Abstract
Acidic ribosomal phosphoproteins P0, P1 and P2 were isolated in soluble form from silkworm ribosomes and tested for their interactions with each other and with RNA fragments corresponding to the GTPase-associated domain of residues 1030–1127 (Escherichia coli numbering) in silkworm 28S rRNA in vitro. Mixing of P1 and P2 formed the P1–P2 heterodimer, as demonstrated by gel mobility shift and chemical crosslinking. This heterodimer, but neither P1 or P2 alone, tightly bound to P0 and formed a pentameric complex, presumably as P0(P1–P2)2, assumed from its molecular weight derived from sedimentation analysis. Complex formation strongly stimulated binding of P0 to the GTPase-associated RNA domain. The protein complex and eL12 (E.coli L11-type), which cross-bound to the E.coli equivalent RNA domain, were tested for their function by replacing with the E.coli counterparts L10.L7/L12 complex and L11 on the rRNA domain within the 50S subunits. Both P1 and P2, together with P0 and eL12, were required to activate ribosomes in polyphenylalanine synthesis dependent on eucaryotic elongation factors as well as eEF-2-dependent GTPase activity. The results suggest that formation of the P1–P2 heterodimer is required for subsequent formation of the P0(P1–P2)2 complex and its functional rRNA binding in silkworm ribosomes.
INTRODUCTION
It is generally accepted that ribosomal proteins modulate the structure and function of rRNAs (1). The acidic stalk protein complex, L10.L7/L12 in procaryotes, binds to a region around residues 1030–1127 in domain II of 23S rRNA, termed the GTPase-associated domain (2–4), and constitutes a part of the functional center, termed the GTPase center (5) or factor-binding center (6). Acidic L7/L12 protein (L7 differs from L12 by an acetylated N-terminus) is an important component of this functional center (7), although there is no evidence for its direct binding to rRNA. This protein has characteristic properties: there are four copies, two homodimers, of L7/L12 per ribosome (8); they are flexible in the ribosome (9–11). The four copies of L7/L12 bind to L10 and form the pentameric complex (12); the L10 moiety of the complex appears to bind directly to the rRNA domain (13). Although it is well known that L7/L12 within the GTPase center participates in interaction of the ribosome with translation factors (G-proteins) (7,14), the functional significance of the four copies has been poorly understood (15–17).
The structure and function of the acidic phosphoproteins in eucaryotic ribosomes have been investigated mainly in rat (18–20), human (21), Artemia salina (22) and yeast (23,24). Animal ribosomes contain two kinds of the acidic proteins, P1 and P2 (reviewed in 25), whereas yeast ribosomes contain two P1-type proteins, P1α and P1β, and two P2-type proteins, P2α and P2β (24). These proteins bind to P0 protein, the equivalent of Escherichia coli L10, and presumably form the pentameric complex in the ribosome (22,26), designated here P0.P1/P2. The P0.P1/P2 complex binds to the GTPase-associated domain of 28S rRNA (27) and plays a crucial role in kingdom-specific interaction between 80S ribosomes with eucaryotic elongation factor 1α (eEF-1α) and elongation factor 2 (eEF-2) (28).
It is interesting that the eucaryotic ribosomes have more than two kinds of acidic proteins, unlike procaryotic ribosomes. There are two major interpretations for the states of P1 and P2 in the ribosome: (i) P1 and P2 form homodimers, as suggested by crosslinking (22,29); or (ii) they form heterodimers, suggested by yeast two-hybrid system (26,29,30) and the other genetic and biochemical studies (26,31–33). Isolated P1 and P2 proteins have a tendency to form oligomeric complexes (29), which may occur because of non-specific interactions. The instability of free P1 and P2 proteins in solution seems to bring ambiguity to the biochemical data. Moreover, the insolubility of isolated P0 protein makes in vitro binding experiments difficult.
We here used the ribosomal proteins from the silkworm Bombyx mori; proteins P1, P2 and P0 could be isolated in soluble form and were used for a study on their interaction in vitro. We show that formation of a P1–P2 heterodimer is significant for the subsequent assembly of a pentameric complex, probably in the form P0(P1–P2)2, and then its rRNA binding. To evaluate the in vitro binding data on the basis of ribosome function, we used a hybrid ribosome system developed recently (28), in which E.coli L10.L7/L12 complex and L11 on the 50S subunit were replaced with the eucaryotic counterparts P0.P1/P2 complex and eL12, respectively. The functions of the hybrid ribosomes carrying the silkworm ribosomal proteins were tested with eucaryotic elongation factors. The results provide evidence that formation of P1–P2 heterodimers is crucial for their binding to P0 and the subsequent interaction with the GTPase-associated domain of rRNA, from which derives factor-dependent ribosome function. We discuss an additional functional role of the P1–P2 heterodimer as a modulator of the RNA-binding protein P0.
MATERIALS AND METHODS
Silkworm ribosomes and ribosomal proteins
The high KCl/puromycin-treated 80S ribosomes were isolated from posterior silk glands of last instar larvae of B.mori (strain C132), according to the method previously described (34). Total proteins were extracted from the ribosome in 66% acetic acid, 33 mM MgCl2 and recovered by precipitation with 7 vol of cold acetone. The proteins were dialyzed against buffer A containing 20 mM sodium acetate, pH 4.5, 7.5 M urea and 5 mM 2-mercaptoethanol and then applied to a column of CM-cellulose (Whatman) equilibrated with the same buffer. Proteins were successively eluted with buffer A containing increasing concentrations of LiCl; P1, P2 and P0 were eluted in solutions containing 0, 0.05 and 0.08 M LiCl, respectively. The protein fractions were concentrated with Centricon YM-10 (Amicon). P1 and P2 fractions were dialyzed against buffer B containing 20 mM sodium phosphate, pH 6.5, 6 M urea, 100 mM LiCl, 5 mM 2-mercaptoethanol and further purified by ion exchange high performance liquid chromatography (HPLC) with DEAE-5PW (Tosoh) in a linear gradient of 100–300 mM LiCl. The P0 fraction was dialyzed against buffer C (buffer B except that the concentration of LiCl was 50 mM) and purified by HPLC with CM-5PW (Tosoh) in a linear gradient of 50–250 mM LiCl. The identities of the P1, P2 and P0 proteins were confirmed by reactivity with anti-P monoclonal antibody (35). P1 and P2 were also tested for partial amino acid sequencing using a protein sequencer (model PPSQ-21; Shimadzu).
