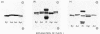Abstract
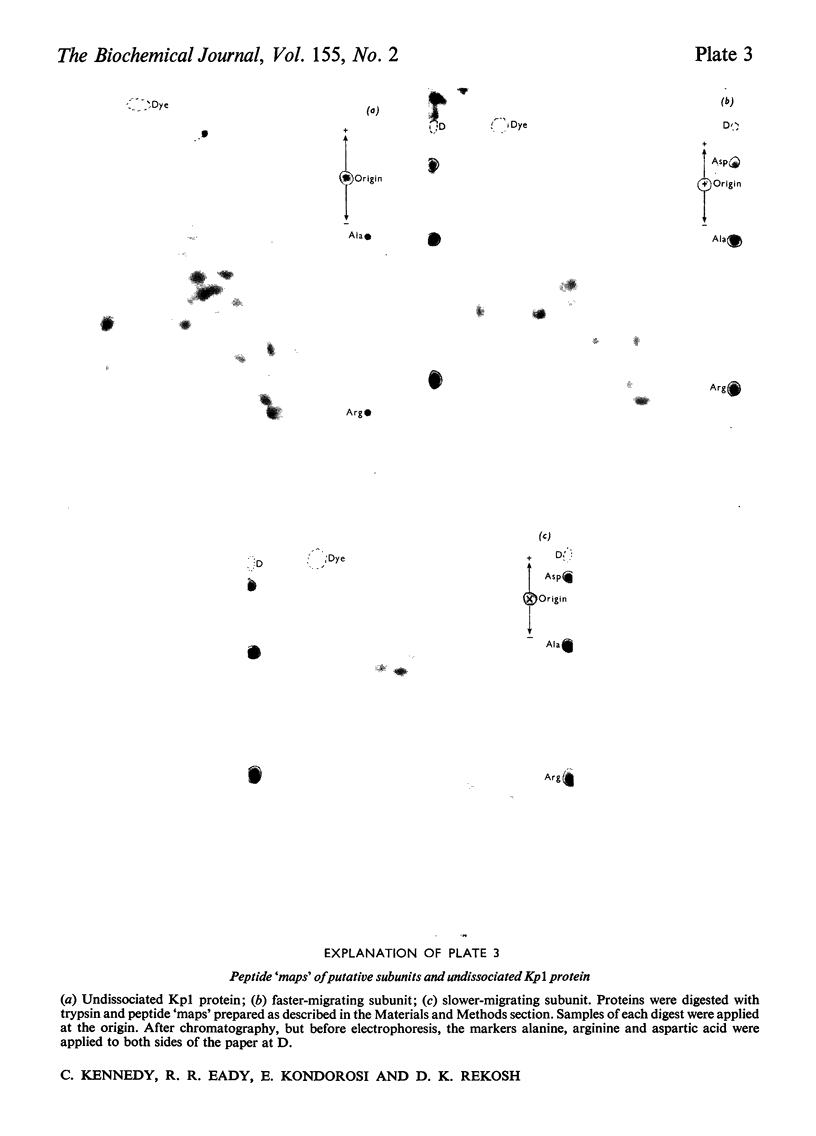
The molybdenum- and iron-containing protein components of nitrogenase purified from Klebsiella pneumoniae, Azotobacter vinelandii, Azotobacter chroococcum and Rhizobium japonicum bacteroids all gave either one or two protein-staining bands after sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, depending on the commercial brand of sodium dodecyl sulphate used. The single band obtained with K. pneumoniae Mo-Fe protein when some commercial brands of sodium dodecyl sulphate were used in the preparation of the electrode buffer was resolved into two bands by the addition of 0.01% (v/v) dodecanol to the buffer. Protein extracted from the two bands obtained after electrophoresis of K. pneumoniae Mo-Fe protein gave unique and distinct peptide 'maps' after tryptic digestion. Undissociated Mo-Fe protein contained both sets of tryptic peptides. These data are consistent with Mo-Fe protein from K. pneumoniae being composed of non-identical subunits. Amino acid analyses of the subunit proteins revealed some clear differences in amino acid content, but the two subunits showed close compositional relatedness, with a different index [Metzer, H., Shapiro, M.B., Mosiman, J.E. & Vinton, J.G. (1968) Nature (London) 219, 1166-1168] of 4.7.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. C., Hardy R. W. Purification of nitrogenase and crystallization of its Mo-Fe protein. Methods Enzymol. 1972;24:480–496. doi: 10.1016/0076-6879(72)24094-0. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Multani J. S., Mortenson L. E. Structural investigation of nitrogenase components from Clostridium pasteurianum and comparison with similar components of other organisms. Biochim Biophys Acta. 1973 May 17;310(1):51–59. doi: 10.1016/0005-2795(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Postgate J. R. Nitrogenase. Nature. 1974 Jun 28;249(460):805–810. doi: 10.1038/249805a0. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Smith R. V. The purification and some properties of the molybdenum-iron protein of Chromatium nitrogenase. Biochim Biophys Acta. 1973 Jun 15;310(2):344–352. doi: 10.1016/0005-2795(73)90114-1. [DOI] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. Differentiation in Nostoc muscorum: nitrogenase is synthesized in heterocysts. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2727–2731. doi: 10.1073/pnas.70.10.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. C., Zumft W. G., Mortenson L. E. Structure of the molybdoferredoxin complex from Clostridium pasteurianum and isolation of its subunits. J Bacteriol. 1973 Feb;113(2):884–890. doi: 10.1128/jb.113.2.884-890.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. W., Howard R. L., Evans H. J., Russell S. A. Purification and characterization of the molybdenum-iron protein component of nitrogenase from soybean nodule bacteroids. J Biol Chem. 1974 Jan 25;249(2):500–508. [PubMed] [Google Scholar]
- Kleiner D., Chen C. H. Physical and chemical properties of the nitrogenase proteins form Azotobacter vinelandii. Arch Mikrobiol. 1974 Jun 7;98(1):93–100. doi: 10.1007/BF00425272. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Nakos G., Mortenson L. Molecular weight and subunit structure of molybdoferredoxin from Clostridium pasteurianum W5. Biochim Biophys Acta. 1971 Feb 16;229(2):431–436. doi: 10.1016/0005-2795(71)90202-9. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stasny J. T., Burns R. C., Korant B. D., Hardy R. W. Electron microscopy of the Mo-Fe protein from Azotobacter nitrogenase. J Cell Biol. 1974 Jan;60(1):311–316. doi: 10.1083/jcb.60.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney J. B., Vande Woude G. F., Bachrach H. L. Sodium dodecylsulfate-dependent anomalies in gel electrophoresis: alterations in the banding patterns of foot-and-mouth disease virus polypeptides. Anal Biochem. 1974 Apr;58(2):337–346. doi: 10.1016/0003-2697(74)90201-2. [DOI] [PubMed] [Google Scholar]
- Tso M. Y. Some properties of the nitrogenase proteins from Clostridium pasteurianum. Molecular weight, subunit structure, isoelectric point and EPR spectra. Arch Microbiol. 1974;99(1):71–80. doi: 10.1007/BF00696223. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whiting M. J., Dilworth M. J. Legume root nodule nitrogenase. Purification, properties, and studies on its genetic control. Biochim Biophys Acta. 1974 Dec 18;371(2):337–351. [PubMed] [Google Scholar]
- Yates M. G., Planqué K. Nitrogenase from Azotobacter chroococcum. Purification and properties of the component proteins. Eur J Biochem. 1975 Dec 15;60(2):467–476. doi: 10.1111/j.1432-1033.1975.tb21025.x. [DOI] [PubMed] [Google Scholar]