Abstract
Objective
To investigate whether there is a difference in hardness and hemoglobin concentration changes in the infrapatellar fat pad (IFP) during isometric quadriceps exercise (IQE) in patients with knee osteoarthritis (KOA) between those with and without knee extension limitation.
Design
In this cross-sectional study, data were collected at an orthopedic clinic from March 2022 to April 2023. Among patients diagnosed with KOA, those with knee joint extension range of motion <0° and >0° were defined as the limited group (n = 16) and non-limited group (n = 13), respectively. Ultrasonography was performed at rest and during IQE to measure IFP hardness based on shear wave velocity. Near-infrared spectroscopy was performed to measure oxygenated hemoglobin (O2Hb), deoxygenated hemoglobin (HHb), and total hemoglobin (cHb) in the IFP before (baseline), during (IQE task), and post IQE. IFP hardness and O2Hb, HHb, and cHb concentration were analyzed using a linear mixed model for the groups and measurement points.
Results
IFP hardness changes during IQE were significantly less in the limited group than in the non-limited group (limited: mean difference [MD] = −0.136, 95 % confidence interval [CI] [−0.475, 0.203]; non-limited: MD = −1.154, 95 % CI [−1.530, −0.778]). O2Hb concentration did not significantly change at post compared with baseline in the limited group but did in the non-limited group (limited: MD = −0.024, 95 % CI [−0.961, 0.913]; non-limited: MD = −4.118, 95 % CI [−5.156, −3.079]).
Conclusions
The limited group revealed no IFP hardness and hemoglobin concentration change following IQE, whereas the opposite was observed for the non-limited group, indicating oxygenation.
Keywords: Knee osteoarthritis, Osteoarthritis, Infrapatellar fat pad, Isometric quadriceps exercise, Knee joint extension limitation, Hemoglobin
1. Introduction
Knee osteoarthritis (KOA) is one of the fastest-growing chronic diseases and the most common form of arthritis worldwide [1,2]. The risk factors for KOA include advanced age; female sex, obesity; knee injury; occupational factors; and varus or valgus alignment [2]; the incidence of KOA is higher in women than in men [1,3]. KOA is characterized by subchondral bone remodeling; meniscal degeneration; and inflammation and fibrosis of the infrapatellar fat pad (IFP) and synovial membrane. Additionally, KOA is a whole-joint disease that affects the entire joint tissue [2]. Prolonged joint pain in patients with KOA reduces activities of daily living and quality of life [4]. The IFP functions as a synovial membrane and anatomo-functional unit [5]; it has been confirmed that inflammation and fibrosis of the IFP are the cause of pain in patients with KOA [6,7], wherein an association between fibrosis of the IFP and long-term knee pain has been suggested [8]. The mechanical behavior of the IFP in patients with KOA shows different behavior compared with subcutaneous and other adipose tissues [9]. In addition, compared with healthy people, patients with KOA show less forward movement of the IFP and less volume change in the infero-posterior-lateral portion during knee joint extension [10], suggesting that KOA might worsen. Consequently, it is necessary to prevent and treat fibrosis of the IFP in patients with KOA.
Kitagawa et al. [11] reported that hypoxia is involved in developing IFP fibrosis in animal experiments, and improving hypoxia can inhibit IFP fibrosis. Additionally, compared to healthy elderly and young participants, those with KOA exhibit lesser variation in hemoglobin concentration and IFP hardness following isometric quadriceps exercise (IQE) [12]. Hence, they may be hypoxic in the IFP due to a lack of mechanical stress loading during exercise [12]. However, the physical factors limiting the application of mechanical stress to IFP remain unclear. Patients with KOA often have knee joint extension limitations [[13], [14], [15]], and the internal pressure of the IFP decreases in the flexed position compared with that in the extended position [16]. Specifically, the presence or absence of knee joint extension limitation in KOA may partially affect hemoglobin concentration and IFP hardness changes induced by IQE. Therefore, we focused on the limitation of knee joint extension in patients with KOA as a factor leading to IFP under hypoxic conditions.
It was hypothesized that patients with KOA with and without knee extension limitation may have differences in IFP stiffness and hemoglobin concentration. This study aimed to determine whether there is a difference in hemoglobin concentration and hardness changes in the IFP between patients with KOA, with and without knee extension limitation.
2. Methods
2.1. Participants
This cross-sectional study compared patients with KOA and knee joint extension limitation (limited group) and patients with KOA without joint extension limitation (non-limited group). The analysis was partially performed using the same KOA cases as those in a previous study [12]. Data were collected at an outpatient orthopedic clinic in Osaka, Japan, from March 2022 to April 2023.