Complex formation of P0, P1 and P2
Isolated proteins in the presence of 6 M urea were mixed together at a molar ratio of P0:P1:P2 of 1:3:3 and dialyzed against 0.3 M KCl, 20 mM Tris–HCl, pH 7.6, at 0°C. The same dialysis was also performed in the absence of P1 or P2. The P1–P2 complex was formed by mixing various ratios of P1 and P2 in 0.3 M KCl, 20 mM Tris–HCl, pH 7.6, at 0°C. Complex formation was confirmed by 6% native PAGE (acrylamide/bisacrylamide ratio 40:1) at 6.5 V/cm with a buffer system containing 5 mM MgCl2, 50 mM KCl and 50 mM Tris–HCl, pH 8.0. Samples were electrophoresed for 10 h at constant voltage and 4°C with buffer recirculation. The gel was stained with Coomassie Brilliant Blue. Bands of the complexes were cut out of the gel and the constituents were separated by SDS–PAGE as described by Laemmli (36), except that the gel contained 22% polyacrylamide and 0.44% bisacrylamide to improve separation of P1 and P2. The P0, P1 or P2 proteins on the gel were identified by immunoblotting using an anti-P monoclonal antibody that cross-reacts with the silkworm proteins. In some experiments, the P0.P1/P2 complex was further purified by gel filtration with G-3000SWXL (Tosoh) in a solution consisting of 100 mM KCl, 0.2 mM dithiothreitol, 20 mM Tris–HCl, pH 7.6.
Gel retardation assays
RNA fragments containing residues 1030–1127 of E.coli 23S rRNA and the equivalent region of B.mori 28S rRNA were synthesized with SP-6 RNA polymerase using cDNA as template (34). The transcripts were purified by gel filtration on a Sephadex G-50 column (Amersham Pharmacia). A solution (10 µl) containing 5 pmol [32P]RNA fragments, 20 mM MgCl2, 0.3 M KCl and 20 mM Tris–HCl, pH 7.6, was preincubated at 65°C for 5 min and then cooled to 30°C over 10 min. After the addition of a protein sample as indicated in the legend to Figure 5, the mixture was further incubated at 30°C for 10 min. RNA–protein binding was examined by 6% native PAGE under the same conditions as described above.
Figure 5.
Binding of P0, P1 and P2 protein mixtures to GTPase-associated RNA domain. The 32P-labeled RNA fragment (5 pmol) containing residues 1030–1127 (E.coli numbering) of B.mori wild-type 28S rRNA (A) and its U1094/A1098 variant (B) were incubated in 10 µl of a solution without protein (lane 1) or with a mixture of P1 and P2 (87 pmol each, lane 2), P0 (29 pmol, lane 3) and the mixtures P0 (29 pmol) + P1 (87 pmol) (lane 4), P0 (29 pmol) + P2 (87 pmol) (lane 5) and P0 (29 pmol) + P1 (87 pmol) + P2 (87 pmol) (lane 6). The samples were analyzed by gel retardation, as described in Materials and Methods. (C) The B.mori U1094/A1098 variant (5 pmol) was incubated in 10 µl of a solution with increasing amounts of isolated P0: 0 (lane 1), 29 (lane 2), 58 (lane 3) and 120 pmol (lane 4). The samples were analyzed by the same gel retardation assay as in (B).
Functional assay using the hybrid system
Escherichia coli ribosomes deficient in L11 were obtained from strain AM68 (37), as described previously (28). The 50S subunits were incubated in a solution containing 50% ethanol and 0.5 M NH4Cl to remove specifically the L10.L7/L12 complex, as described in a previous report (28). The 50S core subunits deficient in L10.L7/L12 and L11 thus obtained were used to study function of the P0.P1/P2 complex. The 50S core subunits were incubated with the silkworm ribosomal proteins P0.P1/P2 (or samples without P1 and/or P2) and eL12, as described in the legend to Figure 7. The resultant E.coli–silkworm hybrid 50S subunit (0.13 µM) was tested for eucaryotic eEF-2-dependent GTPase activity in a solution (20 µl) containing 0.38 µM 30S subunit, 0.25 µM eEF-2, 150 µM [γ-32P]GTP, 10 mM MgCl2, 50 mM NH4Cl, 20 mM Tris–HCl, pH 7.6, and 0.2 mM dithiothreitol. The reaction was performed at 37°C for 10 min. Polyphenylalanine synthetic activity was assayed in a solution (100 µl) containing 0.1 µM hybrid 50S subunit, 0.5 µM 30S subunit, 10 µg poly(U), 0.4 µM E.coli [14C]Phe-tRNA (with 80 µg deacylated total tRNA), 200 µM GTP, 10 mM MgCl2, 75 mM NH4Cl, 50 mM Tris–HCl, pH 7.6, 0.2 mM dithiothreitol and 800 µg silk gland cytosol fraction as a source of elongation factors that was obtained by ammonium sulfate precipitation (40–60%) of the S200 fraction. The reaction was performed at 37°C for 10 min.
Figure 7.
