ABSTRACT
Background:
Sepsis-induced acute kidney injury (AKI) is difficult to prevent because most patients are diagnosed after they develop it. Standard serum and urine creatinine levels are insensitive and nonspecific for detecting kidney injury in its early stages. Glutathione S-transferase (GST) has received little attention as a biomarker in AKI.
Methods:
This study included 65 adult patients with sepsis who developed oliguria within 72 h of admission. Baseline serum creatinine values were recorded at least 1 month before or after intensive care unit (ICU) admission. The clinical endpoints were defined as the occurrence of advanced AKI stages 2 or 3 according to the KDIGO classification. Serum creatinine and urinary GST levels were measured every 6 h from admission until 72 h postoliguria development. The primary objective was to assess the correlation between urinary GST and serum creatinine levels in patients with sepsis-induced AKI.
Results:
Among the 65 patients, 13 (20%) progressed to AKI Grade I, while 52 (80%) progressed to AKI Grade II or III. Both groups exhibited an increasing trend in serum creatinine and urinary GST levels up to 72 h. Significant mean differences between the two AKI groups were observed at 48 and 72 h for serum creatinine (P = 0.021 and P = 0.007, respectively) and at 18 h for urinary GST levels (P = 0.044).
Conclusion:
Urinary GST levels demonstrated an earlier elevation than serum creatinine levels in critically ill sepsis patients, underscoring their utility as a valuable tool for the early diagnosis and predicting AKI following admission to the ICU.
Keywords: Acute kidney injury, creatinine, glutathione S-Transferases, sepsis
INTRODUCTION
The proportion of patients presenting with sepsis-induced acute kidney injury (AKI) in the intensive care unit (ICU) has been reported to be approximately 40%–50%.[1] AKI is an independent risk factor for mortality and increases the risk of developing chronic kidney disease in survivors. Preventing sepsis-induced AKI is difficult because most patients are diagnosed after developing AKI.[2]
AKI is defined if serum creatinine increases by 0.3 mg/dL or more within 48 h or rises to at least 1.5 times the baseline. Standard serum and urine biomarkers are insensitive and nonspecific in detecting kidney injury in its early stages, limiting therapeutic options and compromising outcomes.[3] Due to the half-life of circulating creatinine, an increase in serum creatinine lags behind the decrease in the glomerular filtration rate (GFR) over time. Furthermore, the time required to achieve a new steady-state concentration that fully reflects the degree of GFR loss is delayed by multiples of the prolonged serum creatinine half-life, resulting in changes occurring over days rather than hours.[4]
In critically ill septic patients, hemodilution in those with hypotension following aggressive fluid resuscitation and a positive fluid balance can obscure increases in serum creatinine. This obscuring effect has been linked to a further delay in diagnosing AKI. Moreover, sepsis has been found to decrease muscle production of creatinine, even in the absence of weight loss, which further restricts the effectiveness of serum creatinine as an indicator of septic AKI.[5]
While some novel biomarkers have been proposed, in current clinical practice, the identification and classification of AKI are based on the elevations in serial serum creatinine concentrations, which are delayed and unreliable in the acute setting. There are multiple promising new serum AKI markers; one is glutathione S-transferase (GST). Despite numerous recently published studies and reviews on biomarkers in AKI, the GST protein family has received relatively little attention in the literature.[6]
This study aims to correlate urinary GST and serum creatinine to predict early AKI in septic patients. The primary objective is to determine the correlation between urinary GST and serum creatinine in patients with sepsis and septic shock developing oliguria. The secondary objective is determining these patients’ ICU stays, the sequential organ failure assessment (SOFA) score, the Acute Physiological and Chronic Health Evaluation (APACHE) II score, GFR, and dialysis requirements.
METHODS
Study design
This single-center, prospective and observational study was conducted in a 14-bed ICU at a tertiary care academic hospital and research center in North India from May 20, 2021, to October 31, 2022. The Institutional Ethics Committee approved the study protocol (IEC No. 69/19). Before their inclusion in the study, informed written consent was obtained from the patients, their relatives, or a legal guardian.