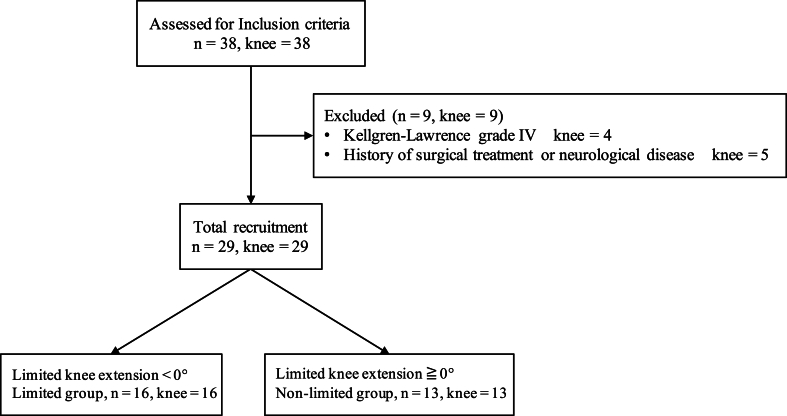
Inclusion criteria were women aged 50 years or older, with unilateral or bilateral KOA diagnosed by an orthopedic surgeon based on the Kellgren-Lawrence (K-L) grade, experiencing walking or stair pain in the anterior or medial knee joint, and patients on physiotherapy for more than 2 months. The exclusion criteria included K-L grade IV, history of knee surgery, trauma to the knee joint, and presence of neurological illness. Among the eligible patients, those with a knee joint extension range of motion of less than 0° and more than 0° were defined as the limited and non-limited groups, respectively.
The study protocol was approved by our institution's Ethics Committee (approval number 2021-149). In accordance with the Declaration of Helsinki, participants provided written informed consent after receiving a full explanation about the objectives, procedures, handling, and application of the results.
2.2. IQE load configurations
This measurement was acquired with the participant wearing a belt to secure the lower leg and in the long sitting position. The popliteal space was subjected to pressure using the strongest IQE achievable, based on a prior study [12]. The manchette of a sphygmomanometer (FC-100, FOCAL CORPORATION Inc., Chiba, Japan), raised to a pressure of 100 mmHg, was placed over the popliteal space. The angle of the knee joint with the manchette in place was 20° of flexion. There were no participants who could not undergo the test considering this knee flexion angle. The 50 % intensity of the highest IQE was determined as the workout intensity using the following formula: (maximum IQE pressure mmHg −100 mmHg) × 0.5. The participants used visual feedback to execute 50 % IQE while viewing the manchette of the blood pressure cuff.
2.3. IFP hardness measurement
Using an 18-MHz transducer (PLI-1205BX, Canon Medical Systems Inc., Tochigi, Japan) of an ultrasound scanner (Aplio i700, Canon Medical Systems Inc., Tochigi, Japan), IFP hardness was assessed, in accordance with prior studies [12,17]. Shear-wave elastography was used to measure IFP hardness after the transducer was positioned along the patellar tendon's long axis. For shear-wave elastography, the IFP served as the region of interest, and the mean of three measurements was designated as the representative value.
2.4. Hemoglobin concentration in the IFP measurement
As previously reported, tissue oxygenation of the IFP was assessed via near-infrared spectroscopy (NIRO-200NX system, Hamamatsu Photonics Inc., Shizuoka, Japan) [12,17]. The light source was a laser diode with three wavelengths (775, 810, and 850 nm), while the light receiver was a photodiode. There were 2.0 s sample intervals. The probe was fastened while straddling the patellar tendon's center, with an estimated depth of 1.5 cm since the maximum depth of near-infrared spectroscopy measurement was 50 % of the distance between the probe's transmitter and receiver, which was set at 3.0 cm in this case [18]. To block noise from ambient light, the measuring device's accessory, a light-shielding sheet, was placed over the probe and secured to the body surface. Spatially resolved spectroscopy was used to calculate the tissue oxygenation index (TOI) of IFP [19]. The modified Beer-Lambert method was used to determine the total hemoglobin (cHb), deoxygenated hemoglobin (HHb), and oxygenated hemoglobin (O2Hb) levels. Each hemoglobin value, which starts at zero, indicates variation from the first measurement. Participants were permitted to take a break until the hemoglobin waveforms stabilized. In the exercise task, they completed 10 sets of 10-s 50 % IQE after a 1-min rest, followed by 3 s of rest. Thereafter, they were instructed to rest once again for 5 min in order to track any changes over time. From the experimental data, O2Hb, HHb, cHb, and TOI data were collected prior to, during, and following the exercise task (Fig. 1). The baseline, which was the mean at rest 1 min before the exercise task, the post, which was the mean at rest 3–4 min after the exercise task, and the mean of the lower limit of each of the ten sets of hemoglobin values that declined during the 50 % IQE (IQE task: IQE-t) were utilized. With the baseline set to zero, the IQE-t and post at each hemoglobin level were computed as the change from baseline.
