Abstract
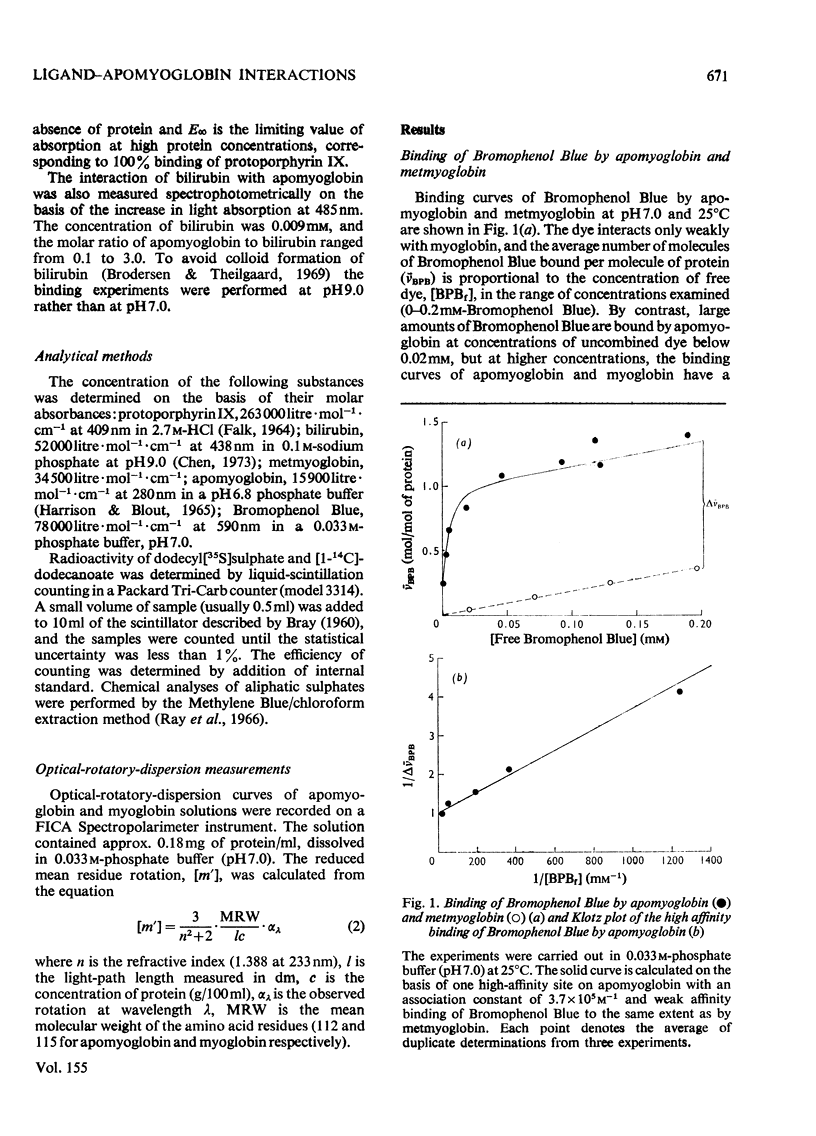
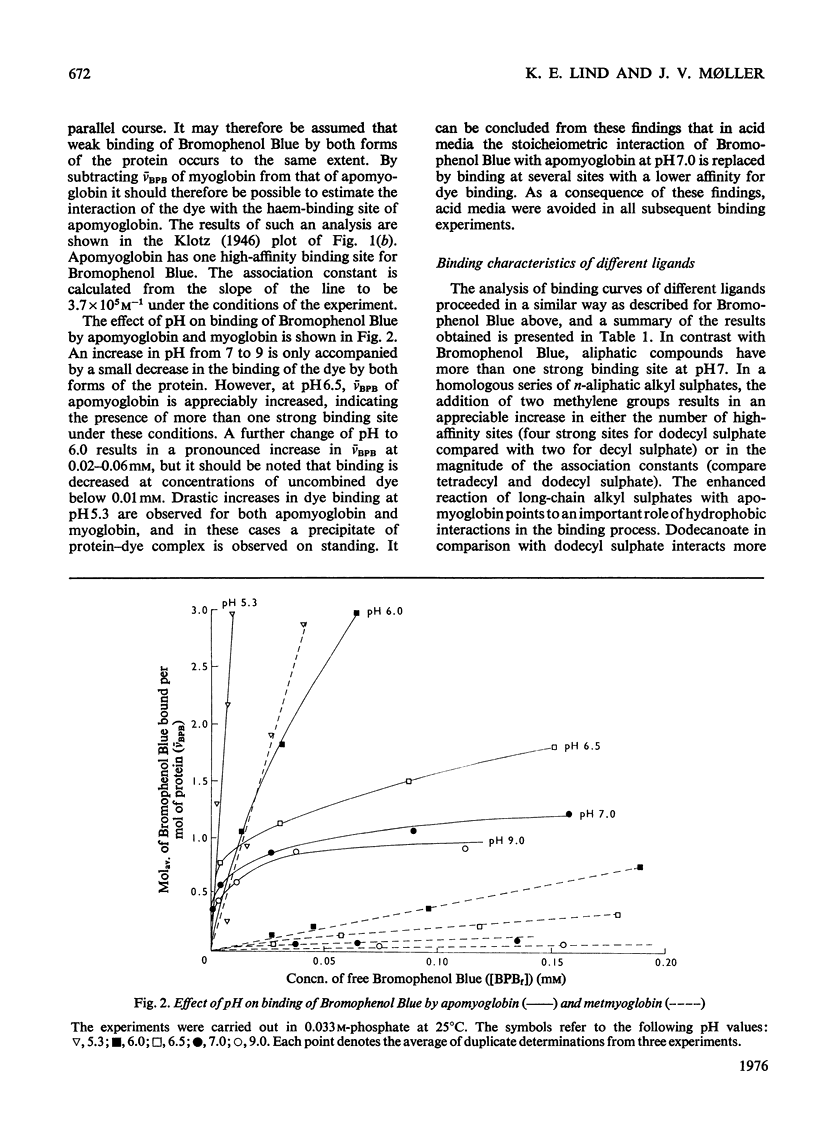
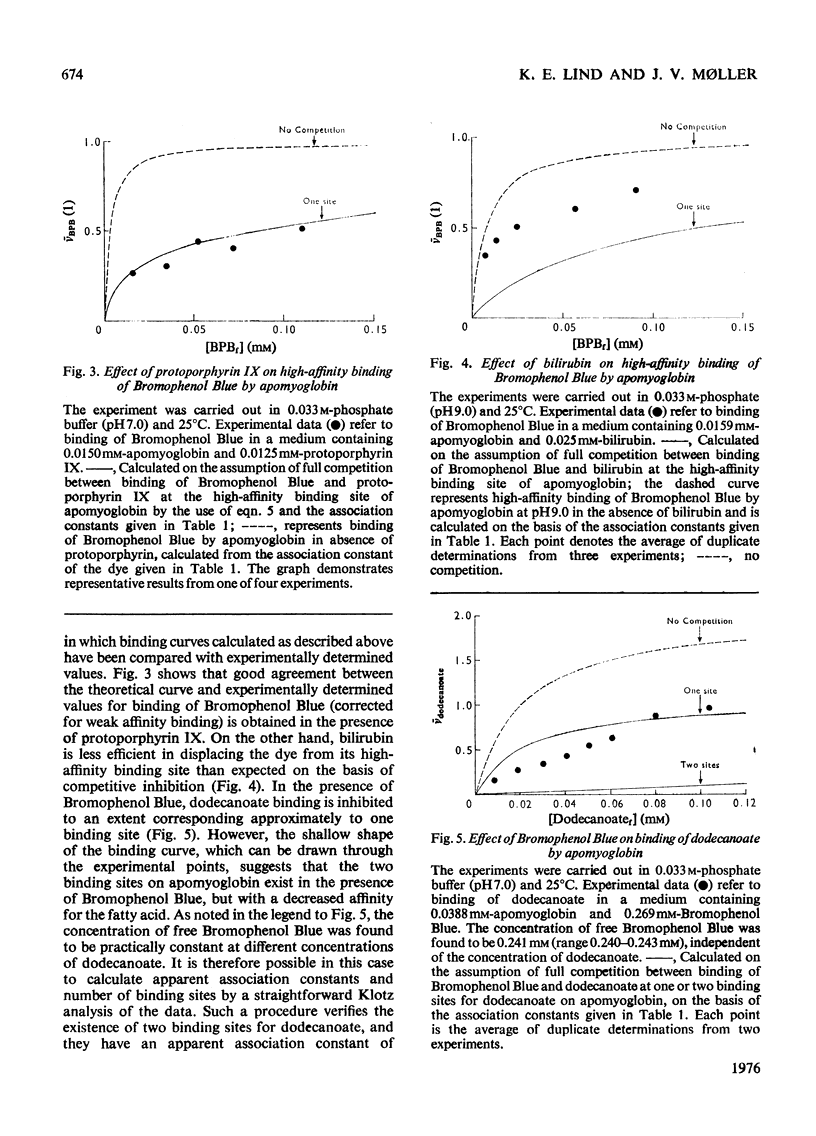
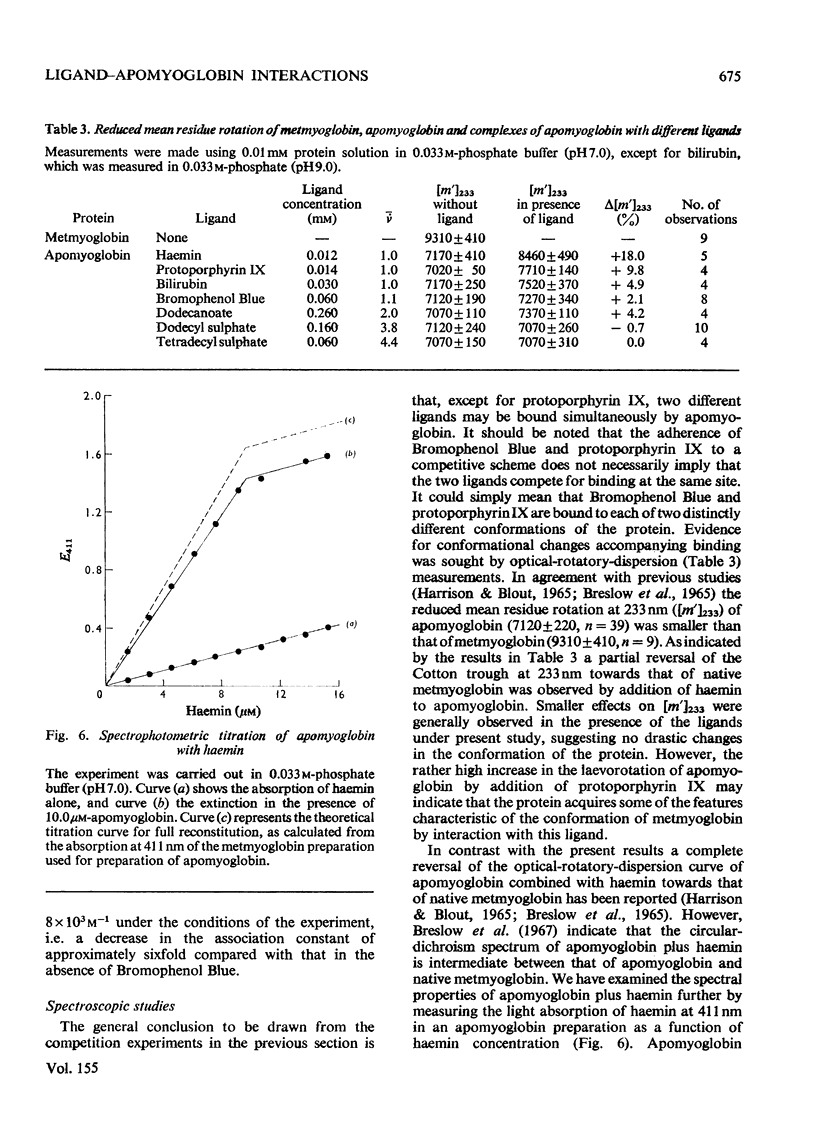
1. The interaction of the haem-binding region of apomyoglobin with different ligands was examined by ultrafiltration, equilibrium dialysis and spectrophotometry, to study unspecific features of protein-ligand interactions such as they occur in, for example, serum albumin binding. 2. Apomyoglobin, in contrast with metmyoglobin, binds at pH 7, with a high affinity, one molecule of Bromophenol Blue, bilirubin and protoporphyrin IX, two molecules of n-dodecanoate and n-decyl sulphate and four molecules of n-dodecyl sulphate and n-tetradecyl sulphate. 3. The number of high-affinity sites and/or association constants for the alkyl sulphates are enhanced by an increase of hydrocarbon length, indicating hydrophobic interactions with the protein. 4. Measurements of the temperature-dependence of the association constants of the high-affinity sites imply that the binding processes are largely entropy-driven. 5. Binding studies in the presence of two ligands show that bilirubin plus Bromophenol Blue and dodecanoate plus Bromophenol Blue can be simultaneously bound by apomyoglobin, but with decreased affinities. By contrast, the apomyoglobin-protoporphyrin IX complex does not react with Bromophenol Blue. 6. Optical-rotatory-dispersion