Functional assays of the P0, P1 and P2 samples in a hybrid system with E.coli ribosomes. (A) Escherichia coli 50S cores deficient in L10.L7/L12 complex and L11 (2.5 pmol) were preincubated in 10 µl of a solution with increasing amounts of the P0.P1/P2 complex (circles), P0/P1 mixture (diamonds), P0/P2 mixture (triangles) and P0 alone (squares), together with 5.7 pmol eL12. The ribosome samples were tested for GTPase activity dependent on eucaryotic eEF-2 purified from pig liver (50), as described in Materials and Methods. (B) Escherichia coli 50S cores (10 pmol) were preincubated in 25 µl of a solution without any P proteins (bar 1) or with 20 pmol P0 (bar 2), P0 (20 pmol) + P1 (61 pmol) (bar 3), P0 (20 pmol) + P2 (61 pmol) (bar 4) and P0 (20 pmol) + P1 (61 pmol) + P2 (61 pmol) (bar 5), together with 23 pmol eL12. The ribosome samples were tested for poly(U)-dependent polyphenylalanine synthesis by adding 800 µg S200 fraction as a source of silkworm elongation factors, as described in Materials and Methods. The activity was also assayed with 10 pmol silkworm 80S ribosomes under the same conditions except that the salt concentrations used were 5 mM MgCl2, 50 mM NH4Cl, 100 mM KCl, 50 mM Tris–HCl, pH 7.6, 0.2 mM dithiothreitol (bar 6). (C) The E.coli 50S cores (10 pmol) were preincubated in 25 µl of a solution with P0.P1/P2 complex (20 pmol) and eL12 (23 pmol) in the presence of increasing amounts of the RNA fragment corresponding to the E.coli GTPase-associated domain (circles). The intact silkworm 80S ribosomes (10 pmol) were also incubated with the same RNA fragments (squares). The samples were assayed for polyphenylalanine synthetic activity, as shown in (B).
Chemical crosslinking
A mixture of P1 and P2 (9.5 nmol each) in 220 µl of 100 mM KCl, 20 mM triethanolamine HCl, pH 7.6, was incubated at 30°C for 10 min. After cooling to 0°C, the proteins were crosslinked with 18 mM 2-iminothiolane as described by Kenny et al. (38). The crosslinked sample was dialyzed against a solution containing 20 mM sodium phosphate, pH 6.5, 7.5 M urea, 100 mM LiCl, 5 mM iodoacetamide and loaded onto a column of DEAE-5PW (Tosoh) equilibrated with the same solution without iodoacetamide. Proteins were separated with a linear gradient of 100–300 mM LiCl (see Fig. 2A). Each fraction was concentrated with Centricon YM-10 (Amicon) and analyzed by SDS–PAGE under non-reducing and reducing conditions.
Figure 2.
Crosslinking between P1 and P2 with 2-iminothiolane. (A) P1 and P2 proteins premixed and crosslinked with 2-iminothiolane were separated into fractions (A–H) using a DEAE-5PW column. (B) A portion of each fraction was analyzed by SDS–PAGE under non-reducing conditions as described in Materials and Methods. Lanes 1–8 correspond to samples of fractions A–H, respectively. (C) Protein samples from fractions C (lane 1), G (lane 2), H (lane 3) and 1.4 pmol silkworm ribosome (lane 4) were subjected to SDS–PAGE under reducing conditions, followed by immunoblotting using anti-P monoclonal antibody.
Analytical ultracentrifugation
Sedimentation equilibrium studies were performed with a Beckman Optima XL-I analytical ultracentrifuge using a double-sector centerpiece and sapphire windows, at three rotor speeds (12 000, 15 000 and 18 000 r.p.m.) and at 20°C. The sample was prepared as mentioned above. Absorbance scans at 280 nm were measured in the radial step mode at 0.001 cm intervals and data were collected taking the average of 16 measurements at each radial distance. Approach to equilibrium was considered to be complete when replicate scans separated by ≥6 h were indistinguishable. The partial specific volume of the protein was assumed to be 0.73 ml/g and the density of the solvent was assumed to be 1.00 g/ml. Analysis of the data was carried out utilizing the program Origin 4.1.
RESULTS
In vitro interaction between isolated silkworm P1 and P2
Animal ribosomes contain two kinds of the L7/L12-like acidic proteins, P1 and P2, unlike procaryotic ribosomes. We purified these proteins from ribosomes of the silkworm B.mori and, in addition, P0, corresponding to procaryotic L10. The identities of these proteins were confirmed by reactivity with monoclonal anti-P antibody (data not shown) and partial amino acid sequencing. The N-terminal sequence of P2 was MRYVAAYLLAVLGGKTTPAA, which is 70% identical to rat P2 (19) and 85% identical to Drosophila melanogaster (39). For P1, we used a peptide produced by V8 protease digestion (40), since the N-terminus of P1 was blocked, as in yeast (41). The sequence was LACVYSALIL, which is 90% identical to residues 7–16 of rat P1 (19) and 70% identical to the same residues of D.melanogaster (42). Isolated P0, P1 and P2 were soluble in a solution containing 0.3 M KCl in the absence of urea.
Isolated P1 and P2 showed characteristic mobilities in native PAGE (Fig. 1A, lanes 1 and 2). A smearing of the P1 sample (lane 1) may be due to instability of this protein under these electrophoresis conditions. By mixing P1 with P2, a new protein band appeared (lane 3) not detected in isolated P1 and P2. Unexpectedly, the gel mobility of the new band was faster than that of isolated P1 and P2 and its mobility did not change regardless of different molar ratios of added P1 and P2 (Fig. 1B). The new band was cut out of the gel and protein constituents were analyzed by SDS–PAGE, followed by immunoblotting using an anti-P monoclonal antibody that reacts with all of P0, P1 and P2 (Fig. 1C). This band contained P1 and P2 (lane 3), suggesting the formation of a complex composed of P1 and P2. Relative intensity between immunostained P1 and P2 components of the complex formed in vitro was apparently comparable with that of the proteins in intact ribosomes (lane 4). To confirm formation of a P1–P2 heterodimer, chemical crosslinking was performed. The P1/P2 mixture crosslinked with 2-iminothiolane was fractionated by ion exchange HPLC (Fig. 2A). Each fraction was analyzed by SDS–PAGE under non-reducing conditions (Fig. 2B). A crosslinked protein complex suggesting a dimer (28 kDa) was detected only in fraction H (lane 8). Crosslinked oligomers suggesting a trimer or tetramer were not formed. On reducing the 28 kDa complex (Fig. 2C), it separated into two protein components (lane 3) corresponding to P1 (lane 2) and P2 (lane 1), detected by immunoblotting analysis. No formation of either P1 or P2 homodimer was detected in this experiment.
Figure 1.