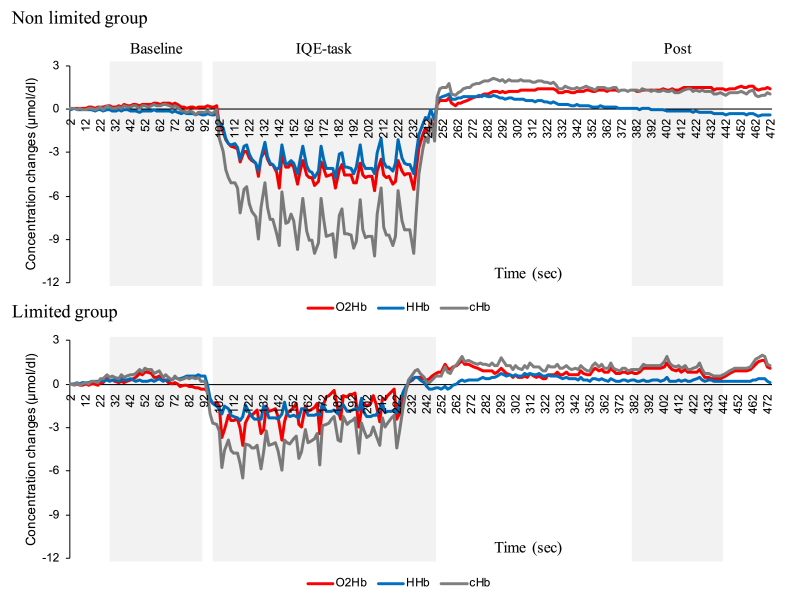
Fig. 1.
Typical time-series change in hemoglobin concentration in IFP following IQE. Typical concentration changes in oxygenated (O2Hb, red line), deoxygenated (HHb, blue line), and total (cHb, gray line) hemoglobin in the non-limited and limited groups are illustrated as time-series waveform data. The data for the statistical analysis before (baseline), during (IQE-task), and after the isometric quadriceps exercise (post) are presented as the mean value at rest 1 min before the task, mean of the 10 lower limits during the task, and mean value at 1 min after 3 min rest after the task, respectively.
2.5. Study size
The sample size was calculated preliminarily based on data of five patients before conducting this study. Using G∗power version 3.1, power analysis revealed that 13 patients in each group were required to identify a difference of 1.19 effect size between the groups, with 80 % power at the 5 % significance level for a two-tailed test.
2.6. Statistical analyses
All statistical analyses were performed using SPSS version 27 (IBM Corp., Armonk, NY, USA).
An unpaired t-test was used to investigate whether there were any differences in the means of age, height, weight and body mass index between the limited and non-limited groups. The effects of measurement points (rest and IQE) and group (limited and non-limited) on IFP hardness were investigated using linear mixed models. Furthermore, the effects of group and measurement points (baseline, IQE-task, and post) on O2Hb, HHb, cHb, and TOI were investigated using linear mixed models. The fit of the linear mixed model was verified visually by assessing the residuals' homoscedasticity and normality. Measurement points, groups, and the interaction between measurement points and groups were considered fixed effects. The covariates included in the analysis were age, body mass index, K-L grade and maximum IQE pressure. Participants comprised the random effects. Using the estimated marginal means of the Bonferroni technique, post hoc testing for IFP hardness were performed for both within-group comparisons (IQE and rest) and between-group comparisons (limited and non-limited group). Baseline, IQE task, and post-test comparisons were included in the post hoc analyses for O2Hb, HHb, cHb, and TOI, which were conducted using estimated marginal means and the Bonferroni technique. The significance level was set at p < 0.05. Bonferroni method corrected for p-values.
3. Results
3.1. Participants
The eligible criteria and characteristics of the participants in this study are shown in Fig. 2 and Table 1.
Fig. 2.
Flowchart of inclusion and exclusion criteria.
Table 1.