measurements show that the laevorotation of apomyoglobin is increased towards that of metmyglobin in the presence of haemin and protoporphyrin IX. Small changes in the optical-rotatory-dispersion spectrum of apomyoglobin are observed in the presence of the other ligands. 7. It is concluded that the binding sites on apomyoglobin probably do not pre-exist but appear to be moulded from predominantly non-polar amino acid residues by reaction with hydrophobic ligands. 8. Comparison with data in the literature indicates that apomyoglobin on a weight basis has a larger hydrophobic area avaialble for binding of ligands than has human serum albumin. On the other hand, the association constants of serum for the ligands used in this study are generally somewhat larger than those of apomyoglobin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., Singhal R. P. Conformational studies on modified proteins and peptides. V. Conformation of myoglobin derivatives modified at two carboxyl groups. J Biol Chem. 1972 Sep 25;247(18):5980–5986. [PubMed] [Google Scholar]
- BANERJEE R. Study of hematin-globin linkage. Determination of equilibrium constants. Biochem Biophys Res Commun. 1962 Jun 19;8:114–119. doi: 10.1016/0006-291x(62)90247-4. [DOI] [PubMed] [Google Scholar]
- BRESLOW E., BEYCHOK S., HARDMAN K. D., GURD F. R. RELATIVE CONFORMATIONS OF SPERM WHALE METMYOGLOBIN AND APOMYOGLOBIN IN SOLUTION. J Biol Chem. 1965 Jan;240:304–309. [PubMed] [Google Scholar]