Formation of the P1–P2 protein complex. (A) Purified P1 (430 pmol, lane 1) and P2 (430 pmol, lane 2) and their mixture (lane 3) in 10 µl of solution were analyzed by 6% native PAGE, as described in Materials and Methods. The P0.P1/P2 complex formed in the presence of P0 (Fig. 3) is also shown in lane 4 as a comparison. (B) P2 (430 pmol) was incubated with increasing amounts of P1: 0 (lane 1), 430 (lane 2), 860 (lane 3) and 1300 pmol (lane 4) in 10 µl of solution. Lane 5, 860 pmol P1 alone. The samples were analyzed with the same 6% polyacrylamide gel. (A and B) The gels were stained with Coomassie Brilliant Blue. A new band which appeared by P1/P2 mixing and was used for further immunoblotting analysis is arrowed. Bands for free P1 and P2 are also indicated. (C) Aliquots of 17 pmol each P1 (lane 1) and P2 (lane 2), a piece of acrylamide gel containing the newly appeared band and 2 pmol silkworm 80S ribosomes (lane 4) were subjected to SDS–PAGE, followed by immunoblotting with anti-P monoclonal antibody (35), as described in Materials and Methods. Appearance of a slight amount of an ∼28 kDa component below the position of P0 in lane 3 is not reproducible. Its origin is unknown. Considering the crosslinking results (Fig. 2), it may be a P1–P2 heterodimer tightly fixed even after SDS treatment of the gel containing the protein complex.
Binding of P1–P2 heterodimer to P0
We then investigated complex formation with isolated silkworm P0, P1 and P2 using native PAGE (Fig. 3A). Although isolated P0 (lane 1) did not run in the gel under the conditions used, complex formation was deducible from the appearance of a new complex band or disappearance of the bands for free P1 (lane 2) and P2 (lane 3). No complex formation was detected in the absence of either P2 (lane 4) or P1 (lane 5). In the presence of both P1 and P2, a stable complex was formed (lane 6). The constituents P0, P1 and P2 of this complex were confirmed by cutting out the band from the gel and analysis by SDS–PAGE, followed by immunoblotting with anti-P antibody (Fig. 3B). The results suggest that P1–P2 interaction is required for both the proteins to assemble tightly on P0. Although the complex composed of P0, P1 and P2 has been reconstituted in vitro with proteins from human (21) and rat (27,32), the stoichiometry of the constituents has not yet been established.
Figure 3.
Binding of P1 and P2 proteins to P0 protein. (A) Purified P0 (145 pmol, lane 1), P1 (430 pmol, lane 2) and P2 (430 pmol, lane 3), as well as the mixtures P0 (145 pmol) + P1 (430 pmol) (lane 4), P0 (145 pmol) + P2 (430 pmol) (lane 5) and P0 (145 pmol) + P1 (430 pmol) + P2 (430 pmol) (lane 6), in 10 µl solutions of 20 mM Tris–HCl, pH 7.6, 0.3 M KCl, 5 mM 2-mercaptoethanol were analyzed by 6% PAGE. The protein complex band used for immunoblotting analysis in (B) is arrowed. (B) The band of protein complex in (A) (lane 6) was cut out of the gel and subjected to SDS–PAGE, followed by immunoblotting with anti-P antibody.
From sedimentation equilibrium experiments, a weight-average molecular weight is estimated by the following equation (43).
Mapp =[2RT/(1 – ![]() ρ)ω2](dlnc/dr2) 1
ρ)ω2](dlnc/dr2) 1
where r is the radius, c is the concentration of the sample, ![]() is the partial specific volume of the sample, ρ is the density of the solvent, ω is the angular velocity of the rotor (in radians/s), R is the universal gas constant, T is the absolute temperature and Mapp is the apparent molecular weight.
is the partial specific volume of the sample, ρ is the density of the solvent, ω is the angular velocity of the rotor (in radians/s), R is the universal gas constant, T is the absolute temperature and Mapp is the apparent molecular weight.
Thus, the absorbance at a specified wavelength and position in the solution column should be given by
A(r) = A(r0)exp[MappH(r2 – r02)] 2
where A(r) represents the absorbance at radius r and A(r0) is the absorbance at r0, the radius at the meniscus, and
Figure 4 shows the results of the sedimentation equilibrium experiments on the P0.P1/P2 complex at 12 000 r.p.m. Considering the facts that the P0.P1/P2 complex was prepared as a fraction with a single symmetrical peak on the gel filtration (data not shown) and that the complex gave a single band in native PAGE, we assume the P0.P1/P2 complex to be a single species. Figure 4 shows the non-linear least squares fitting with this assumption. The symmetrical residuals and the small range of 95% confidence intervals as shown in Figure 4 support this assumption. The non-linear least squares fitting with equation 2 gave the apparent molecular weight as 7.80 ± 0.39 × 104. All the results with various rotor speeds (12 000, 15 000 and 18 000 r.p.m.) gave similar values within the range of experimental error, showing that the further association of solute is not detectable in this range of protein concentrations. The apparent molecular weight is very close to the expected value (80 126) of a complex of P0:P1:P2 (1:2:2) considering the molecular weights of P0 (34 148), P1 (11 451) and P2 (11 538). The results from these sedimentation experiments, together with data from gel analyses (Figs 1–3), strongly suggest that the reconstituted P0.P1/P2 complex is composed of two P1–P2 heterodimers and a monomeric P0, i.e. P0(P1–P2)2.
Figure 4.
Sedimentation equilibrium analysis of P0.P1/P2 complex. A 0.685 mg/ml (8.5 µM) sample of P0.P1/P2 complex was run at 20°C in 100 mM KCl, 0.2 mM dithiothreitol, 20 mM Tris–HCl, pH 7.6. The data were collected at a rotor speed of 12 000 r.p.m.
