Abstract
Purpose
This multicenter, open-label, phase II trial evaluated the efficacy and safety of bortezomib combined with dexamethasone for the treatment of relapsed/refractory cutaneous T-cell lymphoma (CTCL) in previously treated patients across 14 institutions in South Korea.
Materials and Methods
Between September 2017 and July 2020, 29 patients with histologically confirmed CTCL received treatment, consisting of eight 4-week cycles of induction therapy followed by maintenance therapy, contingent upon response, for up to one year. The primary endpoint was the proportion of patients achieving an objective global response.
Results
Thirteen of the 29 patients (44.8%) achieved an objective global response, including two complete responses. The median progression-free survival (PFS) was 5.8 months, with responders showing a median PFS of 14.0 months. Treatment-emergent adverse events were generally mild, with a low incidence of peripheral neuropathy and hematologic toxicities. Despite the trend toward shorter PFS in patients with higher mutation burdens, genomic profiling before and after treatment showed no significant emergence of new mutations indicative of disease progression.
Conclusion
This study supports the use of bortezomib and dexamethasone as a viable and safe treatment option for previously treated CTCL, demonstrating substantial efficacy and manageability in adverse effects. Further research with a larger cohort is suggested to validate these findings and explore the prognostic value of mutation profiles.
Keywords: Cutaneous T-cell lymphoma, Bortezomib, Dexamethasone, Relapsed/refractory, Treatment efficacy, Safety profile
Introduction
Cutaneous T-cell lymphomas (CTCLs) comprise a heterogeneous group of rare extranodal lymphoproliferative disorders primarily affecting the skin, stemming from tissue-resident T cells [1]. Among the various histologic subtypes of CTCLs, the most prevalent is mycosis fungoides (MF) [2]. Treatment approaches for CTCLs are tailored according to the disease stage [3]. Early-stage CTCLs (patch/plaque stage) typically demonstrate an indolent clinical course with an excellent prognosis and can often be effectively managed using skin-directed therapies over an extended period [4]. The therapeutic goal is to alleviate symptoms and enhance quality of life while minimizing treatment-related adverse effects. Conversely, advanced-stage CTCLs tend to follow a progressive clinical course with a poorer prognosis, often necessitating systemic treatment [5]. Advanced diseases commonly exhibit rapid relapse and progression, even following an initial response to systemic therapies, thereby limiting the utility of combination chemotherapy with cytotoxic agents. Consequently, frontline systemic therapy for CTCLs often favors less toxic biologics such as retinoid derivatives and IFN-α over cytotoxic agents [6].
Over the past two decades, a range of systemic agents, including less myelosuppressive cytotoxic drugs, histone deacetylase inhibitors, and mammalian target of rapamycin inhibitors, have been evaluated in phase II trials for their effectiveness against CTCLs [7-10]. However, these agents typically demonstrated only modest efficacy, with response rates below 40% and brief durations of response. Recently, following positive outcomes from two phase III trials, brentuximab vedotin and mogamulizumab received approval and have been incorporated into standard practice for treating relapsed/refractory CTCLs [11-13]. Additionally, emerging immunotherapeutic strategies, such as antagonistic antibodies targeting immune checkpoints and chimeric antigen receptor (CAR) T-cell therapies, have shown early signs of effectiveness against CTCLs [14,15]. Despite these advances, nearly all patients ultimately experience disease relapse and progression. Thus, CTCLs are currently regarded as disorders that are difficult or even impossible to cure. Given this clinical reality, expanding the range of available treatment options would be highly beneficial for patients.
Prior preclinical research has underscored the vital role of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in both the proliferation and survival of lymphoma cells [16]. Specifically, activating mutations in TNFR2 (tumor necrosis factor receptor 2) have been found to lead to persistent NF-κB activation in primary tumor cells from patients with CTCL, closely linking to resistance against apoptosis [17]. Consequently, NF-κB emerges as a compelling therapeutic target. Indeed, a recent phase II study demonstrated significant efficacy of dimethyl fumarate, an NF-κB inhibitor, in treating MF and Sézary syndrome (SS) [18]. Furthermore, bortezomib, a proteasome inhibitor used in various lymphoid malignancies, prevents IκB degradation, thereby inhibiting NF-κB activity and exhibiting antitumor effects in CTCL [19].
