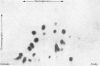
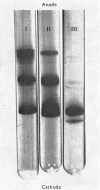
Abstract
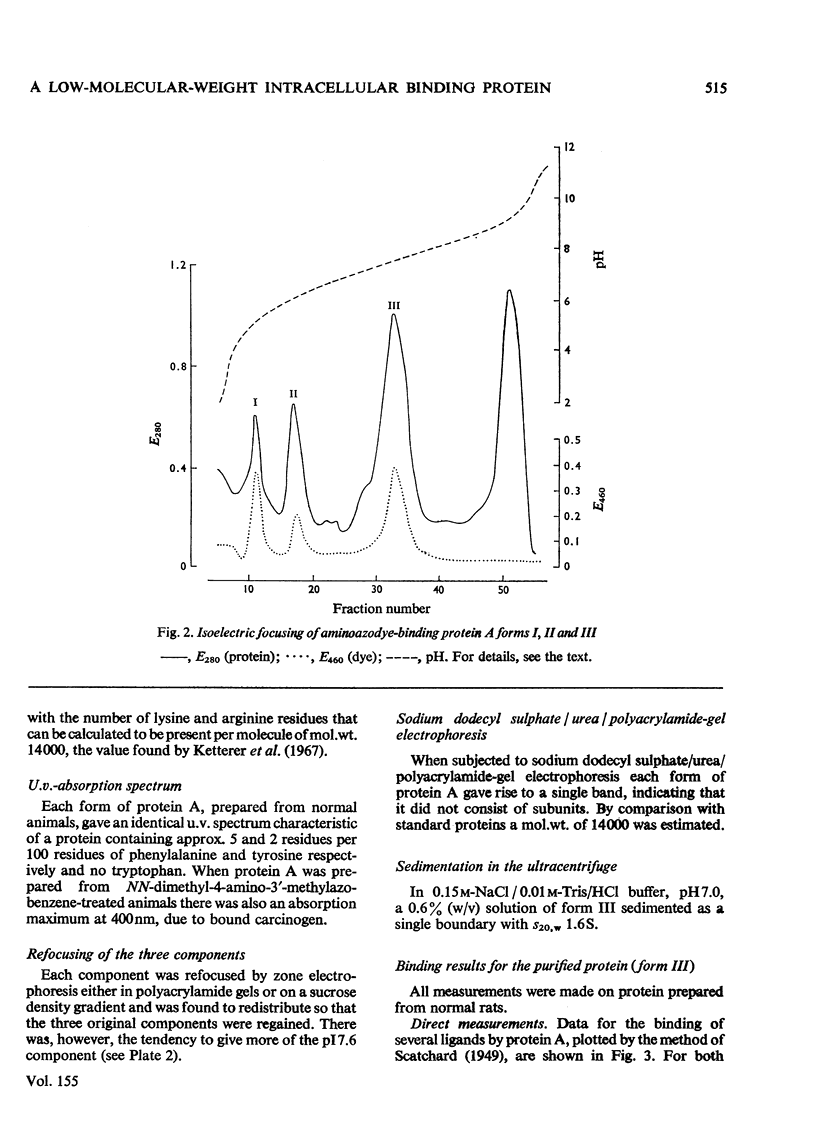
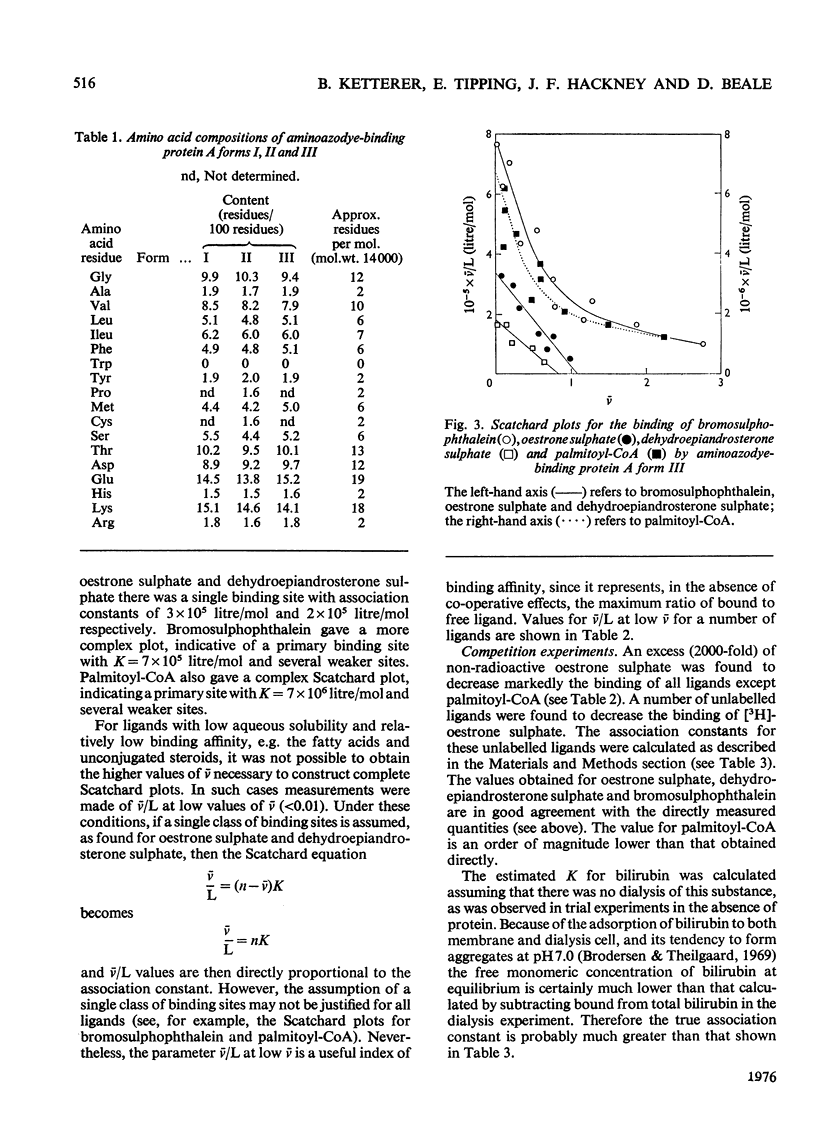
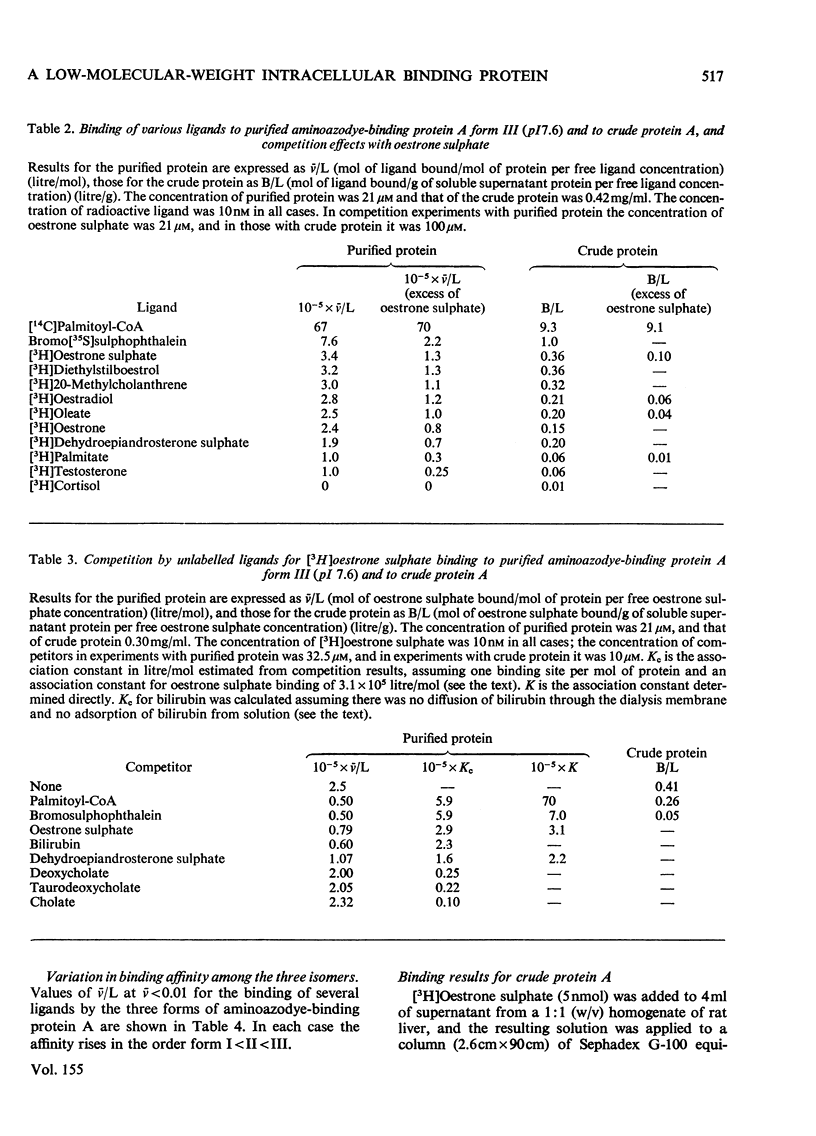
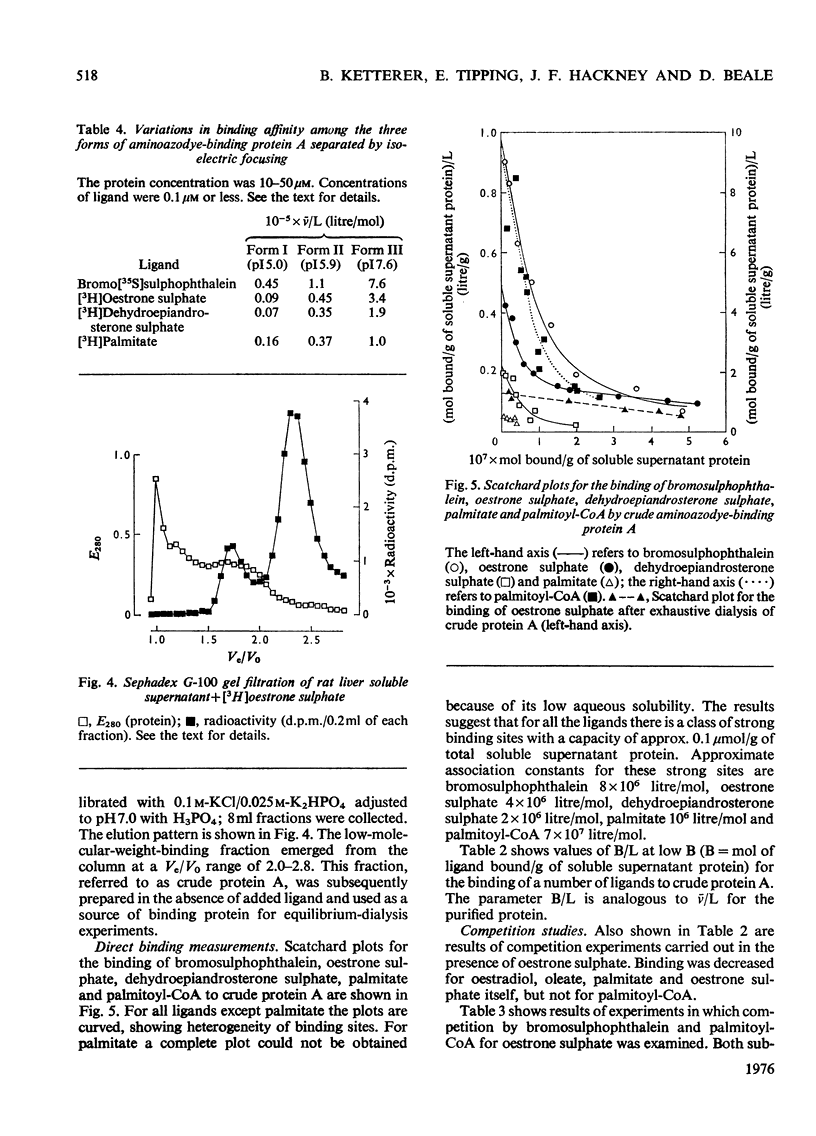
A protein of S20,W 1.6S and mol.wt. 14000, which binds covalently a metabolite of the aminoazodye carcinogen NN-dimethyl-4-amino-3'-methylazobenzene, was isolated from rat liver cytosol from both carcinogen-treated and normal rats. The protein binds non-covalently palmitoyl-CoA, fatty acids, bilirubin, sex steroids and their sulphates, bile acids and salts, bromosulphophthalein, diethylstilboestrol and 20-methylcholanthrene with a wide range of affinities. The protein is isolated as three components with isoelectric points of 5.0, 5.9 and 7.6 by a method involving isoelectric focusing. All three components have closely similar amino acid analyses, tryptic-peptide 'maps' and u.v. spectra. Each single component redistributes into all three on further electrophoresis. However, the three forms differ in their binding characteristics, the form of pI 7.6 having much the highest affinity for compounds bound non-covalently. The protein was identified immunologically in rat liver, small intestine, adipose tissue, skeletal muscle, myocardium and testis. The protein was compared with other hepatic binding-protein preparations of similar molecular weight.