RNA binding of P0.P1/P2 complex
It has been shown that the P0.P1/P2 complex binds to the GTPase-associated domain of 28S rRNA, corresponding to residues 1030–1127 of E.coli 23S rRNA (27), presumably through the P0 moiety. To examine the importance of P1 and P2 in RNA binding, the experiment was performed in the absence of either of these proteins using the silkworm RNA fragment (Fig. 5A). Both P1 and P2, as well as P0, were required for binding to the RNA (lane 6). No RNA binding was detected with P1–P2 heterodimer (lane 2), P0 alone (lane 3), the P0–P1 pair (lane 4) or the P0–P2 pair (lane 5). This experiment was repeated using the U1094/A1098 RNA variant, instead of the wild-type C1094/G1098 RNA, as RNA probe, because the structure of the silkworm wild-type RNA is labile in solution and its protein-binding ability is low compared with the U1094/A1098 RNA variant (34). The same results were obtained (Fig. 5B), except that only very weak binding was detected for the P0–P2 pair (lane 5) using the RNA variant. To confirm that P0, not P1/P2, binds directly to the RNA, a large excess of isolated P0 was added to the U1094/A1098 RNA. A faint complex band appeared when 24-fold P0 was added to the RNA (Fig. 5C), although a P1/P2 mixture of the same amounts showed no binding (data not shown). A stable RNA–protein complex was observed only in the presence of all of P0, P1 and P2, suggesting that the P1–P2 heterodimers greatly increased the binding affinity of P0 to the RNA.
Functional properties of P0.P1/P2 complex
It is important to evaluate the present in vitro binding data in the aspect of ribosome function. To test the function of P0, P1 and P2 proteins, we recently developed a useful hybrid system in which E.coli L10.L7/L12 and L11 bound to the GTPase-associated domain of 23S rRNA were replaced with rat P0.P1/P2 complex and eL12 on the 50S subunit. Accessibility of the hybrid ribosome to eucaryotic elongation factors eEF-1α and eEF-2 has been established (28). We attempted to use this hybrid system to study the function of the silkworm ribosomal proteins. For this purpose, the prepared silkworm samples must cross-bind to the E.coli GTPase-associated RNA domain. As shown in Figure 6, silkworm P0.P1/P2 (lane 3) and L11-like protein eL12 (lane 2) as well as both the proteins (lane 4) bound strongly to the E.coli RNA.
Figure 6.
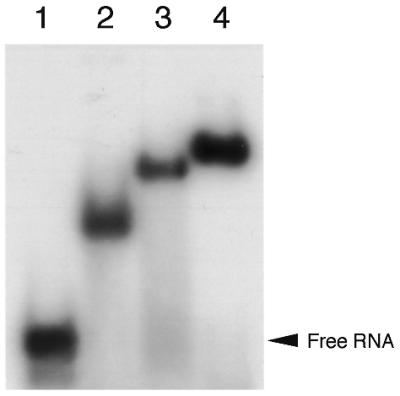
Cross-binding of silkworm P0.P1/P2 complex and eL12 to the E.coli GTPase-associated RNA domain. 32P-labeled RNA fragments (5 pmol) corresponding to the E.coli GTPase-associated RNA domain were incubated in 10 µl of a solution without protein (lane 1) or with eL12 (23 pmol, lane 2), P0.P1/P2 complex (37 pmol, lane 3) and their mixture (lane 4). The samples were analyzed by gel retardation, as described in Materials and Methods.
The silkworm protein samples were added to the core E.coli ribosomes deficient in L10.L7/L12 and L11 (28) and tested for function (Fig. 7). The hybrid ribosomes containing silkworm P0.P1/P2 and eL12 showed eucaryotic eEF-2-dependent GTPase activity (Fig. 7A) and polyphenylalanine synthesis (Fig. 7B). These activities were comparable with those of the previous hybrid sample containing rat ribosomal proteins (28). Removing either P1 or P2 markedly reduced both the activities. In the absence of P1, addition of P0/P2 in 5-fold excess to the E.coli 50S subunit core gave no effect on the GTPase activity (Fig. 7A). Likewise, addition of excess amounts of P0/P1 did not recover the activity in the absence of P2. In the absence of P0, addition of P1/P2 gave no stimulation of the activity (data not shown).
To test whether the activity of the hybrid ribosome is due to binding of the silkworm proteins to the GTPase-associated RNA domain within the E.coli ribosome, a competition study was performed using the E.coli RNA fragment used in the binding assay (Fig. 6) as a competitor. On addition of the RNA competitor, polyphenylalanine synthetic activity of the ribosome sample was reduced to ∼35% of the original activity (Fig. 7C). Addition of RNA itself showed no effect on polyphenylalanine synthesis by the intact silkworm ribosomes. The results indicate that the activity of the hybrid ribosomes was caused by interaction between the silkworm ribosomal proteins and the E.coli GTPase-associated RNA domain.
DISCUSSION
The structure of the ribosomal GTPase center within the large subunit is not resolved well by the current X-ray crystallography (44,45), probably because of its flexible nature. Many lines of biochemical evidence, however, indicate that the pentameric acidic protein complex L10(L7/L12)2(L7/L12)2 binds to the GTPase-associated domain of 23S rRNA and constitutes a major part of the functional center in procaryotic ribosomes. The eucaryotic proteins P0 and P1/P2 are the counterparts of procaryotic L10 and L7/L12, respectively. We here investigated interactions among silkworm P0, P1 and P2 by using the isolated proteins and showed that a P0:P1:P2 (molar ratio 1:2:2) pentameric complex was reconstituted in vitro, which appears to be a functional unit activating ribosomes by its binding to the GTPase-associated RNA domain. A major difference between the eucaryotic and procaryotic acidic proteins is that there are two types, P1 and P2 (three types in some plants; 46), of the proteins in eucaryotes that are expressed from different genes (19,21,24). The present data clearly confirmed the formation of a P1–P2 heterodimer in vitro (Figs 1 and 2). The P1–P2 heterodimer seems to be compact and stable, because the band of the dimer is distinct on native electrophoretic gels and its mobility is higher than isolated P1 and P2 (Fig. 1A). Formation of the heterodimer is consistent with previous data from different approaches (26,29,30,31,33). All of the present results lead to the conclusion that formation of the P1–P2 heterodimer is a key step in assembly of the P0(P1–P)2 pentameric complex and its rRNA binding to constitute the silkworm ribosomal GTPase center.