Therefore, we conducted a phase II study to evaluate the efficacy and safety of bortezomib in combination with high-dose dexamethasone in patients with relapsed or refractory CTCL. This paper presents the results of our study, which includes both pre- and post-treatment targeted sequencing of tumor tissue.
Materials and Methods
1. Participants and eligibility criteria
The research was carried out from 2017 to 2020 across 14 academic institutions throughout South Korea. The study included patients 19 years of age or older who were histologically diagnosed with CTCL. This encompassed several subtypes such as MF, SS, primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and primary cutaneous peripheral T-cell lymphoma unspecified. Eligibility was limited to those who had not responded to at least one prior skin-directed or systemic therapy. Participants were required to have completed any form of anti-lymphoma treatment at least four weeks prior to enrollment and must have been free from any significant treatment-related toxic effects at the time of entry. Additional inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status ranging from 0 to 2, and sufficient organ and bone marrow functionality, evidenced by a leukocyte count of at least 4,000/μL, an absolute neutrophil count of no less than 1,500/μL, and a platelet count exceeding 100,000/μL. Exclusion criteria included pregnancy or lactation, fertile women not employing effective contraception, and individuals with active infections or a past or concurrent secondary neoplasm.
2. Study design and treatment
This phase II, open-label, single-arm trial evaluates the antitumor efficacy and safety of bortezomib combined with dexamethasone in patients with relapsed or refractory CTCL (ClinicalTrials.gov: NCT03487133). The regimen is depicted in S1 Fig. and comprises two treatment phases: (1) Induction therapy: over eight 4-week cycles, patients receive subcutaneous bortezomib (1.6 mg/m2) and dexamethasone (40 mg) administered intravenously or orally on days 1, 8, and 15 of each cycle. Patients exhibiting disease progression at the end of the fourth or eighth cycle are withdrawn from the trial; (2) Maintenance therapy: following the induction phase, patients who achieve at least stable disease continue into the maintenance phase. This involves receiving the same dosages of bortezomib and dexamethasone on day 1 of each subsequent 4-week cycle, with therapy continuing until disease progression or the emergence of intolerable side effects, up to a maximum duration of 1 year.
3. Efficacy and safety assessments
Efficacy and safety assessments were conducted bi-cyclically throughout the treatment duration. The clinical overall response was quantified using the global composite response score, which integrates responses across four anatomical compartments—skin, lymph nodes, viscera, and blood. This scoring adheres to the consensus guidelines established by the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the European Organization for Research and Treatment of Cancer (ISCL-USCLC-EORTC) [20,21]. Skin compartment tumor burden was specifically measured utilizing the modified Severity-Weighted Assessment Tool (mSWAT). For patients presenting with lymph node or visceral involvement, whole-body positron emission tomography–computed tomography and/or contrast-enhanced computed tomography scans were performed at screening, after every two treatment cycles (cycles 2, 4, 6, 8, 11, 14, 17, and 21), and at treatment conclusion. Concurrently, patients with hematologic involvement underwent flow cytometry assessments at identical intervals to the skin evaluations. Adverse events were systematically recorded following the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), ver. 4.3.
4. Study endpoints
The primary endpoint of the study was to establish the overall response rate (ORR) in accordance with the ISCL-USCLC-EORTC response criteria. Among the secondary endpoints, the proportion of patients achieving an objective global response sustained for at least 4 months (ORR4) was evaluated. Additional secondary endpoints included the disease control rate (DCR), defined as the proportion of patients who achieved stable disease or better; the duration of response (DOR); progression-free survival (PFS); overall survival (OS); and safety metrics.
5. Ethical considerations
The study was conducted in strict compliance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice (GCP) guidelines, and the Declaration of Helsinki. The study protocol and informed consent documents received approval from the institutional review boards (IRBs) at all participating institutions. Final approval was also obtained from the Korean Ministry of Food and Drug Safety (MFDS). Written informed consent was obtained from all participants prior to their involvement in the study. Additionally, all experimental procedures involving patient-derived specimens and molecular testing were conducted in accordance with the approved IRB protocol.