Full text
PDF



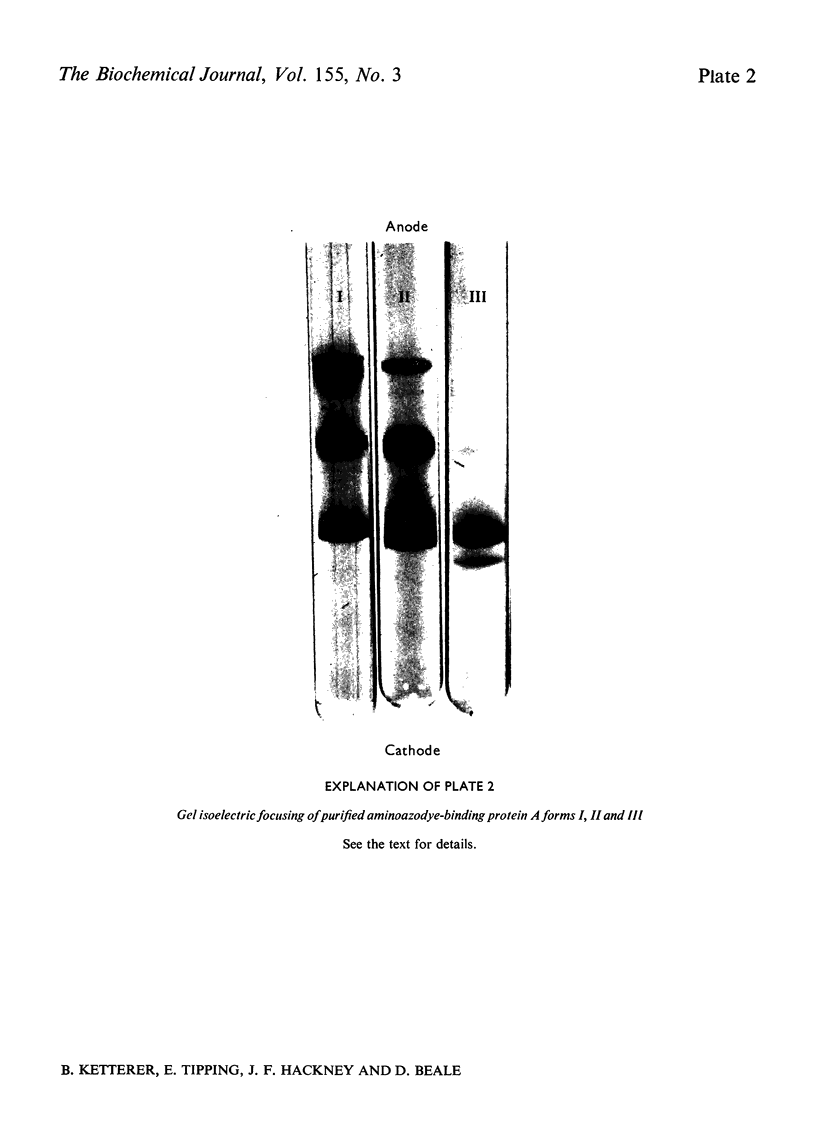


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodersen R., Theilgaard J. Bilirubin colloid formation in neutral aqueous solution. Scand J Clin Lab Invest. 1969 Dec;24(4):395–398. doi: 10.3109/00365516909080178. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Gatmaitan Z., Arias I. M. Circular dichroism analysis of the secondary structure of Z protein and its complexes with bilirubin and other organic anions. Biochim Biophys Acta. 1975 May 30;393(1):24–30. doi: 10.1016/0005-2795(75)90212-3. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Beale D., Hackney J. F. The conjugation of aminoazo dye metabolites with a tripeptide and specific soluble proteins of the liver soluble cytoplasm of the rat. Xenobiotica. 1971 Jul-Oct;1(4):551–552. doi: 10.3109/00498257109041528. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Christodoulides L. Two specific azodye-carcinogen-binding proteins of the rat liver. The identity of amino acid residues which bind the azodye. Chem Biol Interact. 1969 Dec;1(2):173–183. doi: 10.1016/0009-2797(69)90005-2. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Ross-Mansell P., Whitehead J. K. The isolation of carcinogen-binding protein from livers of rats given 4-dimethylaminoazobenzene. Biochem J. 1967 May;103(2):316–324. doi: 10.1042/bj1030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Meuwissen J., Beale D. Ligandin. Biochem Soc Trans. 1975;3(5):626–630. doi: 10.1042/bst0030626. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Stein L., Gatmaitan Z., Arias I. M. The binding of fatty acids to cytoplasmic proteins: binding to Z protein in liver and other tissues of the rat. Biochem Biophys Res Commun. 1972 Jun 9;47(5):997–1003. doi: 10.1016/0006-291x(72)90931-x. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Turcotte R. Stimulation of monoacylglycerophosphate formation by Z protein. Biochem Biophys Res Commun. 