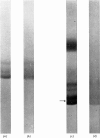
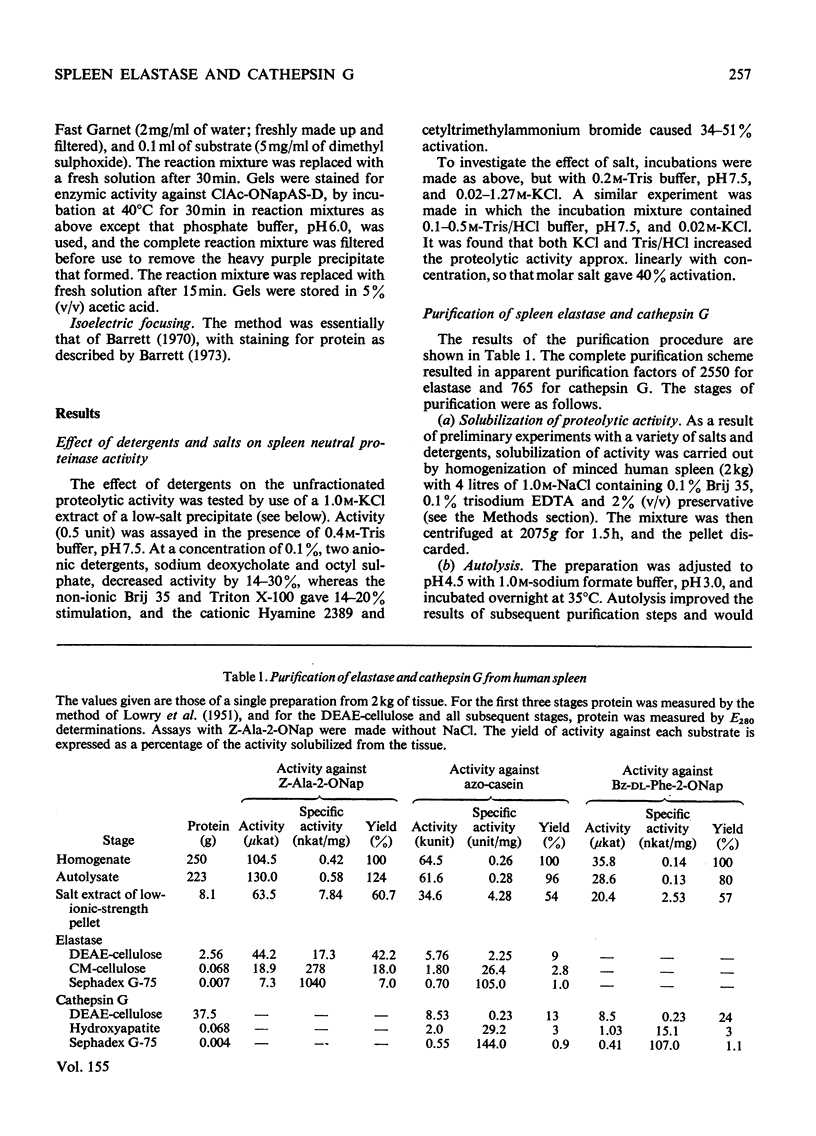
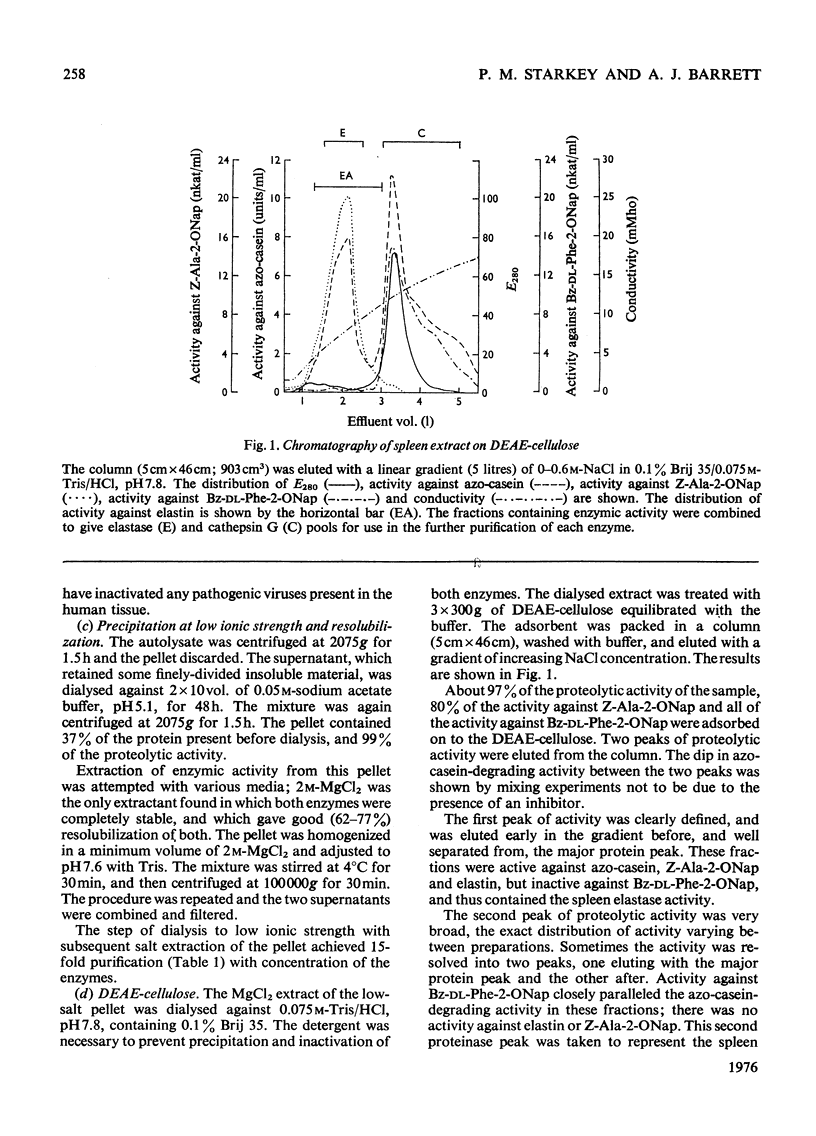
Abstract
1. Human spleen was found to contain proteinases active against azo-casein at neutral and alkaline pH values. 2. The activity was stimulated by high ionic strength and some detergents. 3. Optimal extraction of the proteinases from the tissue was achieved with 1.0M-NaCl containing 0.1% Brij 35 and 0.1% trisodium EDTA. 4. The proteinases were efficiently adsorbed to insoluble material in the absence of salt in the initial stages of purification. 5. Two distinct proteinases were separated by chromatography on DEAE-cellulose, an elastase and a chymotrypsin-like enzyme designated cathepsin G. 6. Both enzymes were highly purified by further column chromatography. 7. The molecular weights of the enzymes were estimated by gel chromatography and sodium dodecyl sulphate-gel electrophoresis. 8. It was shown by isoelectric focusing and gel electrophoresis that both enzymes are cationic proteins that occur in multiple forms.
Full text
PDF




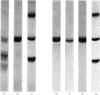
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr K., LoSpalluto J., Ziff M. Degradation of immunoglobulin G by lysosomal acid proteases. J Immunol. 1970 Oct;105(4):973–983. [PubMed] [Google Scholar]
- Gerber A. C., Carson J. H., Hadorn B. Partial purification and characterization of a chymotrypsin-like enzyme from human neutrophil leucocytes. Biochim Biophys Acta. 1974 Sep 11;364(1):103–112. doi: 10.1016/0005-2744(74)90137-5. [DOI] [PubMed] [Google Scholar]
- Hedin S. G. Investigations on the proteolytic enzymes of the spleen of the ox. J Physiol. 1903 Nov 2;30(2):155–175. doi: 10.1113/jphysiol.1903.sp000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Basch R. S. Further studies on elastase-like esterases in human leukocyte granules. Proc Soc Exp Biol Med. 1971 Apr;136(4):1045–1049. doi: 10.3181/00379727-136-35424. [DOI] [PubMed] [Google Scholar]
- Janoff A. Mediators of tissue damage in leukocyte lysosomes. X. Further studies on human granulocyte elastase. Lab Invest. 1970 Mar;22(3):228–236. [PubMed] [Google Scholar]