6. Targeted Sequencing and variant detection
Genomic DNA was isolated from tumor tissues and sheared using a QIAamp DNA Mini kit (Qiagen, Valencia, CA) and Covaris S220 (Covaris, Woburn, MA). The concentration and purity of the DNA were evaluated using a Nanodrop 8000 UV-Vis spectrometer (Thermo Fisher Scientific, Waltham, MA) and a Picogreen fluorescence assay with a Qubit 2.0 Fluorometer (Life Technologies, Foster City, CA). Fragment size distribution was analyzed using a 2200 TapeStation (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instruction (S2 Table). For high-throughput sequencing, HemaSCAN panel, covering the whole exome of 425 genes (S3 Table) linked to hematological malignancies, was employed as previously described [22]. Single-nucleotide variants were called using MuTect version 1.1.4 and Lowfreq ver. 0.6.1 with specific criteria for variant allele frequency set at ≥ 1% or supporting reads greater than four. To enhance sensitivity, the candidate set of variants was formed by combining the variants identified both callers, with the high confidence set from MuTect. Sequencing artifacts were filtered out using a machine learning algorithm analyzing features from the SAM files, as an additional filtering step. Briefly, this process included the application of a logistic regression model trained to discern false-positive calls occurring normal samples, including abnormally aligned, clustered, and strand-biased reads. Additionally, low-confidence variants (supporting reads < 20) were manually inspected using the Integrative Genomics Viewer. Small insertions and deletions were detected using Pindel version 0.2.5a4, with the criterion for variant supporting reads set at > 9. Variants with a minor allele frequency of ≥ 1% in population databases such as the 1000 Genomes Project and the Korean Reference Genome Database were excluded. Copy number variations were assessed, with amplifications defined by a copy number > 5 and deletions by a copy number < 1.2. JuLI was employed to identify deletions greater than 30 bp and structural variants.
7. Statistical methods
The study was designed using Simon’s two-stage phase II optimal design to provide 80% power and maintain a one-sided type I error rate of 0.05. The design aimed to differentiate between a non-effective ORR of 10% and a target ORR of 30%. The initial cohort comprised 10 patients; expansion to 29 patients was contingent upon the occurrence of at least two responders within this group. A positive trial outcome was predetermined if more than 10 out of the 29 patients demonstrated a response. ORR calculations, along with their 95% confidence intervals (CIs), were performed using the Clopper-Pearson exact method. DoR, PFS, and OS were analyzed using the Kaplan-Meier survival estimation technique. Demographic characteristics, other clinical outcomes, and safety data were summarized descriptively. All statistical analyses were conducted using SPSS ver. 25.0 (IBM Corp., Armonk, NY).
