalities of lysosomal enzymes in mucopolysacc- haridoses. Eur J Biochem. 1968 Dec;7(1):34–44. doi: 10.1111/j.1432-1033.1968.tb19570.x. [DOI] [PubMed] [Google Scholar]