Characteristics of participants.
| Limited group | Non-limited group | P-value | |
|---|---|---|---|
| Sex (M:F) | 0:16 | 0:13 | – |
| Age (years) | 67.3 (5.76) | 65.1 (7.35) | 0.687 |
| Height (cm) | 147.4 (6.45) | 146.3 (7.35) | 0.428 |
| Weight (kg) | 62.5 (9.72) | 60.4 (12.8) | 0.843 |
| BMI (kg/m2) | 25.3 (2.71) | 24.0 (3.62) | 0.386 |
| K-L (Ⅰ, Ⅱ, Ⅲ) | 4:7:5 | 5:5:3 | – |
| Knee extension (°) | −10.4 (4.17) | 0 (0) | – |
Variables are presented as mean (standard deviation). An unpaired t-test was used to investigate whether there were any differences in age, height, weight and body mass index between the limited and non-limited groups. BMI: body mass index, K-L: Kellgren–Lawrence grade.
3.2. Hardness of the IFP at rest and during IQE
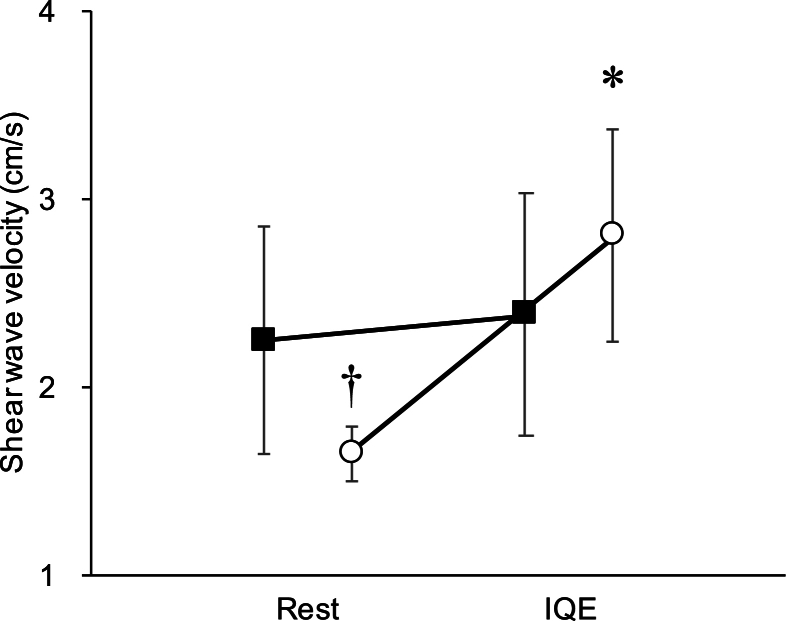
The IFP hardness values at rest and during IQE are presented in Table 2. In a linear mixed model, the main effect between groups was not significant (limited and non-limited group; p = 0.428), whereas the main effect of measurement points was significant (hardness at rest and during IQE; p < 0.001), with an interaction effect (p < 0.001) (Table 2). A post-hoc test revealed no significant difference in the limited group between the rest and IQE groups for IFP hardness (mean difference = −0.136, 95 % confidence interval [CI] [−0.475, 0.203], p > 0.999) (Fig. 3). In the non-limited group, the IFP hardness of the IQE group was significantly higher than that in the rest group (MD = −1.154, 95 % CI [−1.530, −0.778], p < 0.001) (Fig. 3). At rest, the IFP hardness of the limited group was significantly higher than that of the non-limited group (MD = 0.617, 95 % CI [0.239, 0.995], p = 0.014) (Fig. 3).
Table 2.
Infrapatellar fat pad shear wave velocity in rest and during isometric quadriceps exercise.
| Group |
Fixed effects |
Interaction | Covariate | ||||
|---|---|---|---|---|---|---|---|
| Limited | Non-Limited | Group | Measurement points | ||||
| Shear wave velocity (m/s) | Rest | 2.25 (1.94, 2.56) | 1.65 (1.57, 1.74) | 0.428 | <0.001 | <0.001 | Age BMI K-L IQE |
| IQE | 2.39 (2.05, 2.72) | 2.81 (2.48, 3.13) | |||||
Shear wave velocity is from the linear mixed model. Data are shown as mean (95 % confidence intervals). Fixed effects and interaction values indicate p-values. IQE; maximum IQE pressure.
Fig. 3.
Hardness of IFP at rest and during IQE. Squares: limited group. Circles: non-limited group. Asterisks (∗) indicate data that are significantly different from rest (p < 0.05). Daggers (†) indicate data that are significantly different from the limited group (p < 0.05).