Results
1. Demographics and characteristics
Between September 15, 2017, and July 22, 2020, a total of 32 patients were assessed for eligibility. Of these, 29 were enrolled in the phase II study (Fig. 1A). Their demographics and baseline characteristics are detailed in Table 1. The median age of participants was 59 years, with an age range from 26 to 82 years. The cohort included 19 patients diagnosed with MF, six with CD30+ primary cutaneous T-cell lymphoproliferative disorders, and four with unspecified primary cutaneous peripheral T-cell lymphoma. Among the patients with MF, 13 presented with advanced clinical stages (stage IIB or higher) at the start of the study. Patients had a median of two previous lines of systemic therapy before enrollment, with most having been treated with systemic cytotoxic chemotherapy.
Fig. 1.
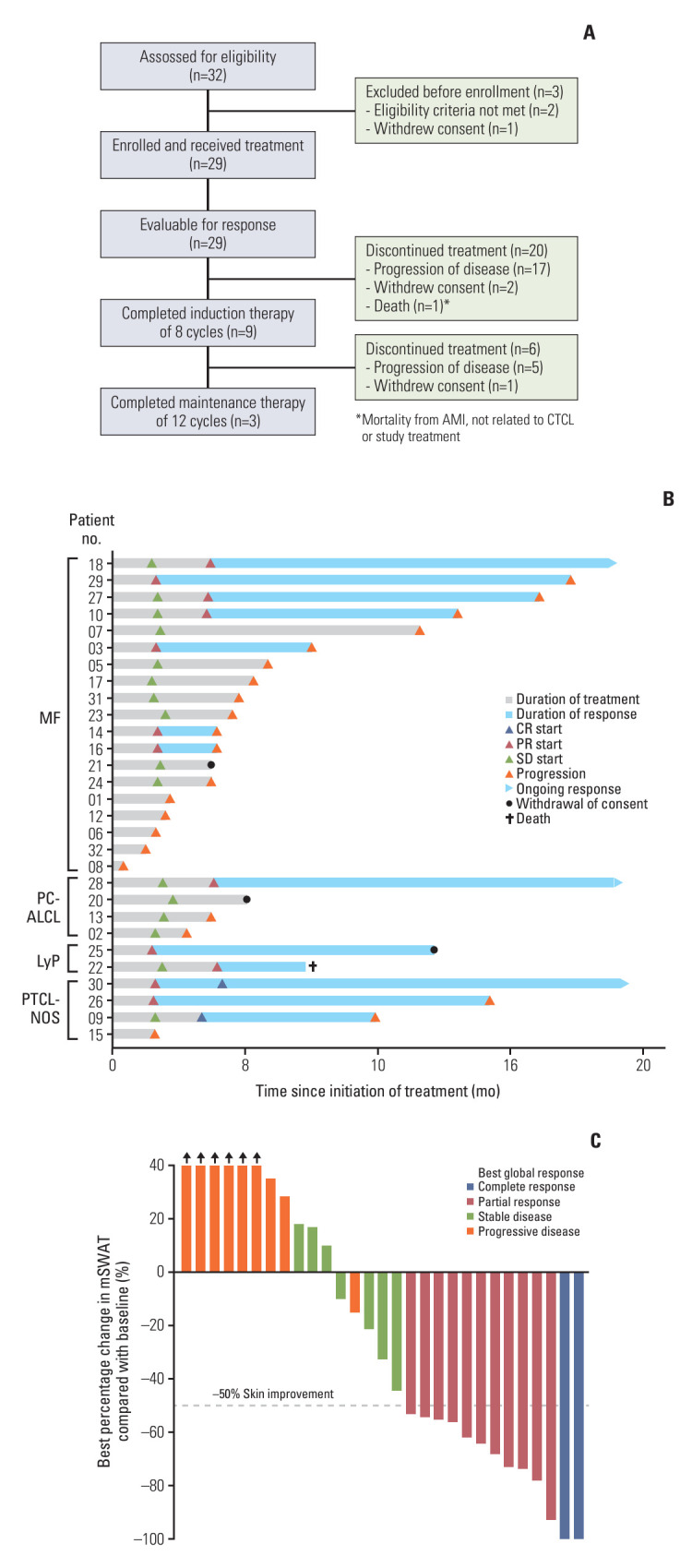
CONSORT diagram and pattern of tumor response. (A) Flowchart of participants in the BIC study. (B) Swimmer plot showing duration of the study treatment and tumor responses. (C) Best change from baseline in skin by mSWAT scoring system. Bar color reflects global response according to global response criteria. AMI, acute myocardial infarction; CR, complete response; CTCL, cutaneous T-cell lymphoma; LyP, lymphomatoid papulosis; MF, mycosis fungoides; mSWAT, modified Severity-Weighted Assessment Tool; PC-ALCL, primary cutaneous anaplastic large cell lymphoma; PR, partial response; PTCL-NOS, peripheral T-cell lymphoma, unspecified; SD, stable disease.
Table 1.
Baseline demographics and disease characteristics
| Characteristic | Patients (n=29) |
|---|---|
| Age (yr) | 59 (26-82) |
| Sex | |
| Male | 14 (48.3) |
| Female | 15 (51.7) |
| ECOG performance status | |
| 0/1 | 13 (44.8)/14 (48.3) |
| 2 | 2 (6.9) |
| Time from initial diagnosis (mo) | 21 (3-193) |
| Histologic subtype | |
| Mycosis fungoides/Sézary syndrome | 19 (65.5)/0 |
| Lymphomatoid papulosis | 2 (6.9) |
| Primary cutaneous anaplastic large cell lymphoma | 4 (13.8) |
| Peripheral T-cell lymphoma, not otherwise specified | 4 (13.8) |
| Clinical stage at study entry | |
| Mycosis fungoides | 19 (100) |
| IA/IB | 1 (5.3)/3 (15.8) |
| IIA/IIB | 2 (10.5)/2 (10.5) |
| IIIA/IIIB | 3 (15.8)/1 (5.3) |
| IVA/IVB | 5 (26.3)/2 (10.5) |
| CD30+ primary cutaneous T-cell lymphoproliferative disordersa) | 6 (100) |
| T1 | 2 (33.3) |
| T2 | 3 (50.0) |
| T3 | 1 (16.7) |
| Prior treatments | |
| Ultraviolet B phototherapy | 9 (31.0) |
| Radiation | 10 (34.5) |
| Systemic therapy | 26 (89.7) |
| Previous lines of systemic therapies | |
| 0-1 | 14 (48.3) |
| 2-3 | 8 (27.6) |
| ≥ 4 | 7 (24.1) |
| Previous systemic treatment received | 26 (100) |
| Retinoids | 2 (7.7) |
| Interferon-α | 7 (26.9) |
| Cytotoxic chemotherapy | 24 (92.3) |
| Brentuximab vedotin | 5 (19.2) |
| Stem cell transplantation | 1 (3.8) |
Values are presented as median (range) or number (%). ECOG, Eastern Cooperative Oncology Group.
