Abstract
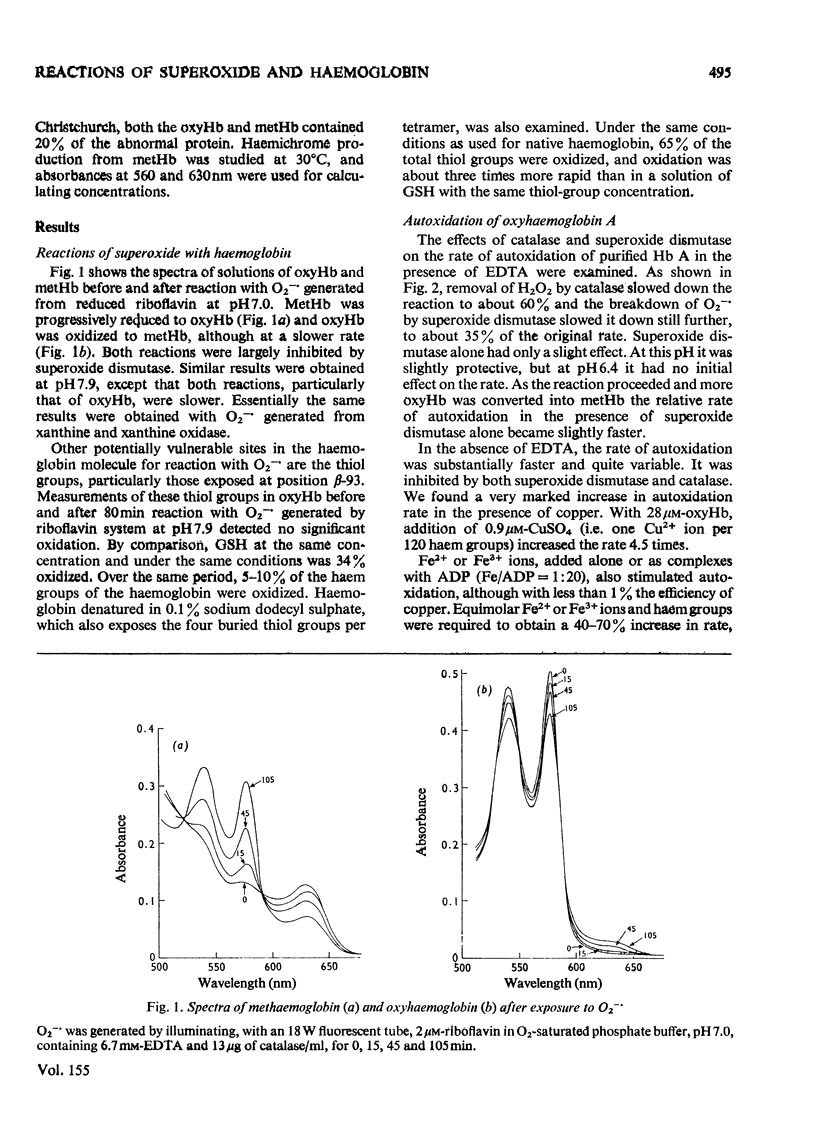
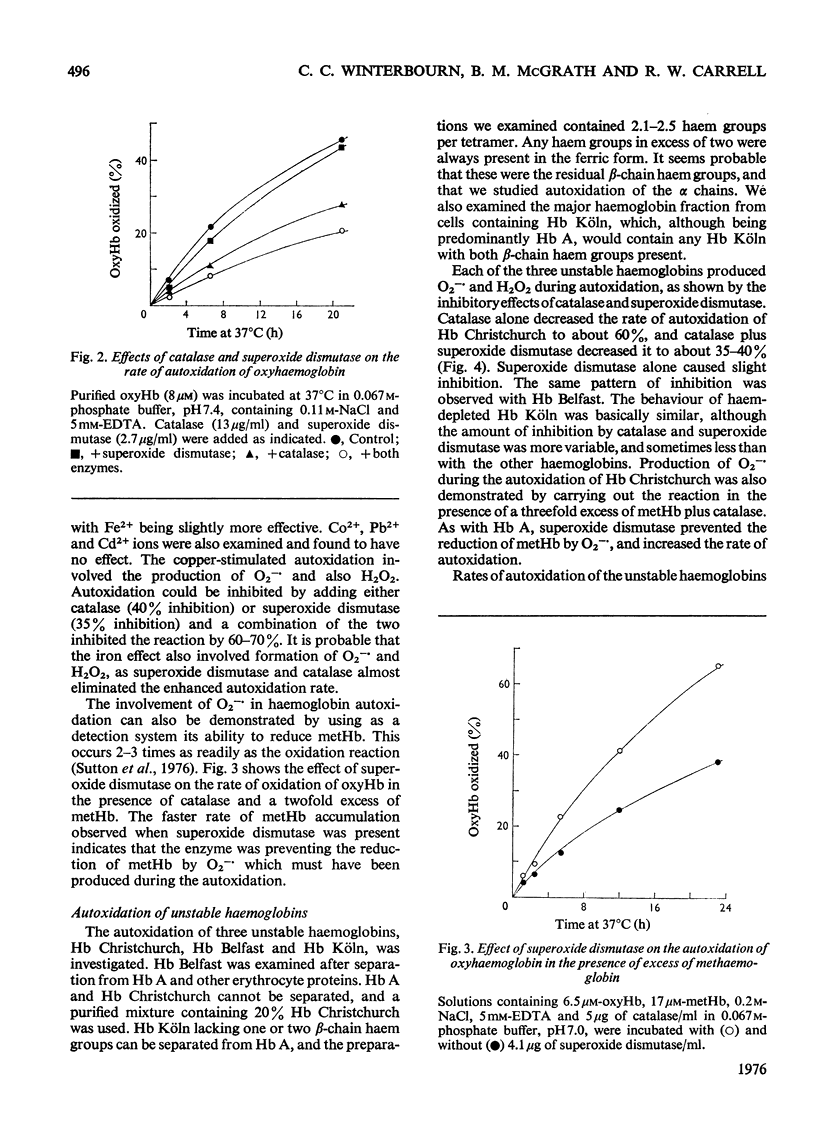
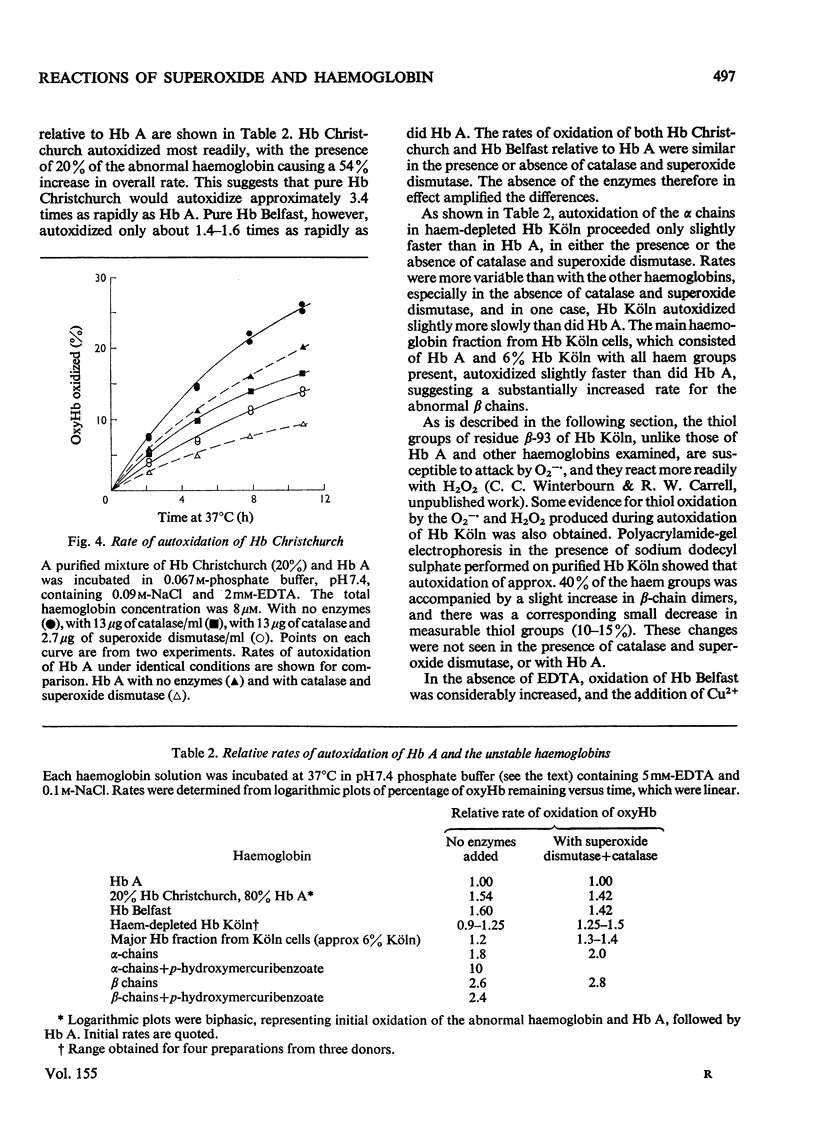
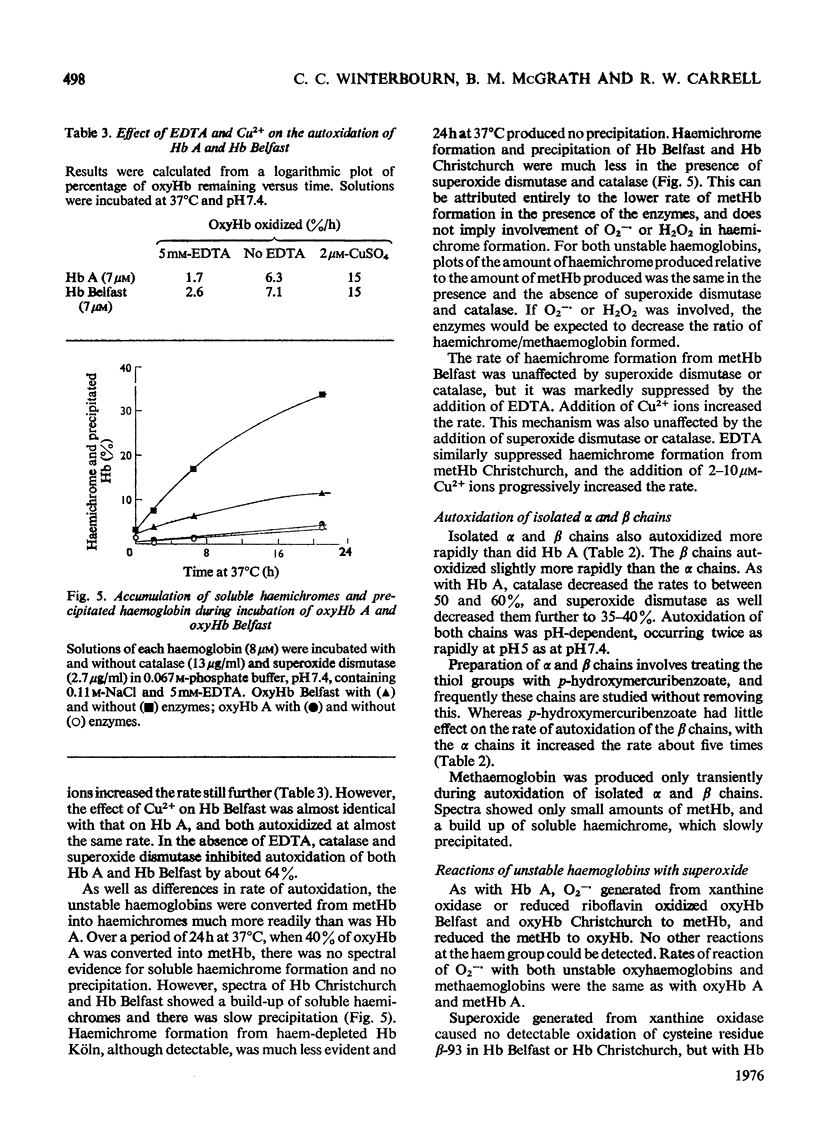
Superoxide ions (O2-) oxidized oxyhaemoglobin to methaemoglobin and reduced methaemoglobin to oxyhaemoglobin. The reactions of superoxide and H2O2 with oxyhaemoglobin or methaemoglobin and their inhibition by superoxide dismutase or catalase were used to detect the formation of superoxide or H2O2 on autoxidation of oxyhaemoglobin. The rate of autoxidation was decreased at about 35% in the presence of both enzymes. The copper-catalysed autoxidation of Hb (haemoglobin) was also shown to involve superoxide production. Superoxide was released on autoxidation of three unstable haemoglobins and isolated alpha and beta chains, at rates faster than with Hb A. Reactions of superoxide with Hb Christchurch and Hb Belfast were identical with those with Hb A, and occurred at the same rate. Hb Koln contrasted with the other haemoglobins in