Study population
This study included all adult (age > 18 years) patients admitted to the ICU with sepsis or septic shock who developed oliguria within 72 h of admission. The exclusion criteria were refusal of consent, preexisting kidney disease, a history of renal replacement therapy, anuric patients, and those already diagnosed with AKI according to the Kidney Disease Improving Global Outcomes (KDIGO) classification definition upon admission.
Patients admitted with sepsis or septic shock were screened for the inclusion in the study. Sepsis was diagnosed based on the clinical criteria or culture reports. All routine laboratory investigations, including serum procalcitonin, were conducted according to the institutional protocols. Disease severity was assessed using the SOFA score and the APACHE II score. Hourly urine output was monitored, and patients who developed oliguria were included in the study. Oliguria was defined as a urine output of < 0.5 mL/kg/h for 6 h.
The diagnosis of AKI was made based on KDIGO criteria[7] [Table 1]. Any available record of serum creatinine values at least 1 month before ICU admission was taken as a baseline. The admission value was used as a baseline if these values were unavailable. The clinical endpoint was defined as the occurrence of advanced AKI stage 2 or 3, as specified by the KDIGO classification. Both urine and creatinine criteria were applied.
Table 1.
Kidney disease improving global outcomes criteria for acute kidney injury
| Stages for AKI | Criteria |
|---|---|
| Stage I | Serum creatinine increased by 1.5–1.9 times baseline Serum creatinine increased by ≥0.3 mg/dL Urine output <0.5 mL/kg/h for 6–12 h |
| Stage II | Serum creatinine increased by 2.0–2.9 times baseline Urine output <0.5 mL/kg/h for ≥12 h |
| Stage III | Serum creatinine increased by 3.0 times baseline Urine output <0.3 mL/kg/h for≥24 h Increase in serum creatinine to t4.0 mg/dL Initiation of renal replacement therapy eGFR decreased to <35 mL/min/1.73 m2 in patients <18 years Anuria for ≥12 h |
AKI: Acute kidney injury, eGFR: Estimated glomerular filtration rate
Sample collection
Blood and urine specimens for serum creatinine and urinary GST were collected using a standardized method. The serum creatinine levels were measured in the hospital’s clinical chemistry laboratory using a Jaffe reaction based on isotope dilution mass spectrometry. Urinary GST levels were determined using the colorimetric method. All urine specimens were centrifuged within 1 h of collection and centrifuged at 800 g at 4°C for 5 min. The supernatant was collected. The results were expressed in ng/mL. The reference urinary π GST and alpha GST values are 3.12–100 ng/mL and 6.25–200 ng/mL, respectively. The average variation coefficient of these assays in this study was 3%–4%.
Data collection
All the data were handled anonymously, and all information remained confidential. All relevant demographic and clinical characteristics, as well as ICU severity scores, were recorded during inclusion. Blood samples for measuring serum creatinine were taken at admission and 6, 12, 18, 24, 48, and 72 h after developing oliguria in patients with sepsis and septic shock. GST activity was also measured and monitored until 72 h following the development of oliguria. The need for dialysis and the duration of the ICU stay were also recorded.
Statistical analysis
The sample size is calculated using the following equation:
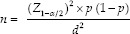
Z1−α/2 is the standard normal variate. At a 5% type I error (P < 0.05), it is 1.96. p is the expected proportion of the population based on the previous study, which was 4%.[8] d is the absolute error or precision. The calculated sample size came out to be 59. Considering the 10% dropout rate during the study, 65 subjects were included as the sample size.
The continuous data following a normal distribution were analyzed using the parametric tests, whereas nonnormal continuous data and discrete or categorical data were analyzed using nonparametric tests. Data were represented as mean ± standard deviation or median (interquartile range). The categorical variables were expressed as frequencies and percentages. The results were analyzed using the descriptive statistics and comparisons among various groups. Chi-square and other appropriate tests were used to find the associations and relationships. Relative risk and logistic regression analyses assessed the risks associated with multiple explanatory variables. To compare the continuous variables, a two-tailed unpaired t-test was utilized for categorical variables, the χ2 or Fisher’s exact test was applied. A receiver operating characteristic (ROC) curve was generated to measure the performance of urinary GST, with further calculation of the area under the curve (AUC). Statistical testing was conducted using the statistical package for IBM SPSS 22.0 (Statistical Package for the Social Sciences, version 22, Chicago, IL, USA).