CD30+ primary cutaneous T-cell lymphoproliferative disorders include lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma.
2. Treatment duration and efficacy outcomes
As illustrated in Fig. 1A, only three patients (10.3%) completed the entire planned 20-cycle course of treatment. The majority, comprising 26 patients (89.7%), discontinued treatment, resulting in a median treatment duration of five cycles. Among those discontinuing, 22 patients (75.9%) ceased treatment due to disease progression, while three patients (10.3%) withdrew their consent (Fig. 1B). At the data cutoff point (median follow-up of 17.0 months [range, 1.53 to 46.6 months]), the ORR was 44.8% (95% CI, 27.6 to 62.1), with two complete responses noted (Table 2). All responders showed improvement in their skin involvement, with the median score of the best change in mSWAT from baseline being –25%, ranging from 58% to –100% (Fig. 1C). Significant tumor responses were observed particularly in patients with advanced MF, including those with erythrodermic and tumor stage manifestations (Fig. 2A). Proportion of patients with tumor response in extracutaneous compartments is also described in Table 3. The response rate remained consistent across different histologic subtypes. Notably, a sustained response of over 4 months (ORR4) was recorded in 34.5% of cases (95% CI, 17.2 to 51.7). The median DOR among these patients was 8.6 months (Fig. 1B), and the DCR was 69.0% (95% CI, 51.7 to 86.2).
Table 2.
Efficacy measures per ISCL-USCLC-EORTC consensus guidelines
| Efficacy | No. (%) (n=29) |
|---|---|
| Best overall response | |
| Complete response | 2 (6.9) |
| Partial response | 11 (37.9) |
| Stable disease | 7 (24.2) |
| Progressive disease | 9 (31.0) |
| Overall response rate | 13 (44.8) |
| ORR4 | 10 (34.5) |
| Disease control rate | 20 (69.0) |
ORR4, an objective global response lasting (from first to last response) at least 4 months. ISCL-USCLC-EORTC, International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the European Organization for Research and Treatment of Cancer.
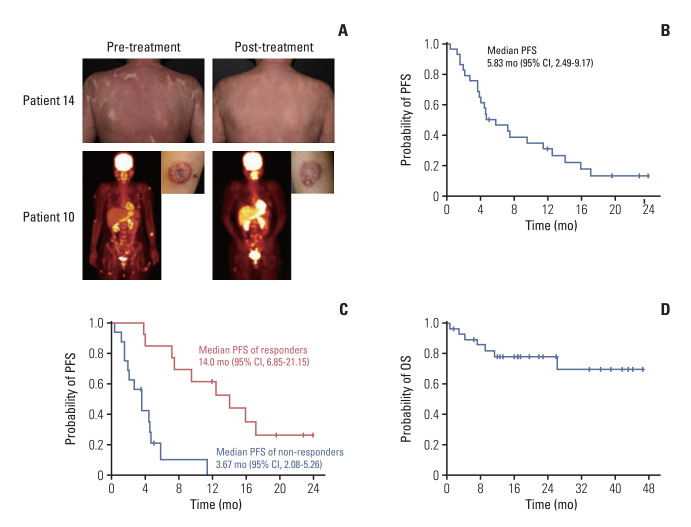
Fig. 2.
Antitumor activity and survival outcomes of the study treatment in patients with cutaneous T-cell lymphomas. (A) Tumor response in representative advanced mycosis fungoides patients with erythrodermic and tumor stage presentations. (B) The Kaplan-Meier curves of progression-free survival (PFS) in the intention-to-treat (ITT) population (n=29). (C) The Kaplan-Meier curves of PFS in responders (n=13) and non-responders (n=16). (D) The Kaplan-Meier curves of overall survival (OS) in the ITT population. CI, confidence interval.
Table 3.
