Abstract
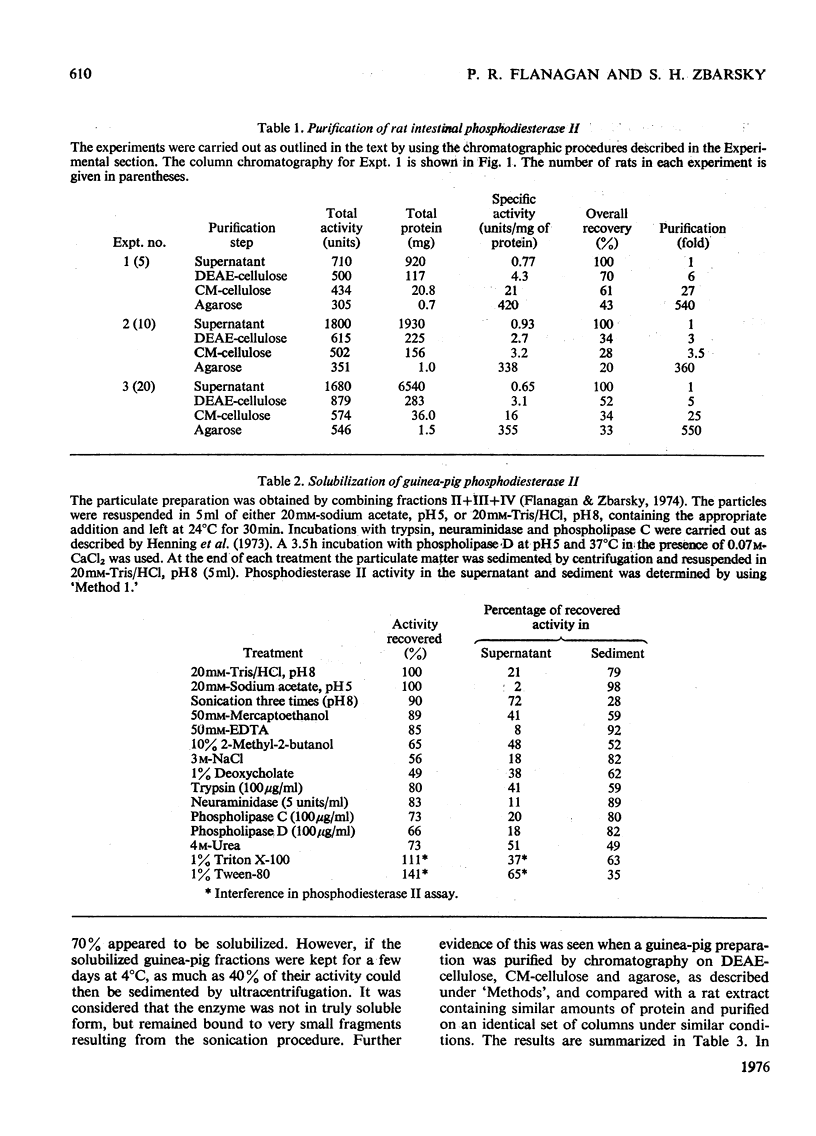
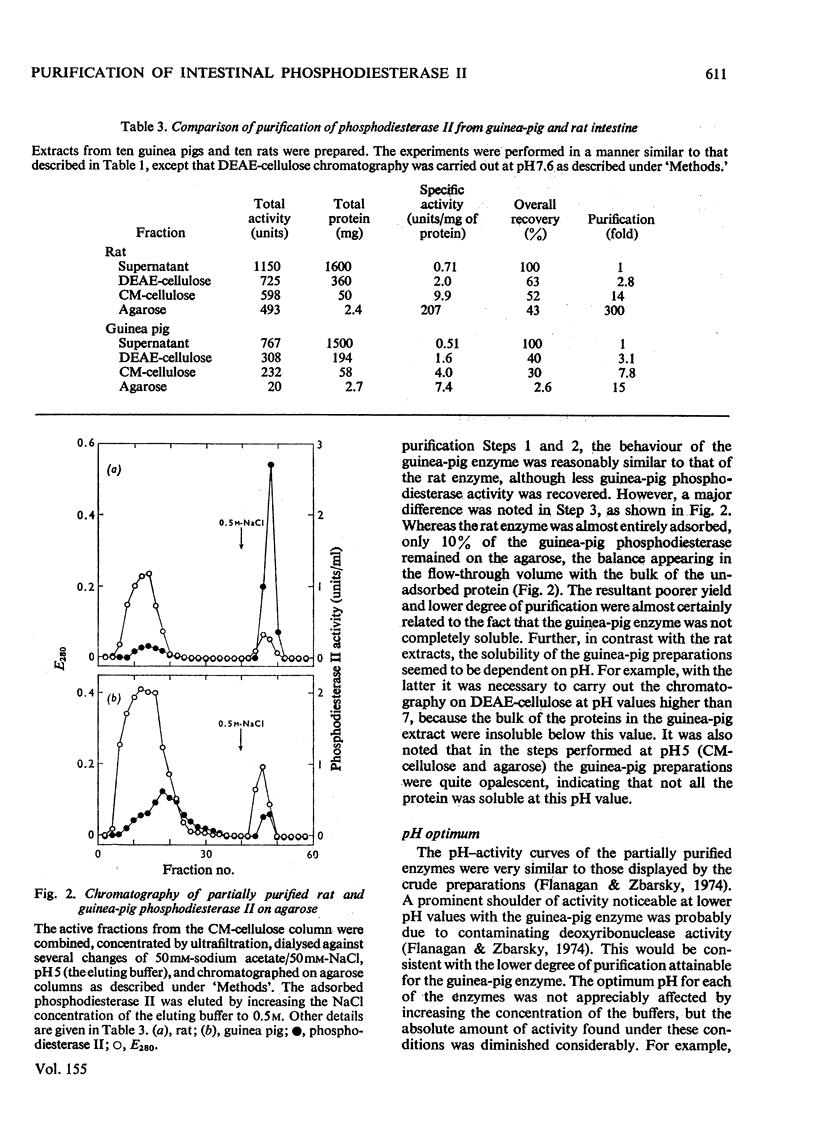
Phosphodiesterase II from extracts of intestinal mucosa of rat and guinea pig was purified by chromatography on DEAE-cellulose, CM-cellulose and agarose. The rat enzyme was purified 350-550-fold, with recoveries ranging up to 46%. The best purification of the guinea-pig enzyme was 15-fold, and the recovery was only 2.6%, the large loss occurring during chromatography on DEAE-cellulose and agarose. The poor results with the guinea-pig enzyme reflect the difficulty in obtaining a truly soluble material. Repeated sonication of the crude guinea-pig preparations yielded material that was initially soluble but tended to re-aggregate quickly. Purification of the rat phosphodiesterase II increased its thermostability, the temperature of half-inactivation being increased from 54degrees to 60degreesC. Both enzymes had a Km value of 4 X 10(-5) M with thymidine 3'-(2,4-dinitrophenyl) phosphate as substrate and showed similar pH optima for activity. Both enzymes were inhibited slightly in 0.1 M-MgC12 or 2M-urea and much more strongly in 2M-(NH4)2SO4 or 6M-NaC1. The guinea-pig enzyme was usually inhibited more than the rat enzyme. The Arrhenius plots of the two enzymes differed slightly in slope, but both were biphasic, showing breaks between 30degrees and 40degreesC. It was concluded that the two enzymes were markedly similar in behaviour and that the differences found were related to the different degrees of purification attained by the procedures described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FIERS W., KHORANA H. G. STUDIES ON POLYNUCLEOTIDES. XXII. ENZYMIC DEGRADATION. AN EXONUCLEASE FROM LACTOBACILLUS ACIDOPHILUS R26. A. PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITY. J Biol Chem. 1963 Aug;238:2780–2788. [PubMed] [Google Scholar]