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Breslow E., Koehler R., Girotti A. W. Properties of protoporphyrin-apomyoglobin complexes and related compounds. J Biol Chem. 1967 Sep 25;242(18):4149–4156. [PubMed] [Google Scholar]
- Brodersen R., Theilgaard J. Bilirubin colloid formation in neutral aqueous solution. Scand J Clin Lab Invest. 1969 Dec;24(4):395–398. doi: 10.3109/00365516909080178. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., Hay L., Stoner H. B. The binding of L-tryptophan to serum albumins in the presence of non-esterified fatty acids. Biochem J. 1975 Mar;146(3):653–658. doi: 10.1042/bj1460653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E., Weber G. Cooperative effects in binding by bovine serum albumin. I. The binding of 1-anilino-8-naphthalenesulfonate. Fluorimetric titrations. Biochemistry. 1966 Jun;5(6):1893–1900. doi: 10.1021/bi00870a016. [DOI] [PubMed] [Google Scholar]
- Fairclough G. F., Jr, Fruton J. S. Peptide-protein interaction as studied by gel filtration. Biochemistry. 1966 Feb;5(2):673–683. doi: 10.1021/bi00866a038. [DOI] [PubMed] [Google Scholar]
- HARRISON S. C., BLOUT E. R. REVERSIBLE CONFORMATIONAL CHANGES OF MYOGLOBIN AND APOMYOGLOBIN. J Biol Chem. 1965 Jan;240:299–303. [PubMed] [Google Scholar]