1974 Sep 9;60(1):376–381. doi: 10.1016/0006-291x(74)90215-0. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Turcotte R. The binding of long chain fatty acid CoA to Z, a cytoplasmic protein present in liver and other tissues of the rat. Biochem Biophys Res Commun. 1974 Apr 8;57(3):918–926. doi: 10.1016/0006-291x(74)90633-0. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974 Aug;54(2):326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A., Poppenhausen R. B., Ho W. K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972 Jul 7;177(4043):56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Herbert S., Polet H., Steinhardt J. The binding of divers detergent anions to bovine serum albumin. Biochemistry. 1967 Mar;6(3):937–947. doi: 10.1021/bi00855a038. [DOI] [PubMed] [Google Scholar]
- SOROF S., COHEN P. P., MILLER E. C., MILLER J. A. Electrophoretic studies on the soluble proteins from livers of rats fed aminoazo dyes. Cancer Res. 1951 May;11(5):383–387. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sorof S., Kish V. M., Sani B. Purification and properties of the principal liver protein conjugate of a hepatic carcinogen. Biochem Biophys Res Commun. 1972 Aug 21;48(4):860–865. doi: 10.1016/0006-291x(72)90687-0. [DOI] [PubMed] [Google Scholar]
- Tasseron J. G., Diringer H., Frohwirth N., Mirvish S. S., Heidelberger C. Partial purification of soluble protein from mouse skin to which carcinogenic hydrocarbons are specifically bound. Biochemistry. 1970 Mar 31;9(7):1636–1644. doi: 10.1021/bi00809a025. [DOI] [PubMed] [Google Scholar]
- Warner M., Neims A. H. Studies on Z-Fraction. I. Isolation and partial characterization of low molecular weight ligand-binding protein from rat hepatic cytosol. Can J Physiol Pharmacol. 1975 Jun;53(3):493–500. doi: 10.1139/y75-069. [DOI] [PubMed] [Google Scholar]
- Wyman J. Facilitated diffusion and the possible role of myoglobin as a transport mechanism. J Biol Chem. 1966 Jan 10;241(1):115–121. [PubMed] [Google Scholar]