3.3. Hemoglobin concentration and TOI in the IFP
The hemoglobin concentration and TOI results for the IFP are illustrated in Table 3. For O2Hb in a linear mixed model, the main effect between groups was not significant (p = 0.183), whereas the main effect of measurement points was significant (baseline, IQE-task, and post; p < 0.001), with an interaction effect (p < 0.001) (Table 3).
Table 3.
Hemoglobin concentration and tissue oxygenation index in the infrapatellar fat pad at each measurement point.
| Group |
Fixed effects |
Interaction | Covariate | ||||
|---|---|---|---|---|---|---|---|
| Limited | Non-Limited | Group | Measurement points | ||||
| O2Hb (mol/dl) | Baseline | 0 | 0 | 0.183 | <0.001 | <0.001 | Age BMI K-L IQE |
| IQE-task | −1.27 (−2.47, −0.07) | −1.97 (−3.12, −0.82) | |||||
| Post | 0.27 (−0.38, 0.92) | 2.15 (1.80, 2.50) | |||||
| HHb (mol/dl) | Baseline | 0 | 0 | 0.196 | <0.001 | 0.003 | Age BMI K-L IQE |
| IQE-task | −0.09 (−0.68, 0.50) | −1.47 (−2.31, −0.64) | |||||
| Post | −0.12 (−0.60, 0.36) | 0.31 (−0.21, 0.83) | |||||
| cHb (mol/dl) | Baseline | 0 | 0 | 0.646 | <0.001 | <0.001 | Age BMI K-L IQE |
| IQE-task | −1.36 (−3.08, 0.36) | −3.44 (−5.02, −1.86) | |||||
| Post | 0.14 (−0.82, 1.10) | 2.46 (1.84, 3.09) | |||||
| TOI (%) | Baseline | 72.7 (70.4, 74.9) | 77.6 (72.7, 82.5) | 0.021 | <0.001 | 0.232 | Age BMI K-L IQE |
| IQE-task | 71.8 (69.5, 74.0) | 77.6 (72.4, 82.7) | |||||
| Post | 73.1 (70.6, 75.7) | 79.3 (73.7, 84.9) | |||||
Results of linear mixed model for each hemoglobin concentration and tissue oxygenation index (TOI). Data are shown as mean (95 % confidence intervals). Fixed effects and interaction values indicate p-values. IQE; maximum IQE pressure.
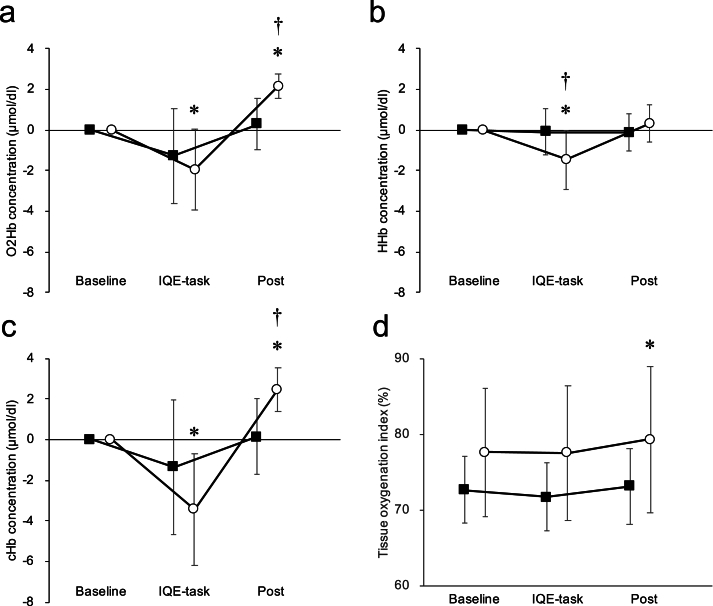
O2Hb concentration in the IQE task and post were not significantly different from baseline in the limited group (baseline vs. IQE task: MD = 1.268, 95 % CI [−0.331, 2.205], p = 0.157; baseline vs. post: MD = −0.024, 95 % CI [−0.961, 0.913], p > 0.999) (Fig. 4). In the non-limited group, IQE task O2Hb concentration was significantly lower than baseline. Post O2Hb concentration was significantly higher than baseline (baseline vs. IQE task: MD = 1.968, 95 % CI [0.929, 3.001], p = 0.007; baseline vs. post: MD = −4.118, 95 % CI [−5.156, −3.079], p < 0.001) (Fig. 4).