RESULTS
During the study period, 220 patients were admitted to the ICU. One hundred and thirty-four patients had or developed sepsis/septic shock and were screened for the inclusion in the study. Fourteen were excluded based on the exclusion criteria. Among 120 patients, 65 developed oliguria. Finally, data from 65 patients were included in this study for the analysis.
The mean age of the patients was 52.60 ± 11.78 years. Forty-six (70.77%) patients were male and nineteen (29.23%) were female. Thirteen (20%) patients developed AKI Grade I, while 52 (80%) progressed to AKI grades II and III. No association was found between age (P = 0.120), gender (P = 0.562), hemoglobin (P = 0.227), or albumin (P = 0.845) and the development of AKI [Table 2].
Table 2.
Patient characteristics
| AKI I | AKI II/III | P | |
|---|---|---|---|
| Age | 53.66±15.66 | 52.28±10.53 | 0.12 |
| Gender | |||
| Male | 12 | 34 | 0.562 |
| Female | 1 | 18 | |
| Hb (g/dL) | 8.94±0.52 | 9.00±0.64 | 0.227 |
| Albumin (g/dL) | 2.80±0.39 | 2.95±0.39 | 0.845 |
| SOFA score | 1.06±0.25 | 2.10±0.30 | 0.022 |
| APACHE II score | 6.61±0.50 | 6.75±0.51 | 0.127 |
| U/O (mL/h) | 349.33±62.10 | 261.80±95.00 | 0.011 |
| ICU stay (28 days) | 30.53±2.00 | 30.12±1.58 | 0.521 |
AKI: Acute kidney injury, Hb: Hemoglobin, SOFA: Sequential organ failure assessment, ICU: Intensive care unit, APACHE: Acute physiological and chronic health evaluation, U/O: Urinary output
Table 2 also compares the scores between the two groups, indicating a significant correlation of the SOFA score between the non-AKI or AKI Stage I group and the AKI Stage II and III groups, with a P = 0.022. ICU stay between the two groups was found to be nonsignificant (P = 0.521).
Table 3 compares serum creatinine values between two groups at different time intervals. Both groups showed an increase in serum creatinine values until 72 h. The mean differences were significant at 48 and 72 h, with P values of 0.021 and 0.007, respectively. Table 3 also shows the comparison of GFR between the two groups. A decreasing trend in GFR was observed in both groups. This difference was significant at 36 h, 48 h, and 72 h, with P values of 0.045, 0.040, and 0.035, respectively. Table 3 also compares GST levels between the two groups. Although both groups showed an increasing trend in GST until 72 h, the mean difference in GST was significant very early, at 18 h (P = 0.044).
Table 3.
Comparison of serum creatinine, urinary glutathione S-transferase, and glomerular filtration rate in acute kidney injury grades
| At 6 h | At 12 h | At 18 h | At 24 h | At 36 h | At 48 h | At 72 h | |
|---|---|---|---|---|---|---|---|
| Serum creatinine | |||||||
| AKI I | 1.61±0.47 | 2.01±0.58 | 2.19±0.54 | 2.37±0.42 | 2.52±0.43 | 2.78±0.58 | 2.89±0.60 |
| AKI II/III | 1.46±0.57 | 2.02±0.61 | 2.45±0.52 | 2.55±0.60 | 2.77±0.73 | 3.18±1.19 | 3.42±1.61 |
| P | 0.68 | 0.795 | 0.861 | 0.099 | 0.139 | 0.021 | 0.007 |
| GFR | |||||||
| AKI I | 41.89±15.03 | 34.80±15.04 | 30.20±8.83 | 25.75±10.40 | 25.50±4.07 | 23.34±5.54 | 23.04±5.32 |