- Jacobsen J. Binding of bilirubin to human serum albumin - determination of the dissociation constants. FEBS Lett. 1969 Oct 21;5(2):112–114. doi: 10.1016/0014-5793(69)80307-8. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- KENDREW J. C. Side-chain interactions in myoglobin. Brookhaven Symp Biol. 1962 Dec;15:216–228. [PubMed] [Google Scholar]
- Kragh-Hansen U., Moller J. V., Sheikh M. I. A spectrophotometric micromethod for the determination of binding of phenol red to plasma proteins of various species. Pflugers Arch. 1972;337(2):163–176. doi: 10.1007/BF00587838. [DOI] [PubMed] [Google Scholar]
- Lind K. E., Kragh-Hansen U., Moller J. V. Protein binding of small molecules. V. Binding of bromphenol blue by chemical modifications of human serum albumin. Biochim Biophys Acta. 1974 Dec 18;371(2):451–461. [PubMed] [Google Scholar]
- Lovrien R., Sturtevant J. M. Calorimetric determination of the enthalpies of binding of ions to deionized bovine serum albumin. Biochemistry. 1971 Oct 12;10(21):3811–3815. doi: 10.1021/bi00797a001. [DOI] [PubMed] [Google Scholar]
- Luk C. K. Energy transfer between tryptophans and aromatic ligands in apomyoglobin. Biopolymers. 1971;10(8):1317–1329. doi: 10.1002/bip.360100806. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Girotti A. W. Photooxidation of the protoporphyrin-apomyoglobin complex. Biochemistry. 1973 Aug 14;12(17):3187–3193. doi: 10.1021/bi00741a007. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Reynolds J. A., Tanford C. The interaction of a cationic detergent with bovine serum albumin and other proteins. J Biol Chem. 1974 Jul 25;249(14):4452–4459. [PubMed] [Google Scholar]