Fig. 4.
Changes in each hemoglobin concentration at baseline, IQE-task, and post IQE. Squares: limited group. Circles: non-limited group. a: Oxygenated hemoglobin (O2Hb). b: Deoxygenated hemoglobin (HHb). c: Total hemoglobin (cHb). d: TOI. Asterisks (∗) indicate data that are significantly different from baseline (p < 0.05). Daggers (†) indicate data that are significantly different from the limited group (p < 0.05).
For HHb in a linear mixed model, the main effect between groups was not significant (p = 0.196). In contrast, the main effect of measurement points was significant (p < 0.001), with an interaction effect (p = 0.003) (Table 3). HHb concentration in the IQE task was significantly lower than baseline in the non-limited group (MD = 1.475, 95 % CI [0.800, 2.148], p = 0.001) (Fig. 4).
For cHb in a linear mixed model, the main effect between groups was not significant (p = 0.646). In contrast, the main effect of measurement points was significant (p < 0.001), with an interaction effect (p < 0.001) (Table 3). The cHb concentration in the IQE task and post were not significantly different from baseline in the limited group (baseline vs. IQE task: MD = 1.584, 95 % CI [−0.279, 2.889], p = 0.314; baseline vs. post: MD = 0.099, 95 % CI [−1.206, 1.405], p > 0.999) (Fig. 4). In the non-limited group, IQE task cHb concentration was significantly lower than baseline. Post was significantly higher than baseline cHb concentration (baseline vs. IQE task: MD = 3.442, 95 % CI [1.994, 4.891], p < 0.001; baseline vs. post: MD = −5.903, 95 % CI [−7.352, −4.455], p < 0.001) (Fig. 4).
For TOI in a linear mixed model, the main effect between groups (p = 0.021) and measurement points (p < 0.001) was significant, whereas the interaction effect was not significant (p = 0.232) (Table 3). TOI of the IQE task and post was not significantly different from baseline in the limited group (baseline vs. IQE task: MD = 0.924, 95 % CI [−0.038, 1.887], p = 0.974; baseline vs. post: MD = −0.472, 95 % CI [−1.434, 0.491], p > 0.999) (Fig. 4). In the non-limited group, IQE task TOI concentrations were not significantly different from baseline, but post was significantly higher than baseline TOI (baseline vs. IQE task: MD = 0.052, 95 % CI [−1.014, 1.118], p > 0.999; baseline vs. post: MD = 1.759, 95 % CI [−2.825, −0.692], p = 0.031) (Fig. 4).
4. Discussion
This study revealed that the IFP hardness of the non-limited group following IQE significantly increased, whereas the limited group showed no significant increase. The non-limited group's IFP O2Hb and cHb concentrations increased in the post compared to baseline, and significantly decreased in the IQE task. In contrast, the limited group showed no discernible variations. Specifically, the limitation of knee extension affected the IFP hemoglobin concentration or IFP hardness following IQE.
Several studies have investigated the characteristics of KOA with knee joint extension limitations [[13], [14], [15]]. Campbell et al. reported that the extent of knee joint extension limitation in KOA was associated with worse pain and joint function [13]; the presence of knee joint extension limitation was a risk factor for the progression of radiographic findings, early conversion to total knee arthroplasty, pain, stiffness, and decreased functional capacity [15]. Knee joint extension limitation was associated with contralateral knee joint extension limitation [14]. KOA with limited knee extension was already known to lead to knee pain and be a risk factor for KOA progression; further to this, we found that KOA with knee joint extension limitation influences the IFP hemoglobin concentration or IFP hardness following IQE.
After IQE, the limited group exhibited no changes in IFP hemoglobin concentration or IFP hardness, whereas the non-limited group presented with changes. The IFP is compressed by the patellar tendon, femur, and tibia during IQE [17], increasing the IFP hardness; however, knee joint extension limitation may be less susceptible to this mechanical stress. Without knee joint extension limitations, mechanical stress is repeated, and the capillaries in a similar region are compressed and released, increasing blood flow and causing reactive hyperemia [20]. As a result, the oxygenation of the IFP is considered to be enhanced. This is supported by the results of this study, wherein the TOI of the IFP in the non-limited group increased after IQE.
