Abstract
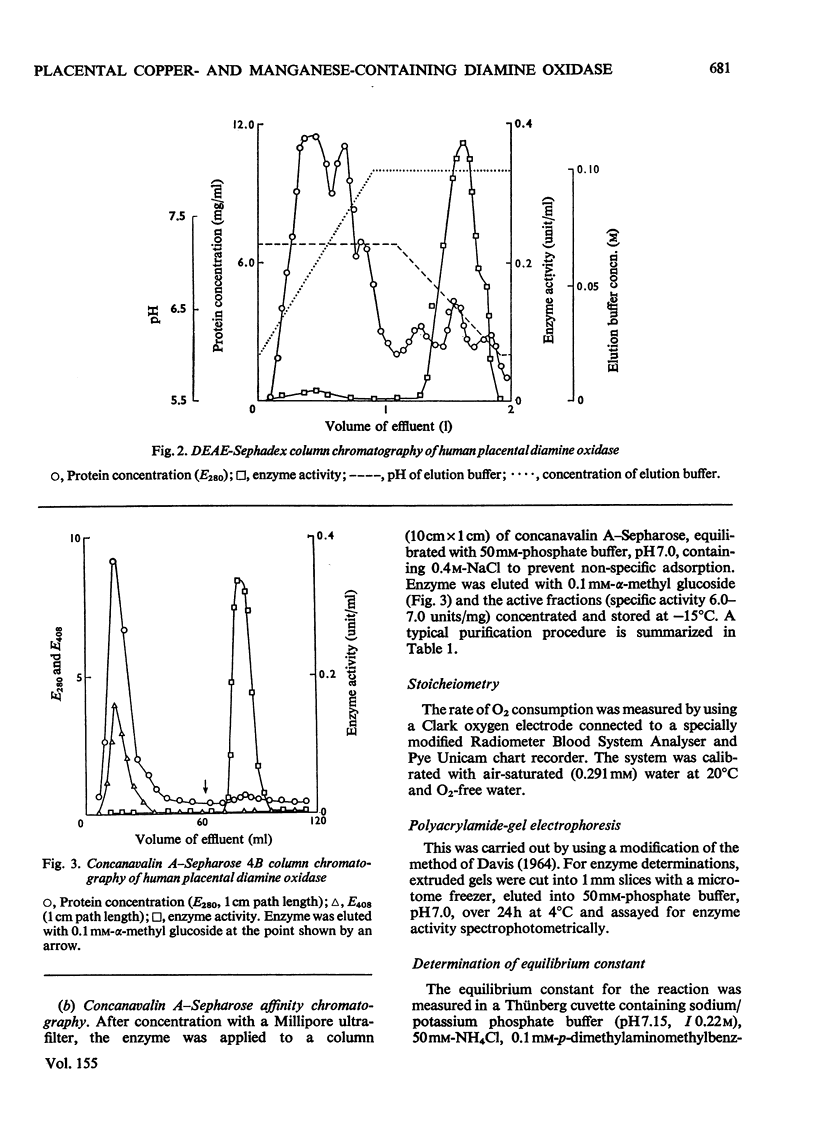
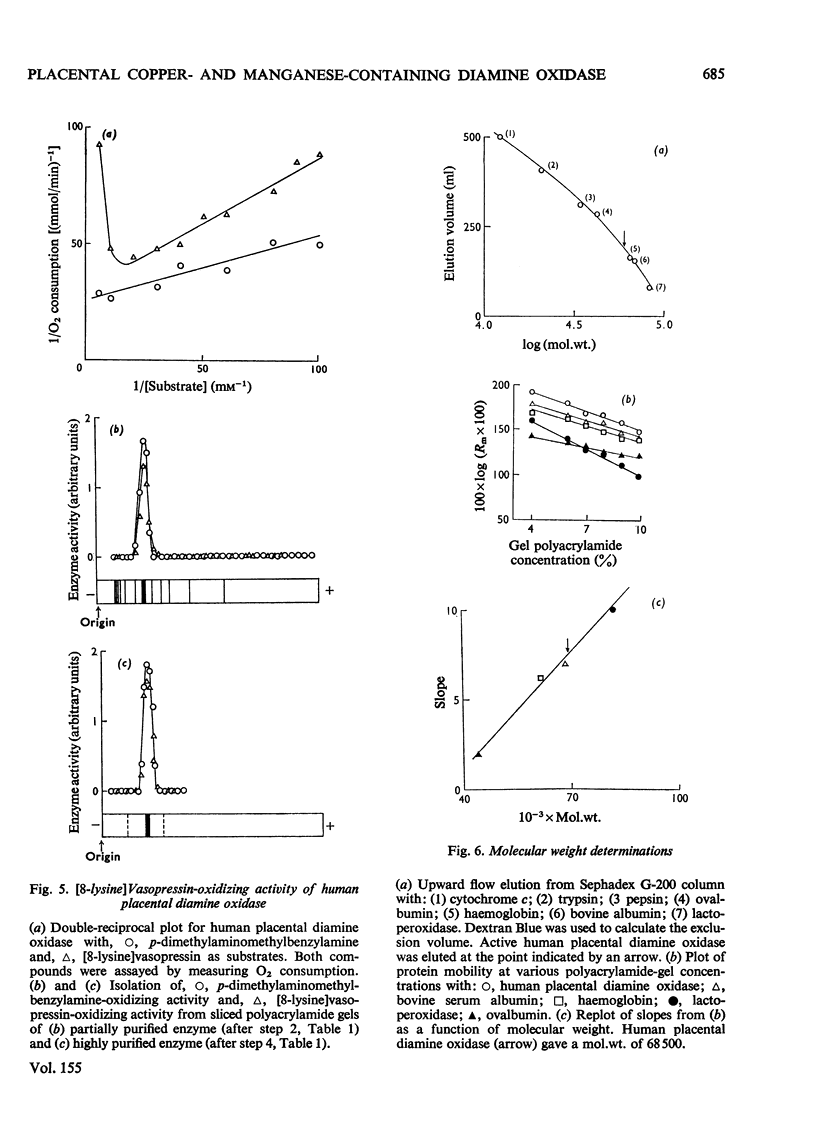
1. Isoelectric focusing studies of human placental diamine oxidase showed the pI value of the active enzyme to be 6.5. This information was used in modifying the enzyme purification by incorporating column chromatography on DEAE-Sephadex with ionic strength and pH gradient elution and this, together with affinity chromatography on concanavalin A--Sepharose, gave a highly purified preparation, with a specific activity of 7.0 units/mg. 2. The enzyme gave the expected stoicheiometry with p-dimethylaminomethylbenzylamine as substrate (Keq. 2700) and also oxidized [8-arginine]vasopressin, [8-lysine]vasopressin, collagen and tropocollagen. Polyacrylamide gel slices showed identical migration of diamine-oxidizing and [8-lysine]vasopressin-oxidizing activity. 3. The molecular weight, determined by ultracentrifugation, sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, variable polyacrylamide-gel electrophoresis and Sephadex G-200 column chromatography, was estimated to be approx. 70000. 4. E.s.r. spectroscopy showed that copper and manganese were present in the purified enzyme. This result was confirmed by atomic absorption spectroscopy, which indicated a stoicheiometry for copper and manganese of approx. 1.0 and 1.2g-atom respectively/70000mol.wt. unit. 5. The e.s.r. spectral intensity did not decrease nor did the spectral line shape change when excess of p-dimethylaminomethylbenzylamine was added to the enzyme. 6. Addition of K13CN to the enzyme eliminated the copper e.s.r. signal without affecting the manganese signal. 