- Ray A., Reynolds J. A., Polet H., Steinhardt J. Binding of large organic anions and neutral molecules by native bovine serum albumin. Biochemistry. 1966 Aug;5(8):2606–2616. doi: 10.1021/bi00872a019. [DOI] [PubMed] [Google Scholar]
- Reynolds J., Herbert S., Steinhardt J. The binding of some long-chain fatty acid anions and alcohols by bovine serum albumin. Biochemistry. 1968 Apr;7(4):1357–1361. doi: 10.1021/bi00844a016. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Hermans J., Jr Kinetics of conformation change of sperm-whale myoglobin. 3. Folding and unfolding of apomyoglobin and the suggested overall mechanism. Biochemistry. 1972 May 9;11(10):1845–1849. doi: 10.1021/bi00760a018. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Steinhardt J., Krijn J., Leidy J. G. Differences between bovine and human serum albumins: binding isotherms, optical rotatory dispersion, viscosity, hydrogen ion titration, and fluorescence effects. Biochemistry. 1971 Oct 26;10(22):4005–4015. doi: 10.1021/bi00798a001. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Tanford C. Hydrophobic free energy, micelle formation and the association of proteins with amphiphiles. J Mol Biol. 1972 Jun 14;67(1):59–74. doi: 10.1016/0022-2836(72)90386-5. [DOI] [PubMed] [Google Scholar]