that the thiol groups of residue beta-93 as well as the haem groups reacted with superoxide. Haemichrome formation from methaemoglobin occurred very rapidly with Hb Christchurch and Hb Belfast, as well as the isolated chains, compared with Hb A. The process did not involve superoxide production or utilization. The relative importance of autoxidation and superoxide production compared with haemichrome formation in the haemolytic process associated with these abnormal haemoglobins and thalassaemia is considered.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal Biochem. 1973 Sep;55(1):245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Spöttl R., Michel C. The relevance of the superoxide anion radical in biological systems. Curr Top Radiat Res Q. 1974 May;9(3):247–309. [PubMed] [Google Scholar]
- Brown W. D., Mebine L. B. Autoxidation of oxymyoglobins. J Biol Chem. 1969 Dec 25;244(24):6696–6701. [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Teitelbaum H. D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):150–158. doi: 10.1016/0006-291x(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Jacob H. S. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol. 1970 Jul;7(3):341–354. [PubMed] [Google Scholar]
- Jacob H. S., Winterhalter K. H. The role of hemoglobin heme loss in Heinz body formation: studies with a partially heme-deficient hemoglobin and with genetically unstable hemoglobins. J Clin Invest. 1970 Nov;49(11):2008–2016. doi: 10.1172/JCI106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Rachmilewitz E. A. Denaturation of the normal and abnormal hemoglobin molecule. Semin Hematol. 1974 Oct;11(4):441–462. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Blumberg W. E. Studies on the stability of oxyhemoglobin A and its constituent chains and their derivatives. J Biol Chem. 1971 May 25;246(10):3356–3366. [PubMed] [Google Scholar]
- Rifkind J. M. Copper and the autoxidation of hemoglobin. Biochemistry. 1974 Jun 4;13(12):2475–2481. doi: 10.1021/bi00709a003. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Falcioni G., Fioretti E., Brunoir M. Decay of oxyperoxidase and oxygen radicals; a possible role for myeloperoxidase. Biochem J. 1975 Feb;145(2):405–407. doi: 10.1042/bj1450405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton H. C., Roberts P. B., Winterbourn C. C. The rate of reaction of superoxide radical ion with oxyhaemoglobin and methaemoglobin. Biochem J. 1976 Jun 1;155(3):503–510. doi: 10.1042/bj1550503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Studies of hemoglobin denaturation and Heinz body formation in the unstable hemoglobins. J Clin Invest. 1974 Sep;54(3):678–689. doi: 10.1172/JCI107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]
- Wittenberg J. B., Noble R. W., Wittenberg B. A., Antonini E., Brunori M., Wyman J. Studies on the equilibria and kinetics of the reactions of peroxidase with ligands. II. The reaction of ferroperoxidase with oxygen. J Biol Chem. 1967 Feb 25;242(4):626–634. [PubMed] [Google Scholar]