Binding of P0 to the GTPase-associated RNA domain appears to be the most crucial step linked directly to ribosome function. It is likely that the RNA-binding site lies in the N-terminal half of P0 protein (47,48) and the P1/P2-binding site in the C-terminal region (24) (Fig. 8). However, it has been very hard to investigate the molecular details of the RNA-binding mechanism of P0, because of its instability in the isolated state. Although rat P0 protein has been prepared from ribosomes (27) and by overexpression in E.coli cells (32), the isolated protein samples are insoluble without P1 and P2. In the present studies, we could isolate P0 protein in a soluble state from silkworm ribosomes. The amino acid sequence of silkworm P0 shows 69% identity with rat P0 (Fig. 8). One or some of the divergent parts of the molecule seem to contribute to the solubility of silkworm P0. From the fact that the very low RNA-binding ability of isolated P0 is significantly increased by addition of both P1 and P2 (Fig. 5), it is conceivable that the conformation of the RNA-binding site of P0 apparently changes on binding of P1–P2 heterodimers to the C-terminal side of the molecule. This allosteric conformational change induced by P1–P2 heterodimers may be important for RNA binding and ribosome function. We propose here that the P1–P2 heterodimer, but neither P1 nor P2 alone, plays a role in modulating the structure and function of P0 protein at least in the case of silkworm proteins.
Figure 8.
Comparison of amino acid sequence for silkworm (Bmo) P0 with those for the rat (Rno) and yeast (Sce) homologs. The sequence of silkworm P0 was derived by assembling several partial sequence data (http://www.ab.a.u-tokyo.ac.jp/silkbase/) of the B.mori cDNA library (51). The conserved arginine-rich region (positions 44–67), which is most likely to participate in RNA binding (47), and the P1/P2-binding region (positions 182–293) (24) are indicated.
There is a major difference between the present study and that of yeast mutants. The yeast mutant deficient in P1 and P2 was made by disruption of genes for both proteins (for P1α, P1β, P2α and P2β) (24). This P1/P2-deficient yeast ribosome retained P0 protein and showed a reduced level of polyphenylalanine synthetic activity. Growth of this strain was 3-fold slower compared with the wild-type (24). In contrast, our results indicate that isolated silkworm P0 hardly binds to the GTPase-associated RNA domain without P1 and P2 and does not activate the ribosome. One explanation for this discrepancy may be the difference in experimental conditions between in vitro and in vivo studies. In yeast cells, there may be components compensating for the P1–P2 modulation of P0. A certain ribosomal protein other than P1/P2 may play such a role. A second possible explanation is species differences. Unlike animal P0, yeast P0 may bind to the RNA without the help of other proteins, although this has not yet been tested. Modes of interaction between P0 and P1/P2 and their functional significance may have diverged during evolution. In fact, identity of amino acid sequence around the P1/P2-binding region (28.6% identity) on P0 is lower than that of full-length P0 (44.2%) as well as the N-terminal arginine-rich region (62.5% identity) among yeast, silkworm and rat (Fig. 8).
Some possible models of protein topography of P0, P1 and P2 on animal ribosomes have been presented (32): (i) P1–P1 and P2–P2 homodimers bind separately to P0; (ii) two P1–P2 heterodimers bind to P0 so that the two dimers come close to each other; (iii) as (ii), but the two dimers do not come close to each other. Our present results strengthen the idea of heterodimer formation and support model (ii) or (iii). The two E.coli L7/L12 homodimers are known to bind very close to each other on the C-terminus of L10 (17); if the same is true in animal ribosomes, then model (ii) may be the actual molecular arrangement. Previous P1–P1 and P2–P2 crosslinking with 2-iminothiolane (22) may be explained by model (ii).
Eucaryotic translation is highly regulated. For instance, the GTPase turnover by eucaryotic 80S ribosomes and the translocase eEF-2 is 10-fold slower than that by procaryotic 70S ribosomes and EF-G (49). Our previous study demonstrated that the P0.P1/P2 complex participates in the eucaryotic characteristics (49). The presence of two kinds of the acidic proteins and their interaction may be involved in the characteristics. Strong binding of P0.P1/P2 complex to the GTPase-associated RNA domain, compared with the E.coli equivalent L10.L7/L12 complex (49), may be also related to the eucaryotic-specific properties in the translation process.
Acknowledgments
ACKNOWLEDGEMENTS
We thank K. Mita (National Institute of Radiological Sciences, Japan) for providing sequence data of silkworm ribosomal proteins and S. Uchiyama (Osaka University, Japan) for helpful discussion on equilibrium sedimentation. We also thank Gene Research Center Shinshu University for giving full facilities for the research. This work was supported by grants-in-aid for scientific research (12660053 and 13033015) and for COE research (10CE2003) from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Noller H.F. (1991) Ribosomal RNA and translation. Annu. Rev. Biochem., 60, 191–227. [DOI] [PubMed] [Google Scholar]
- 2.Beauclerk A.A.D., Cundliffe,E. and Dijk,J. (1984) The binding site for ribosomal protein complex L8 within 23 S ribosomal RNA of Escherichia coli. J. Biol. Chem., 259, 6559–6563. [PubMed] [Google Scholar]
- 3.Egebjerg J., Douthwaite,S., Liljas,A. and Garrett,R.A. (1990) Characterization of the binding sites of protein L11 and the L10.(L12)4 pentameric complex in the GTPase domain of 23S ribosomal RNA from Escherichia coli. J. Mol. Biol., 213, 275–288. [DOI] [PubMed] [Google Scholar]
- 4.Rosendahl G. and Douthwaite,S. (1993) Ribosomal proteins L11 and L10.(L12)4 and the antibiotic thiostrepton interact with overlapping regions of the 23 S rRNA backbone in the ribosomal GTPase centre. J. Mol. Biol., 234, 1013–1020. [DOI] [PubMed] [Google Scholar]
- 5.Cundliffe E. (1986) Involvement of specific portions of ribosomal RNA in defined ribosomal function: a study utilizing antibiotics. In Hardesty,B. and Kramer,G. (eds), Structure, Function and Genetics of Ribosomes. Springer-Verlag, New York, NY, pp. 586–604. [Google Scholar]
- 6.Ban N., Nissen,P., Hansen,J., Capel,M., Moore,P.B. and Steitz,T.A. (1999) Placement of protein and RNA structures into a 5 Å-resolution map of the 50S ribosomal subunit. Nature, 400, 841–847. [DOI] [PubMed] [Google Scholar]
- 7.Möller W. (1974) The ribosomal components involved in EF-G- and EF-Tu-dependent GTP hydrolysis. In Nomura,M., Tissières,A., Lengyel,P. and Kramer,G. (eds), Ribosomes. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 711–731.