Tumor response in extracutaneous compartments
| Response in compartment |
|||
|---|---|---|---|
| Lymph node | Viscera | Blood | |
| Complete response | 0 | 0 | 0 |
| Partial response | 3 (10.3) | 0 | 0 |
| Stable disease | 4 (13.8) | 2 (6.9) | 1 (3.4) |
| Progressive disease | 4 (13.8) | 2 (6.9) | 1 (3.4) |
| Not involved | 18 (62.1) | 25 (86.2) | 27 (93.2) |
Values are presented as number (%).
3. Survival analysis
Progression or death occurred in 23 patients (79.3%). The median PFS was calculated at 5.83 months (95% CI, 2.49 to 9.17) (Fig. 2B). Notably, in the 13 patients who responded to treatment, the median PFS extended to 14.0 months (95% CI, 6.85 to 21.15) (Fig. 2C). OS events were recorded in seven patients (24.1%), and the median OS had not been reached at the time of data analysis (Fig. 2D).
4. Safety and tolerability
Throughout the study, no treatment-related fatalities were reported. A significant proportion of the participants, 24 patients (82.8%), experienced at least one treatment-emergent adverse event (TEAE), as detailed in Table 4. The most frequently reported TEAEs included peripheral neuropathy (20.7%), fatigue (17.2%), and skin rash (17.2%). Severe adverse events, categorized as grade 3/4 TEAEs, were relatively uncommon, affecting only four patients (13.8%). This included a singular case (3.4%) of grade 4 thrombocytopenia. Additionally, two patients (6.9%) experienced serious infections; one of these was a case of pneumonia that necessitated hospitalization. Overall, the combination of bortezomib and dexamethasone demonstrated manageable toxicities, with most TEAEs being of grade 1 or 2 severity. According to the study protocol, dose delay and reduction were required in only one case, which was due to grade 4 thrombocytopenia. Importantly, none of the adverse events led to the discontinuation of study treatment.
Table 4.
Treatment-emergent adverse events
| Adverse event | Grade 1-2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Hematologic events | |||
| Thrombocytopenia | 2 (6.9) | 0 | 1 (3.4) |
| Neutropenia | 1 (3.4) | 0 | 0 |
| Anemia | 4 (13.8) | 0 | 0 |
| Gastrointestinal events | |||
| Diarrhea | 3 (10.3) | 1 (3.4) | 0 |
| Nausea | 4 (13.8) | 0 | 0 |
| Neurologic events | |||
| Peripheral neuropathy | 6 (20.7) | 0 | 0 |
| Seizure | 1 (3.4) | 0 | 0 |
| Infections | |||
| Pneumonia | 0 | 1 (3.4) | 0 |
| Eye infection | 2 (6.9) | 0 | 0 |
| Skin infection | 0 | 1 (3.4) | 0 |
| Upper respiratory tract infection | 3 (10.3) | 0 | 0 |
| Other conditions | |||
| Fatigue | 5 (17.2) | 0 | 0 |
| Skin rash | 5 (17.2) | 0 | 0 |
| Pyrexia | 4 (13.8) | 0 | 0 |
| Elevated aminotransferase | 2 (6.9) | 0 | 0 |
| Hypertension | 2 (6.9) | 0 | 0 |
Values are presented as number (%).