7. The placental enzyme therefore appears to differ from other amine oxidases in terms of its metal cofactor requirement, molecular weight and substrate specificity, and possible roles in vivo for this enzyme are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achee F. M., Chervenka C. H., Smith R. A., Yasunobu K. T. Amine oxidase. XII. The association and dissociation, and number of subunits of beef plasma amine oxidase. Biochemistry. 1968 Dec;7(12):4329–4336. doi: 10.1021/bi00852a027. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- BUFFONI F., BLASCHKO H. BENZYLAMINE OXIDASE AND HISTAMINASE: PURIFICATION AND CRYSTALLIZATION OF AN ENZYME FROM PIG PLASMA. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:153–167. doi: 10.1098/rspb.1964.0086. [DOI] [PubMed] [Google Scholar]
- Bardsley W. G., Ashford J. S., Hill C. M. Synthesis and oxidation of aminoalkyl-onium compounds by pig kidney diamine oxidase. Biochem J. 1971 May;122(4):557–567. doi: 10.1042/bj1220557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Childs R. E., Crabbe M. J. Inhibition of enzymes by metal ion-chelating reagents. The action of copper-chelating reagents on diamine oxidase. Biochem J. 1974 Jan;137(1):61–66. doi: 10.1042/bj1370061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S. Kinetics of the diamine oxidase reaction. Biochem J. 1973 Mar;131(3):459–469. doi: 10.1042/bj1310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B., Margolis S. Purification of histaminase (diamine oxidase) from human pregnancy plasma by affinity chromatography. Biochim Biophys Acta. 1975 Aug 26;397(2):294–306. doi: 10.1016/0005-2744(75)90119-9. [DOI] [PubMed] [Google Scholar]
- Bradsley W. G., Crabbe M. J., Scott I. V. The amine oxidases of human placenta and pregnancy plasma. Biochem J. 1974 Apr;139(1):169–181. doi: 10.1042/bj1390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffoni F., Corte L. D., Knowles P. F. The nature of copper in pig plasma benzylamine oxidase. Biochem J. 1968 Jan;106(2):575–576. doi: 