- 8.Pettersson I. and Liljas,A. (1979) The stoichiometry and reconstitution of a stable protein complex from Escherichia coli ribosomes. FEBS Lett., 98, 139–144. [DOI] [PubMed] [Google Scholar]
- 9.Hamman B.D., Oleinikove,A.V., Jokhadze,G.G., Traut,R.R. and Jameson,D.M. (1996) Rotational and conformational dynamics of Escherichia coli ribosomal protein L7/L12. Biochemistry, 35, 16672–16679. [DOI] [PubMed] [Google Scholar]
- 10.Dey D., Bochkariov,D.E., Jokhadze,G.G. and Traut,R.R. (1998) Cross-linking of selected residues in the N- and C-terminal domains of Escherichia coli protein L7/L12 to other ribosomal proteins and the effect of elongation factor Tu. J. Biol. Chem., 273, 1670–1676. [DOI] [PubMed] [Google Scholar]
- 11.Stark H., Rodnina,M.V., Wieden,H.-J., van Heel,M. and Wintermeyer,W. (2000) Large-scale movement of elongation factor G and extensive conformational change of the ribosome during translocation. Cell, 100, 301–309. [DOI] [PubMed] [Google Scholar]
- 12.Liljas A. and Gudkov,A.T. (1987) The structure and dynamics of ribosomal protein L12. Biochimie, 69, 1043–1047. [DOI] [PubMed] [Google Scholar]
- 13.Petterson I. (1979) Studies on the RNA and protein binding sites of the E.coli ribosomal protein L10. Nucleic Acids Res., 6, 2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel E., Koka,M. and Nakamoto,T. (1972) Requirement of an Escherichia coli 50S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J. Biol. Chem., 247, 805–814. [PubMed] [Google Scholar]
- 15.Möller W., Schrier,P.I., Maassen,J.A., Zantema,A., Schop,E., Reinalda,H., Cremers,A.F. and Mellema,J.E. (1983) Ribosomal proteins L7/L12 of Escherichia coli. Localization and possible molecular mechanism in translation. J. Mol. Biol., 63, 553–573. [DOI] [PubMed] [Google Scholar]
- 16.Oleinikov A.V., Jokhadze,G.G. and Traut,R.R. (1998) A single-headed dimer of Escherichia coli ribosomal protein L7/L12 supports protein synthesis. Proc. Natl Acad. Sci. USA, 95, 4215–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griaznova O. and Traut,R.R. (2000) Deletion of C-terminal residues of Escherichia coli ribosomal protein L10 causes the loss of binding of one L7/L12 dimer: ribosomes with one L7/L12 dimer are active. Biochemistry, 39, 4075–4081. [DOI] [PubMed] [Google Scholar]
- 18.MacConnell W.P. and Kaplan,N.O. (1982) The activity of the acidic phosphoproteins from the 80S rat liver ribosome. J. Biol. Chem., 257, 5359–5366. [PubMed] [Google Scholar]
- 19.Wool I.G., Chan,Y.L., Glück,A. and Suzuki,K. (1991) The primary structure of rat ribosomal proteins P0, P1 and P2 and a proposal for a uniform nomenclature for mammalian and yeast ribosomal proteins. Biochimie, 73, 861–870. [DOI] [PubMed] [Google Scholar]
- 20.Vard C., Guillot,D., Bargis,P., Lavergne,J.P. and Reboud,J.P. (1997) A specific role for the phosphorylation of mammalian acidic ribosomal protein P2. J. Biol. Chem., 272, 20259–20262. [DOI] [PubMed] [Google Scholar]
- 21.Rich B.E. and Steitz,J. (1987) Human acidic ribosomal phosphoproteins P0, P1 and P2: analysis of cDNA clones, in vitro synthesis and assembly. Mol. Cell. Biol., 7, 4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchiumi T., Whaba,A.J. and Traut,R.R. (1987) Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by crosslinking. Proc. Natl Acad. Sci. USA, 84, 5580–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remacha M., Saenz-Robles,M.T., Vilella,M.D. and Ballesta,J.P.G. (1988) Independent genes coding for three acidic proteins of the large ribosomal subunit from Saccharomyces cerevisiae. J. Biol. Chem., 263, 9094–9101. [PubMed] [Google Scholar]
- 24.Remacha M., Jimenez-Diaz,A., Santos,C., Briones,E., Zambrano,R., Gabriel,M.A.R., Guarinos,E. and Ballesta,J.P.G. (1995) Proteins P1, P2 and P0, components of the eukaryotic ribosome stalk. New structural and functional aspects. Biochem. Cell Biol., 73, 959–968. [DOI] [PubMed] [Google Scholar]
- 25.Wool I.G., Chan,Y.L. and Glück,A. (1995) Structure and evolution of mammalian ribosomal proteins. Biochem. Cell Biol., 73, 933–947. [DOI] [PubMed] [Google Scholar]
- 26.Ballesta J.P.G., Guarinos,E., Zurdo,J., Parada,P., Nusspaumer,G., Lalioti,V.S., Perez-Fernandez,J. and Remacha,M. (2000) Structure of the yeast ribosomal stalk. In Garret,R.A., Douthwaite,S.R., Liljas,A., Matheson,A.T., Moore,P.B. and Noller,H.F. (eds), The Ribosome: Structure, Function, Antibiotics and Cellular Interactions. ASM Press, Washington, DC, pp. 115–125.