A previous study [12] has reported that measuring the hardness of the IFP and changes in hemoglobin following IQE could indicate local microcirculatory disorders of the IFP in patients with KOA. However, the reason why the IFP in KOA cases showed local microcirculatory dysfunction was unclear. Since this local microcirculatory disturbance might lead to fibrosis of the IFP via hypoxia, elucidating the cause was necessary. We hypothesized that the reason why the IFP could show local microcirculatory disturbance was due to the lack of mechanical stress during IQE. Therefore, we focused on knee joint extension restriction as a factor that would inhibit mechanical stress on the IFP during IQE. Previous studies [[13], [14], [15]] have confirmed that knee extension limitation is a characteristic feature of KOA. Moreover, when comparing the presence or absence of knee extension limitation in this study, changes in IFP hardness and hemoglobin occurred when there was no knee extension limitation. Based on these results, it is suggested that knee extension limitation is involved in the local microcirculation of the IFP in patients with KOA wherein treatment of knee extension limitation might be important. Despite the clinical significance of the IFP, no exercise therapy can prevent IFP fibrosis. Hence, future studies are needed to determine whether IQE can provide IFP oxygenation if knee extension limitation in KOA improves. We believe that the results of this study may validate this hypothesis.
4.1. Study limitations
This study had several limitations. First, sex differences were not evident because only women were included. Second, the study focused on the range of motion of knee joint extension as a factor in the lack of change in IFP hemoglobin concentration and IFP hardness following IQE but not on muscular strength factors such as the quadriceps muscle. Third, since we did not compare between K-L grades, whether IFP hardness or hemoglobin concentration changed as K-L grade progressed remains unclear. Fourth, since the load of IQE was set at 50 % of the participants' maximum IQE pressure, the load was not uniform between the two groups. This might have influenced the results. However, we accounted for this by adding maximum IQE pressure as a covariate in the linear mixed model. Fifth, in this study, we performed IQE at 50 % intensity with a knee flexion angle of 20°. However, to what extent the hip extension muscles were involved in addition to the knee extension muscles during IQE remains unclear. In addition, the results might have differed when IQE was performed at different knee flexion angles. In the future, investigating how IFP hardness and hemoglobin concentration change when IQE is performed at different knee flexion angles and the extent to which 50 % intensity IQE is a functionally significant load will be necessary. Finally, given that this was a cross-sectional study, whether IQE exercise without knee extension limitation prevents IFP fibrosis in KOA remains unclear. Longitudinal studies are recommended to verify these findings.
In conclusion, the limited group revealed no change in IFP hemoglobin concentration and IFP hardness following IQE, whereas the opposite was observed for the non-limited group, indicating oxygenation.
Author contributions
Each author made a significant contribution to this research. T.K., T.M., and M.W. oversaw the study's final approval and critical revision for significant intellectual content. S.N. was data collection and assembly, analysis and interpretation, drafting. S.K. and M.T. were responsible for the study's conception and design, data analysis and interpretation, critical revision for significant intellectual content, and final approval. From the beginning to the end, S.K. was in charge of ensuring the work's integrity.
Data statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Role of the funding source
This study was supported by the JSPS KAKENHI Grant Number JP24K02805, and the Morinomiya University Presidents Research Encouragement Award 2023MPA1.
Declaration of competing interest
There are no conflicts of interest to declare for the authors.
Acknowledgments
We are very grateful to the staff of Wada Orthopedic Clinic and Kudo Laboratory for their cooperation, comments, and ideas.
