Abstract
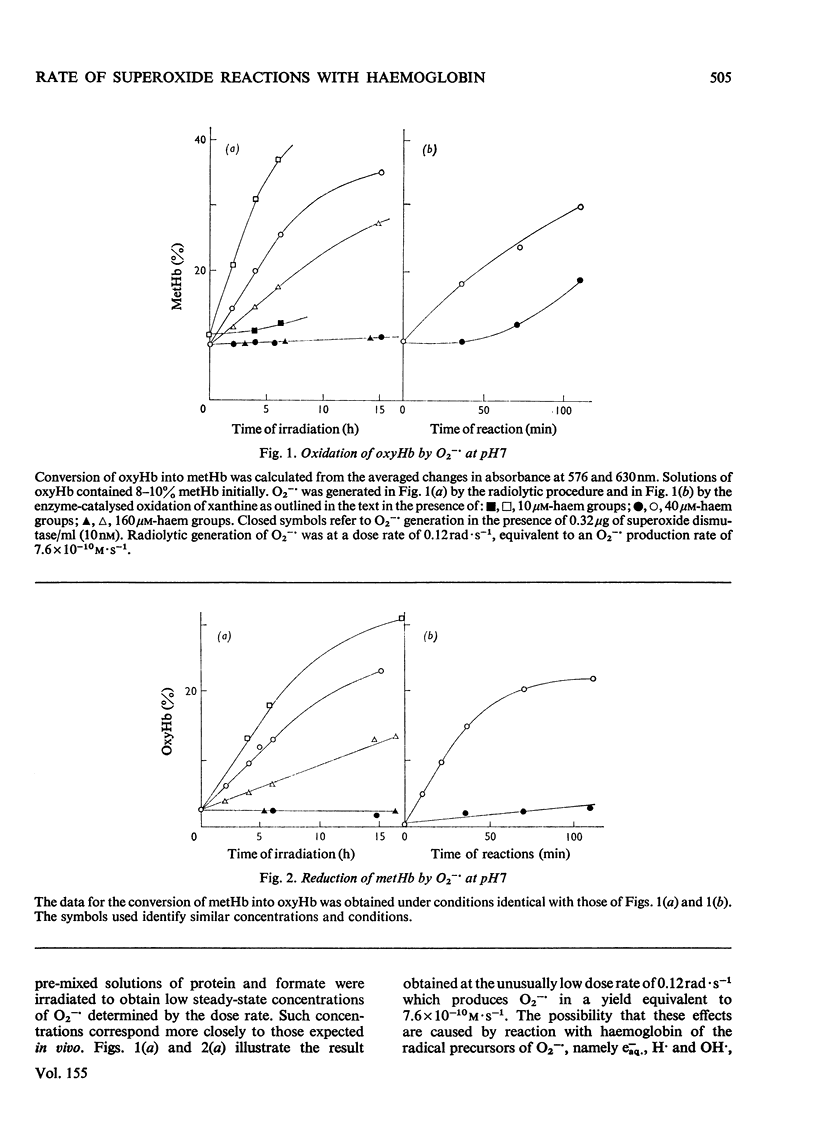
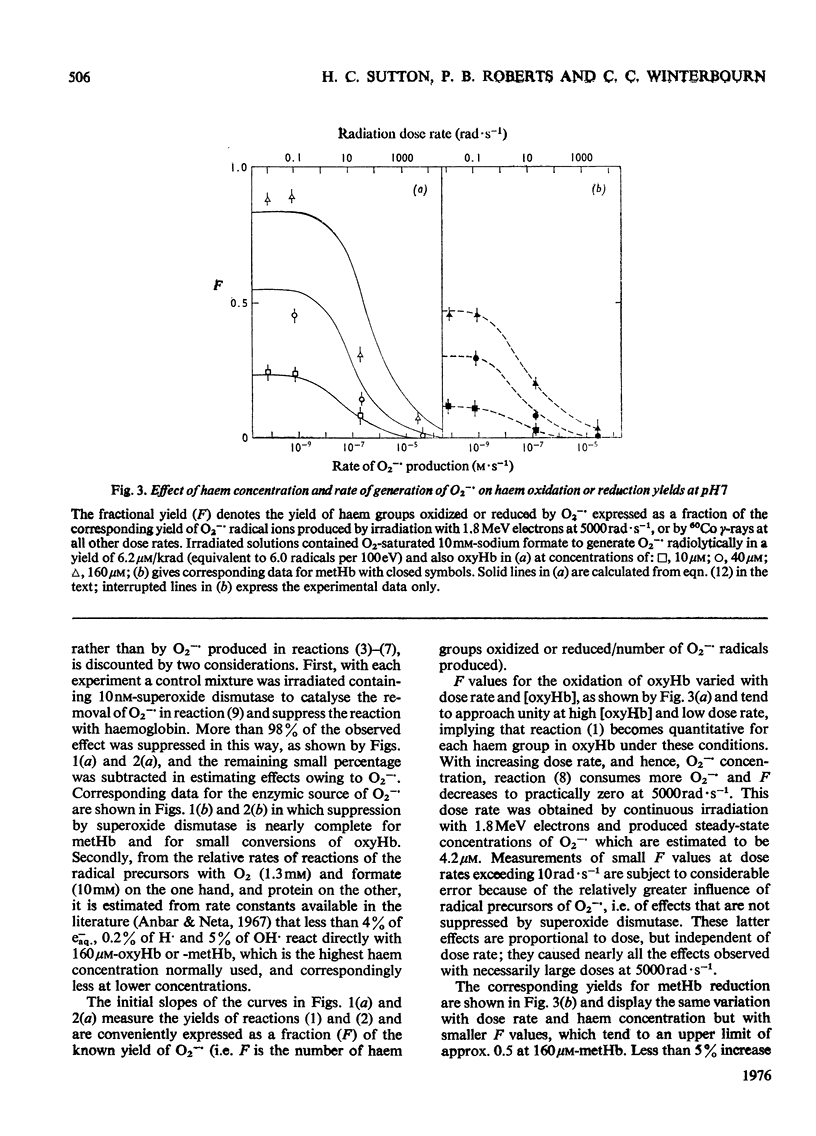
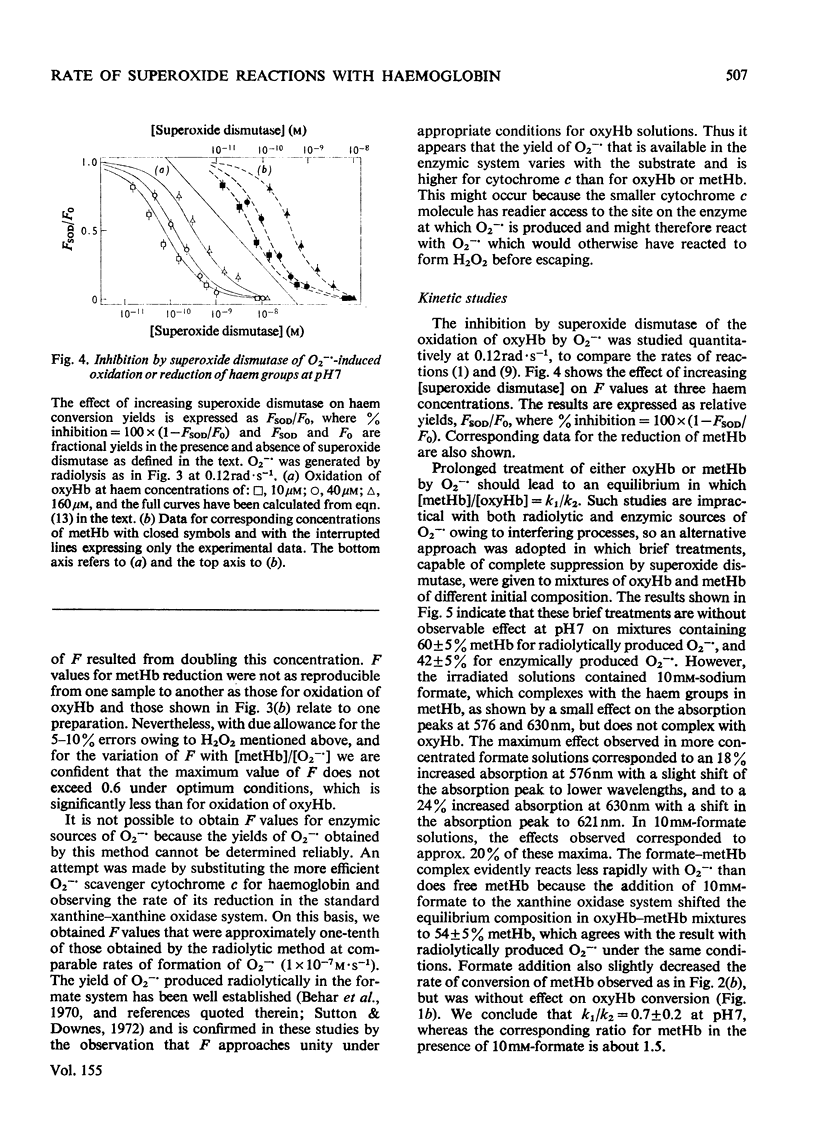
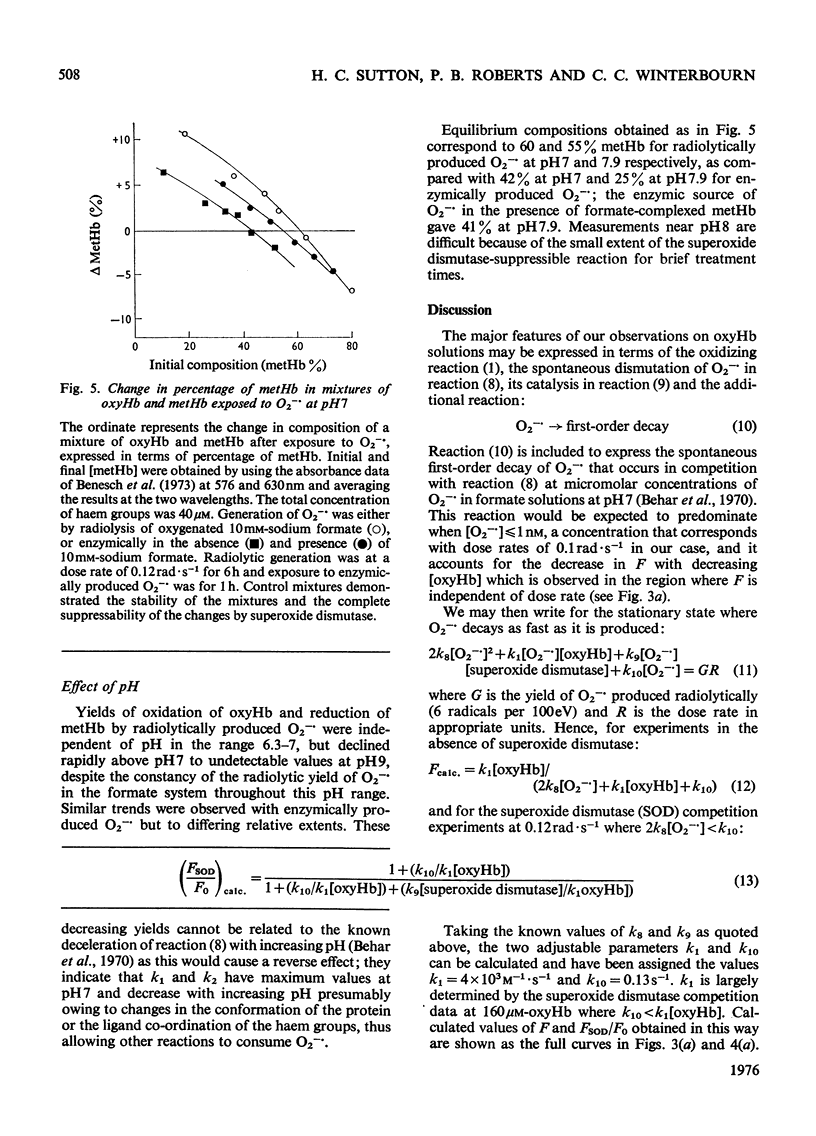
Superoxide radical ions (O2-) produced by the radiolytic reduction of oxygenated formate solutions and by the xanthine oxidase-catalysed oxidation of xanthine were shown to oxidize the haem groups in oxyhaemoglobin and reduce those in methaemoglobin as in reactions (1) and (2): (see articles) Reaction (1) is suppressed by reaction (8) when [O2-]exceeds 10 muM, but consumes all the O2- generated in oxyhaemoglobin solutions when [oxyhaemoglobin] greater than 160 muM and [O2-]less than 1 nM at pH 7. The yield of reaction (2) is also maximal in methaemoglobin solutions under similar conditions, but less than