10.1042/bj1060575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. E., Bardsley W. G. The steady-state kinetics of peroxidase with 2,2'-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J. 1975 Jan;145(1):93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe M. J., Bradsley W. G. The inhibition of human placental diamine oxidase by substrate analogues. Biochem J. 1974 Apr;139(1):183–189. doi: 10.1042/bj1390183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe M. J., Childs R. E., Bardsley W. G. Time-dependent inhibition of diamine oxidase by carbonyl-group reagents and urea. Eur J Biochem. 1975 Dec 15;60(2):325–333. doi: 10.1111/j.1432-1033.1975.tb21007.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Mann P. J. Further properties of the diamine oxidase of pea seedlings. Biochem J. 1964 Apr;91(1):171–182. doi: 10.1042/bj0910171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz W., Kusche J., Werle E. Uber eine neue Methode zur Bestimmung der Diaminoxydase-Aktivität. Hoppe Seylers Z Physiol Chem. 1967 May;348(5):561–567. [PubMed] [Google Scholar]
- Mondovì B., Rotilio G., Costa M. T., Finazzi-Agrò A., Chiancone E., Hansen R. E., Beinert H. Diamine oxidase from pig kidney. Improved purification and properties. J Biol Chem. 1967 Mar 25;242(6):1160–1167. [PubMed] [Google Scholar]
- Siegel R. C., Pinnell S. R., Martin G. R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970 Nov 10;9(23):4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. II. Copper, one of the prosthetic groups of plasma monoamine oxidase. J Biol Chem. 1962 Oct;237:3077–3082. [PubMed] [Google Scholar]
- Yamada H., Kumagai H., Kawasaki H., Matsui H., Ogata K. Crystallization and properties of diamine oxidase from pig kidney. Biochem Biophys Res Commun. 1967 Dec 15;29(5):723–727. doi: 10.1016/0006-291x(67)90277-x. [DOI] [PubMed] [Google Scholar]