Handling Editor: H Madry
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Sharma L. Osteoarthritis of the knee. N. Engl. J. Med. 2021;384:51–59. doi: 10.1056/NEJMcp1903768. [DOI] [PubMed] [Google Scholar]
- 3.Muraki S., Akune T., Oka H., Ishimoto Y., Nagata K., Yoshida M., Tokimura F., Nakamura K., Kawaguchi H., Yoshimura N. Incidence and risk factors for radiographic knee osteoarthritis and knee pain in Japanese men and women: a longitudinal population-based cohort study. Arthritis Rheum. 2012;64:1447–1456. doi: 10.1002/art.33508. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M., Saika A., Yoshida H., Suzuki T., Yamamoto S., Ishibashi H., Kawaguchi H., Nakamura K., Akune T. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metabol. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 5.Emmi A., Stocco E., Boscolo-Berto R., Contran M., Belluzzi E., Favero M., Ramonda R., Porzionato A., Ruggieri P., De Caro R., Macchi V. Infrapatellar fat pad-synovial membrane anatomo-fuctional unit: microscopic basis for piezo1/2 mechanosensors involvement in osteoarthritis pain. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.886604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng N., Yan Z.P., Chen X.Y., Ni G.X. Infrapatellar fat pad and knee osteoarthritis. Aging Dis. 2020;11:1317–1328. doi: 10.14336/AD.2019.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belluzzi E., Stocco E., Pozzuoli A., Granzotto M., Porzionato A., Vettor R., De Caro R., Ruggieri P., Ramonda R., Rossato M., Favero M., Macchi V. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/6390182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onuma H., Tsuji K., Hoshino T., Inomata K., Udo M., Nakagawa Y., Katagiri H., Miyatake K., Watanabe T., Sekiya I., Muneta T., Koga H. Fibrotic changes in the infrapatellar fat pad induce new vessel formation and sensory nerve fiber endings that associate prolonged pain. J. Orthop. Res. 2020;38:1296–1306. doi: 10.1002/jor.24580. [DOI] [PubMed] [Google Scholar]
- 9.Fontanella C.G., Belluzzi E., Pozzuoli A., Favero M., Ruggieri P., Macchi V., Carniel E.L. Mechanical behavior of infrapatellar fat pad of patients affected by osteoarthritis. J. Biomech. 2022;131 doi: 10.1016/j.jbiomech.2021.110931. [DOI] [PubMed] [Google Scholar]
- 10.Okita Y., Miura R., Morimoto M., Sadamatsu T., Kawahara T., Gamada K. Three-dimensional volume and shape of the infrapatellar fat pad during quasi-static knee extension from 30° to 0°: comparisons of patients with osteoarthritic knees and young, healthy individuals. J. Phys. Ther. Sci. 2023;35:507–514. doi: 10.1589/jpts.35.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa T., Kawahata H., Aoki M., Kudo S. Inhibitory effect of low-intensity pulsed ultrasound on the fibrosis of the infrapatellar fat pad through the regulation of HIF-1α in a carrageenan-induced knee osteoarthritis rat model. Biomed. Rep. 2022;17:79. doi: 10.3892/br.2022.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanishi S., Tsutsumi M., Kitano M., Kitagawa T., Miyashita T., Wada M., Kudo S. Effect of isometric quadriceps exercise on local microcirculation of the infrapatellar fat pad in female patients with knee osteoarthritis. Osteoarthr. Carti. 2024;32:1319–1326. doi: 10.1016/j.joca.2024.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Campbell T.M., Ramsay T., Trudel G. Knee flexion contractures are associated with worse pain, stiffness, and function in patients with knee osteoarthritis: data from the osteoarthritis Initiative. Pharm. Manag. PM R. 2021;13:954–961. doi: 10.1002/pmrj.12497. [DOI] [PubMed] [Google Scholar]
- 14.Campbell T.M., Trudel G. Knee flexion contracture associated with a contracture and worse function of the contralateral knee: data from the osteoarthritis Initiative. Arch. Phys. Med. Rehabil. 2020;101:624–632. doi: 10.1016/j.apmr.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Campbell T.M., McGonagle D. Flexion contracture is a risk factor for knee osteoarthritis incidence, progression and earlier arthroplasty: data from the osteoarthritis Initiative. Ann. Phys. Rehabil. Med. 2021;64 doi: 10.1016/j.rehab.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Bohnsack M., Hurschler C., Demirtas T., Rühmann O., Stukenborg-Colsman C., Wirth C.J. Infrapatellar fat pad pressure and volume changes of the anterior compartment during knee motion: possible clinical consequences to the anterior knee pain syndrome. Knee Surg. Sports Traumatol. Arthrosc. 2005;13:135–141. doi: 10.1007/s00167-004-0561-1. [DOI] [PubMed] [Google Scholar]
- 17.Katayama N., Noda I., Fukumoto Y., Kawanishi K., Kudo S. Effects of isometric contraction of the quadriceps on the hardness and blood flow in the infrapatellar fat pad. J. Phys. Ther. Sci. 2021;33:722–727. doi: 10.1589/jpts.33.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K., Homma S., Okada E., Matsushita K., Homma S., Okada E. Influence of adipose tissue on muscle oxygenation measurement with an NIRS instrument. SPIEL. 1997;3194:159–165. [Google Scholar]
- 19.Matcher S.J., Kirkpatrick P.J., Nahid K., Cope M., Delpy D.T. Absolute quantification methods in tissue near infrared spectroscopy. SPIE Proceedings. SPIE. 1995;2389:486–495. doi: 10.1117/12.209997. [DOI] [Google Scholar]
- 20.Rosenberry R., Nelson M.D. Reactive hyperemia: a review of methods, mechanisms, and considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R605–R618. doi: 10.1152/ajpregu.00339.2019. [DOI] [PubMed] [Google Scholar]