| AKI II/III | 48.88±25.44 | 32.19±13.26 | 24.67±6.19 | 21.98±4.99 | 18.53±4.29 | 15.87±5.59 | 13.30±7.80 |
| P | 0.142 | 0.098 | 0.214 | 0.222 | 0.045 | 0.04 | 0.035 |
| Urinary GST | |||||||
| AKI I | 2.22±0.59 | 2.39±0.54 | 2.45±0.49 | 2.71±0.39 | 2.90±0.30 | 3.03±0.33 | 3.19±0.27 |
| AKI II/III | 2.24±0.50 | 2.49±0.45 | 2.93±0.52 | 3.01±0.55 | 3.06±0.52 | 3.43±0.88 | 3.58±1.44 |
| P | 0.098 | 0.077 | 0.044 | 0.099 | 0.12 | 0.111 | 0.098 |
AKI: Acute kidney injury, GFR: Glomerular filtration rate, GST: Glutathione S-transferase
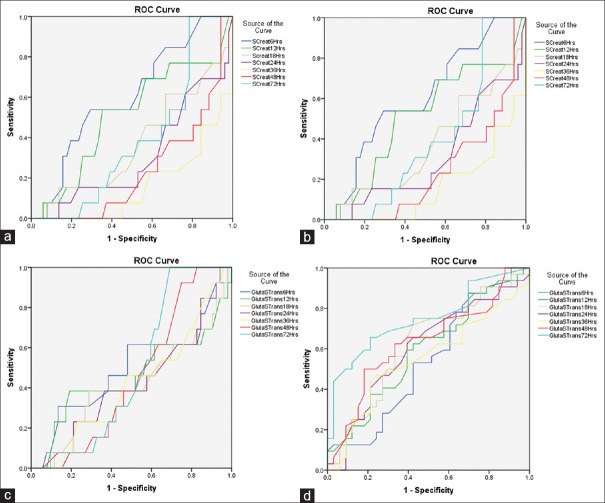
Figure 1 depicts the ROC curve of serum creatinine and glutathione S transferase for the development of AKI at different time intervals (at 6, 12, 18, 24, 36, 48, and 72 h) and the corresponding areas under the curve, with respective P values mentioned in Table 4. The maximum area of the curve with a significant P value for serum creatinine in patients with and without AKI occurred at 48 and 72 h. Meanwhile, for glutathione S, the maximum area of the curve with a significant P value in patients with or without AKI was reached much earlier, at 18 h.
Figure 1.
Receiver operating characteristic curves (ROC) of serum creatinine and urinary glutathione S-transferase (GST) levels for predicting advanced acute kidney injury (AKI). (a) ROC curve of serum creatinine for No/Grade I AKI (b) ROC curve of serum creatinine for Grade II/III AKI, (c) ROC curve of urinary GST for No/Grade I AKI (d) ROC curve of urinary GST for Grade II/III AKI
Table 4.
The area under the curve for serum creatinine and urinary glutathione S-transferase
| Test variables (h) | The AUC (P), test outcome | |||
|---|---|---|---|---|
|
| ||||
| No/grade I AKI | Grade II/III AKI | |||
|
|
|
|||
| Serum creatinine | GST | Serum creatinine | GST | |
| At 6 | 0.606 (0.243) | 0.495 (0.954) | 0.593 (0.202) | 0.540 (0.577) |
| At 12 | 0.511 (0.900) | 0.458 (0.640) | 0.469 (0.667) | 0.591 (0.208) |
| At 18 | 0.373 (0.159) | 0.819 (0.027) | 0.387 (0.119) | 0.818 (0.031) |
| At 24 | 0.310 (0.036) | 0.416 (0.350) | 0.578 (0.768) | 0.603 (0.153) |
| At 36 | 0.16 (0.056) | 0.416 (0.350) | 0.517 (0.814) | 0.55 (0.491) |
| A 48 | 0.738 (0.004) | 0.460 (0.658) | 0.714 (0.046) | 0.632 (0.068) |
| At 72 | 0.791 (0.026) | 0.487 (0.889) | 0.854 (0.034) | 0.448 (0.211) |
AUC: Area under the curve, GST: Glutathione S-transferase, AKI: Acute kidney injury
DISCUSSION
AKI remains a significant problem for ICU patients with sepsis. AKI, studied by the Beginning and Ending Supportive Therapy for the Kidney, shows a high hospital mortality of 60.3% with sepsis.[9] We evaluated the prediction of AKI within 72 h of oliguria detection in sepsis or septic shock by correlating urinary GST with serum creatinine. We observed that in patients who developed AKI, serum creatinine started to differ significantly from those who did not develop AKI only after 48 h. GST differed significantly between the two groups much earlier, at 18 h.