- 27.Uchiumi T. and Kominami,R. (1997) Binding of mammalian ribosomal protein complex P0.P1.P2 and protein L12 to the GTPase-associated domain of 28S ribosomal RNA and effect on the accessibility to anti-28S RNA autoantibody. J. Biol. Chem., 272, 3302–3308. [DOI] [PubMed] [Google Scholar]
- 28.Uchiumi T., Honma,S., Nomura,T., Dabbs,E.R. and Hachimori,A. (2002) Translation elongation by a hybrid ribosome in which proteins at the GTPase center of the Escherichia coli ribosome are replaced with rat counterparts. J. Biol. Chem., 277, 3857–3862. [DOI] [PubMed] [Google Scholar]
- 29.Tchórzewski M., Boguszewska,A., Dukowski,P. and Grankowski,N. (2000) Oligomerization properties of the acidic ribosomal P-proteins from Saccharomyces cerevisiae: effect of P1A protein phosphorylation on the formation of the P1A-P2B hetero-complex. Biochim. Biophys. Acta, 1499, 63–73. [DOI] [PubMed] [Google Scholar]
- 30.Tchórzewski M., Boldyreff,B., Issinger,O.G. and Grankowski,N. (2000) Analysis of the protein-protein interactions between the human acidic ribosomal P-proteins: evaluation by the two hybrid system. Int.J. Biochem. Cell Biol., 32, 737–746. [DOI] [PubMed] [Google Scholar]
- 31.Zurdo J., González,C., Sanz,J.M., Rico,M., Remacha,M. and Ballesta,J.P.G. (2000) Structural differences between Saccharomyces cerevisiae ribosomal stalk proteins P1 and P2 support their functional diversity. Biochemistry, 39, 8935–8943. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalo P., Lavergne,J.P. and Reboud,J.P. (2001) Pivotal role of the P1 N-terminal domain in the assembly of the mammalian ribosomal stalk and in the proteosynthetic activity. J. Biol. Chem., 276, 19762–19769. [DOI] [PubMed] [Google Scholar]
- 33.Garinos E., Remacha,M. and Ballesta,J.P.G. (2001) Asymmetric interactions between the acidic P1 and P2 proteins in the Saccharomyces cerevisiae ribosomal stalk. J. Biol. Chem., 276, 32474–32479. [DOI] [PubMed] [Google Scholar]
- 34.Uchiumi T., Nomura,T., Shimizu,T., Katakai,Y., Mita,K., Koike,Y., Nakagaki,M., Taira,H. and Hachimori,A. (2000) A covariant change of the two highly conserved bases in the GTPase-associated center of 28S rRNA in silkworms and other moths. J. Biol. Chem., 275, 35116–35121. [DOI] [PubMed] [Google Scholar]
- 35.Uchiumi T., Traut,R.R. and Kominami,R. (1990) Monoclonal antibodies against acidic phosphoproteins P0, P1 and P2 of eukaryotic ribosomes as functional probes. J. Biol. Chem., 265, 89–95. [PubMed] [Google Scholar]
- 36.Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 37.Dabbs E.R. (1979) Selection for Escherichia coli mutants with proteins missing from the ribosome. J. Bacteriol., 140, 734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenny J.W., Lambert,J.M. and Traut,R.R. (1979) Cross-linking of ribosomes using 2-iminothiolane (methyl 4-mercaptobutyrimidate) and identification of cross-linked proteins by diagonal polyacrylamide/sodium dodecyl sulfate gel electrophoresis. Methods Enzymol., 59, 534–550. [DOI] [PubMed] [Google Scholar]
- 39.Qian S., Zhang,J.Y., Kay,M.A. and Jacobs-Lorena,M. (1987) Structural analysis of the Drosophila rpA1 gene, a member of the eucaryotic ‘A’ type ribosomal protein family. Nucleic Acids Res., 15, 987–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleveland D.W., Fischer,S.G., Kirschner,M.W. and Laemmli,U.K. (1977) Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem., 252, 1102–1106. [PubMed] [Google Scholar]
- 41.Santos C., Ortiz-reyes,B., Naranda,T., Remacha,M. and Ballesta,J.P.G. (1993) The acidic phosphoproteins from Saccharomyces cerevisiae ribosomes. NH2-terminal acetylation is a conserved difference between P1 and P2 proteins. Biochemistry, 32, 4231–4236. [DOI] [PubMed] [Google Scholar]
- 42.Wigboldus J.D. (1987) cDNA and deduced amino acid sequence of Drosophila rp21C, another ‘A’-type ribosomal protein. Nucleic Acids Res., 15, 10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schachman H.K. (1959) Ultracentrifugation in Biochemistry. Academic Press, New York, NY.
- 44.Ban N., Nissen,P., Hansen,J., Moore,P.B. and Steitz,T.A. (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science, 289, 905–920. [DOI] [PubMed] [Google Scholar]
- 45.Harms J., Schluenzen,F., Zarivach,R., Bashan,A., Gat,S., Agmon,I., Bartels,H., Franceschi,F. and Yonath,A. (2001) High resolution structure of the large ribosomal subunit from a mesophilic eubacterium. Cell, 107, 679–688. [DOI] [PubMed] [Google Scholar]
- 46.Szick K., Springer,M. and Bailey-Serres,J. (1998) Evolutionary analyses of the 12-kDa acidic ribosomal P-proteins reveal a distinct protein of higher plant ribosomes. Proc. Natl Acad. Sci. USA, 95, 2378–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsui K., Nakagawa,T. and Tsurugi,K. (1989) The gene and the primary structure of acidic ribosomal protein A0 from yeast Saccharomyces cerevisiae which shows partial homology to bacterial ribosomal protein L10. J. Biochem. (Tokyo), 106, 223–227. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Gabriel M.A., Remacha,M. and Ballesta,J.P.G. (2000) The RNA interacting domain but not the protein interacting domain is highly conserved in ribosomal protein P0. J. Biol. Chem., 275, 2130–2136. [DOI] [PubMed] [Google Scholar]
- 49.Uchiumi T., Hori,K., Nomura,T. and Hachimori,A. (1999) Replacement of L7/L12.L10 protein complex in Escherichia coli ribosomes with the eukaryotic counterpart changes the specificity of elongation factor binding. J. Biol. Chem., 274, 27578–27582. [DOI] [PubMed] [Google Scholar]
- 50.Iwasaki K. and Kaziro,Y. (1979) Polypeptide chain elongation factors from pig liver. Methods Enzymol., 60, 657–676. [DOI] [PubMed] [Google Scholar]
- 51.Mita K., Morimyo,M., Okano,K., Shimada,T. and Maeda,S. (1999) The construction of EST database for genome analysis of Bombyx mori. Riken Rev., 22, 63–67. [Google Scholar]