one haem group is reduced per O2- radical. From studies of (a) the yield of reactions (1) and (2) at variable [haemoglobin] and rates of production of O2-, (b) their suppression by superoxide dismutase, and (c) equilibria observed with mixtures of oxyhaemoglobin and methaemoglobin, it is shown that k1/k2=0.7 +/- 0.2 and k1 = (4 +/- 1) X 10(3) M-1-S-1 At pH7, and k1 and k2 decrease with increasing pH. Concentrations and rate constants are expressed in terms of haem-group concentrations. Concentrations of superoxide dismutase observed in normal erythrocytes are sufficient to suppress reactions (1) and (2), and hence prevent the formation of excessive methaemoglobin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BRUNORI M., TAYLOR J. F., ROSSI-FANELLI A., CAPUTO A. STUDIES ON THE OXIDATION-REDUCTION POTENTIALS OF HEME PROTEINS. I. HUMAN HEMOGLOBIN. J Biol Chem. 1964 Mar;239:907–912. [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal Biochem. 1973 Sep;55(1):245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Spöttl R., Michel C. The relevance of the superoxide anion radical in biological systems. Curr Top Radiat Res Q. 1974 May;9(3):247–309. [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972 Aug 10;247(15):4839–4842. [PubMed] [Google Scholar]
- Land E. J., Swallow A. J. One-electron reactions in biochemical systems as studied by pulse radiolysis. V. Cytochrome c. Arch Biochem Biophys. 1971 Jul;145(1):365–372. doi: 10.1016/0003-9861(71)90049-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Muir Wood P. The redox potential of the system oxygen--superoxide. FEBS Lett. 1974 Aug 15;44(1):22–24. doi: 10.1016/0014-5793(74)80297-8. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Bray R. C., Fielden E. M. A pulse radiolysis study of superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):605–609. doi: 10.1016/0005-2744(72)90359-2. [DOI] [PubMed] [Google Scholar]
- Stansell M. J., Deutsch H. F. The levels of catalase and of erythrocuprein in human erythrocytes. Clin Chim Acta. 1966 Nov;14(5):598–607. doi: 10.1016/0009-8981(66)90183-5. [DOI] [PubMed] [Google Scholar]
- Steinman H. M., Naik V. R., Abernethy J. L., Hill R. L. Bovine erythrocyte superoxide dismutase. Complete amino acid sequence. J Biol Chem. 1974 Nov 25;249(22):7326–7338. [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]