We studied 65 patients and divided them based on the KDIGO criteria into two groups. Group 1 was no/stage 1 AKI and Group 2 was stage II/III AKI. Serum creatinine differed significantly between the two groups at 48 h, while urinary GST differed much earlier, with the maximum AUC (AUC = 0.81, P = 0.027) at 18 h. Our study suggests a potential role for urinary GSTs in predicting AKI in sepsis or septic shock. Like our study, Yavuz et al. showed that α GST had reasonable accuracy when discriminating AKI from non-AKI patients.[10] Kashani et al. also reported that GST was a highly sensitive AKI predictor for sepsis and septic shock (AUC = 0.81, P = 0.027).[11]
The expression pattern of GST before the rise in serum creatinine is early, and its predictive power also increases closer to the AKI presentation time. This observation suggests that the time-to-injury relationship is essential and should be obtained to correctly interpret its AKI predictive value. Shu et al. studied 141 patients and observed that urinary π-GST levels predict advanced AKI.[1] Koyner et al., in their study, reported that π-GST could predict the progression to stage 3 AKI at 6 h postsurgery in 123 adults undergoing cardiac surgery (AUC = 0.78).[12]
In our analysis, we determined that urinary GST could predict AKI in septic shock earlier than serum creatinine. The upregulated proteins GST gradually increase in concentration before serum creatinine rises. The AUCs is used to predict the development of AKI for each biomarker at different time points. GST displayed the most consistent predictive performance, starting 18 h before the AKI presentation (0.819, P = 0.027) and increasing as it approached the AKI endpoint (AUC = 0.787, P = 0.003). At that exact time, serum creatinine levels rose for the first time at 48 h (AUC = 0.738, P = 0.010).
Our study suggests a potential role for urinary GSTs in the clinical diagnostic evaluation of AKI.
The SOFA score was more accurate in predicting mortality for critically ill patients with AKI undergoing CRRT than the APACHE-II score. This study performed a curve-fitting analysis to explore the relationship between the SOFA score and the prognosis of patients with AKI.
There are a few limitations. First, we used only GST to predict AKI early, while many other markers were also available. A multiple-marker combined study is significantly better than a single-marker study for improving the discrimination of AKI in sepsis and septic shock patients. Second, the small sample size and nonrandomized study design necessitate further studies with improved designs to validate the results.
CONCLUSIONS
This study concludes that the level of urinary GST was raised earlier than the level of serum creatinine in critically ill sepsis or septic shock patients. Our data demonstrate that urinary GST is a valuable tool (because of its high sensitivity and specificity) for the early diagnosis and prediction of AKI after admission to the ICU, which reduces morbidity and mortality.
Research quality and ethics statement
This study was approved by the Institutional Ethics Committee at Dr. RMLIMS, Lucknow, Uttar Pradesh (Approval # IEC number – 69/19). The authors followed the applicable EQUATOR Network (http://www.equator-network.org/) guidelines during this research project, specifically the STROBE Guidelines.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil
REFERENCES
- 1.Shu KH, Wang CH, Wu CH, Huang TM, Wu PC, Lai CH, et al. Urinary π-glutathione S-transferase predicts advanced acute kidney injury following cardiovascular surgery. Sci Rep. 2016;6:26335. doi: 10.1038/srep26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–99. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 4.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–37. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Jenq CC, Tian YC, Chang MY, Lin CY, Chang CC, et al. Rifle classification for predicting in-hospital mortality in critically ill sepsis patients. Shock. 2009;31:139–45. doi: 10.1097/SHK.0b013e31817d419e. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–93. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: A consensus statement. JAMA Netw Open. 2020;3:e2019209. doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 8.Walshe CM, Odejayi F, Ng S, Marsh B. Urinary glutathione S-transferase as an early marker for renal dysfunction in patients admitted to intensive care with sepsis. Crit Care Resusc. 2009;11:204–9. [PubMed] [Google Scholar]
- 9.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 10.Yavuz I, Asgun FH, Bolcal C, Bingol H, Yokusoglu M, Baysan O, et al. Importance of urinary measurement of glutathione S-transferase in renal dysfunction patients after on- and off-pump coronary artery bypass surgery. Thorac Cardiovasc Surg. 2009;57:125–9. doi: 10.1055/s-2008-1038663. [DOI] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–65. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]