- Flanagan P. R., Zbarsky S. H. Phosphodiesterase II in epithelial cells from guinea-pig and rat small intestine. Biochem J. 1974 Sep;142(3):545–553. doi: 10.1042/bj1420545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILMOE R. J. Purification and properties of spleen phosphodiesterase. J Biol Chem. 1960 Jul;235:2117–2121. [PubMed] [Google Scholar]
- HJERTEN S. A new method for preparation of agaraose for gel electrophoresis. Biochim Biophys Acta. 1962 Aug 27;62:445–449. doi: 10.1016/0006-3002(62)90224-x. [DOI] [PubMed] [Google Scholar]
- Henning R., Plattner H., Stoffel W. Nature and localization of acidic groups on lysosomal membranes. Biochim Biophys Acta. 1973 Nov 30;330(1):61–75. doi: 10.1016/0005-2736(73)90284-8. [DOI] [PubMed] [Google Scholar]
- Kumamoto J., Raison J. K., Lyons J. M. Temperature "breaks" in Arrhenius plots: a thermodynamic consequence of a phase change. J Theor Biol. 1971 Apr;31(1):47–51. doi: 10.1016/0022-5193(71)90120-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Menon K. M., Smith M. Exonuclease (phosphodiesterase) from the testes of Chinook salmon (Oncorhynchus tschawytscha). Biochemistry. 1970 Mar 31;9(7):1584–1592. doi: 10.1021/bi00809a017. [DOI] [PubMed] [Google Scholar]
- RAUENBUSCH E. C., ALTMAN K. I. Urinary sulfate ion as a naturally occurring inhibitor of DNase I activity. Proc Soc Exp Biol Med. 1960 Jul;104:385–388. doi: 10.3181/00379727-104-25845. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Studies on polynucleotides. X. Enzymic degradation. Some properties and mode of action of spleen phosphodiesterase. J Biol Chem. 1961 Apr;236:1144–1149. [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- von Tigerstrom R. G., Smith M. Preparation of the 2,4-dinitrophenyl esters of thymidine 3'-and thymidine 5'-phosphate and their use as substrates fof phosphodiesterases. Biochemistry. 1969 Jul;8(7):3067–3070. doi: 10.1021/bi00835a056. [DOI] [PubMed] [Google Scholar]