- Janoff A. Purification of human granulocyte elastase by affinity chromatography. Lab Invest. 1973 Oct;29(4):458–464. [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis, IV. Design and analysis of discontinuous buffer systems with a digital computer. Ann N Y Acad Sci. 1973 Jun 15;209:477–496. doi: 10.1111/j.1749-6632.1973.tb47551.x. [DOI] [PubMed] [Google Scholar]
- Kawiak J., Vensel W. H., Komender J., Barnard E. A. Non-pancreatic proteases of the chymotrypsin family. I. A chymotrypsin-like protease from rat mast cells. Biochim Biophys Acta. 1971 Apr 14;235(1):172–187. doi: 10.1016/0005-2744(71)90045-3. [DOI] [PubMed] [Google Scholar]
- LAPRESLE C., WEBB T. Study of a proteolytic enzyme from rabbit spleen. Biochem J. 1960 Sep;76:538–543. doi: 10.1042/bj0760538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- LoSpalluto J. J., Fehr K., Ziff M. Degradation of immunoglobulins by intracellular proteases in the range of neutral pH. J Immunol. 1970 Oct;105(4):886–897. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler-Ludwig R., Braunsteiner H. Cationic proteins from human neutrophil granulocytes. Evidence for their chymotrypsin-like properties. Biochim Biophys Acta. 1975 Feb 27;379(2):606–617. doi: 10.1016/0005-2795(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Schumacher G. F., Schill W. B. Radial diffusion in gel for micro determination of enzymes. II. Plasminogen activator, elastase, and nonspecific proteases. Anal Biochem. 1972 Jul;48(1):9–26. doi: 10.1016/0003-2697(72)90165-0. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human cathepsin G. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):273–278. doi: 10.1042/bj1550273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human lysosomal elastase. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):265–271. doi: 10.1042/bj1550265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman F., Ornstein L. Electrophoresis of elastase-like esterases from human neutrophils. J Histochem Cytochem. 1974 May;22(5):327–339. doi: 10.1177/22.5.327. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H., JONES M. E., FRUTON J. S. On the proteolytic enzymes of animal tissues. X. Beef spleen cathepsin C. J Biol Chem. 1952 Feb;194(2):793–805. [PubMed] [Google Scholar]