5. Mutation analysis and genomic landscape comparison
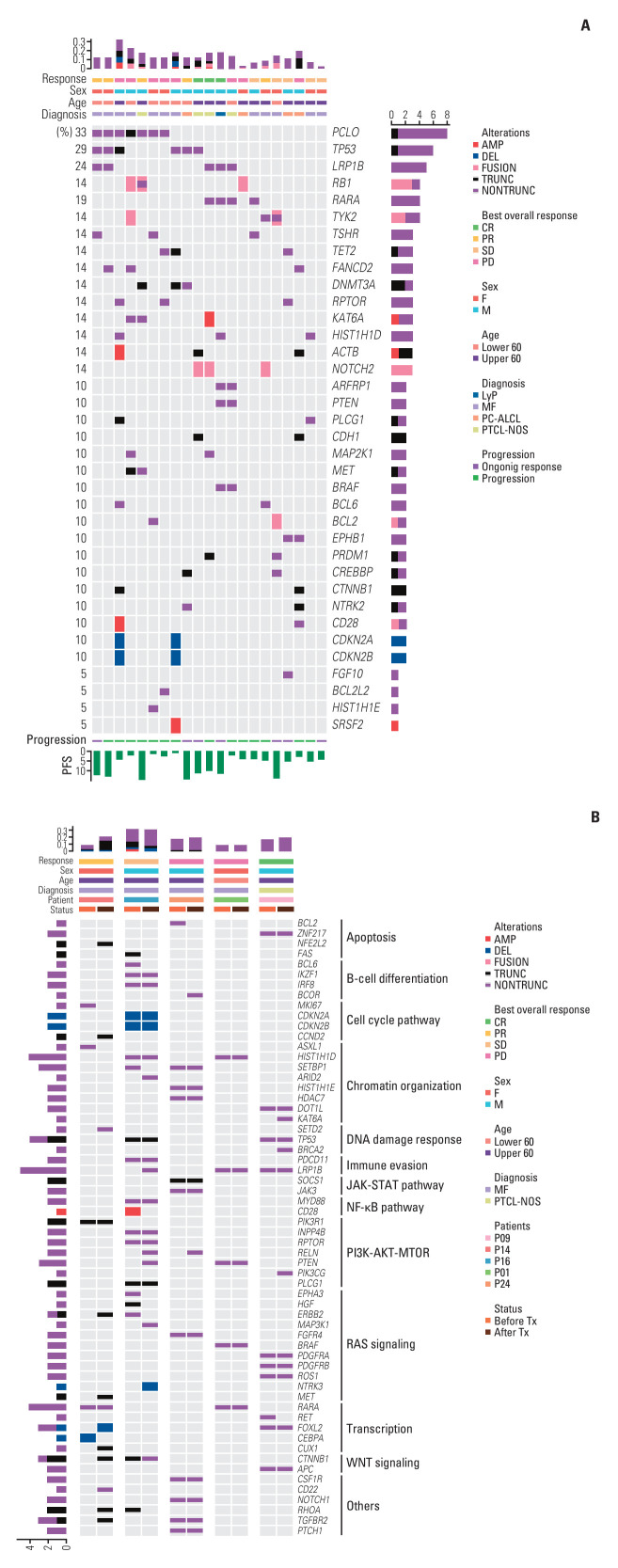
Following enrollment, pre-treatment tissue samples from patients were collected and preserved for targeted sequencing to evaluate the mutation profiles of relapsed or refractory CTCL before starting treatment with bortezomib and dexamethasone (S4 Table). Of the 29 patients enrolled, targeted sequencing was performed on 21 whose tissue samples were available, as shown in Fig. 3A. The most frequent mutations in the cohort were found in the PCLO (33%, n=7), TP53 (29%, n=6), and LRP1B (24%, n=5) genes. Common copy number alterations included deletions in CDKN2A and CDKN2B (both 10%, n=2 each). Among the six patients with TP53 mutations, two also had concurrent deletions in CDKN2A and CDKN2B. Additionally, mutations were observed in several genes involved in chromatin organization, including CREBBP, DNMT3A, and TET2. These mutations, however, did not correlate with the therapeutic response to bortezomib and dexamethasone. There was a trend toward shorter PFS in patients with higher mutation burdens, though this did not reach statistical significance (Fig. 3A). A comparative genomic analysis before and after treatment indicated no significant changes or emergence of new mutations suggestive of disease progression. However, one patient (P14) showed an increase in mutation number in the post-treatment biopsy compared to the pre-treatment tissue, as detailed in Fig. 3B. This patient developed de novo truncating mutations in several genes including NFE2L2, CCND2, ERBB2, MET, CTNNB1, RHOA, and TGFBR2, the impact of which on disease progression remains to be further explored.
Fig. 3.
Landscape of somatic alterations in patients with cutaneous T-cell lymphomas (CTCLs). (A) Heatmap showing the most frequent genetic alteration in patients with relapsed or refractory CTCLs prior to initiating treatment with bortezomib and dexamethasone. Each column represents a distinct patient. (B) Longitudinal analysis of treatment-induced genomic profile changes. The heatmap illustrates the evolution of genomic profiles under the influence of bortezomib and dexamethasone treatment in patients with relapsed or refractory CTCLs. CR, complete response; LyP, lymphomatoid papulosis; MF, mycosis fungoides; MTOR, mammalian target of rapamycin; PC-ALCL, primary cutaneous anaplastic large cell lymphoma; PD, progressive disease; PFS, progression-free survival; PI3K, phosphoinositide 3-kinase; PR, partial response; PTCL-NOS, peripheral T-cell lymphoma, unspecified; SD, stable disease; Tx, therapy.
Discussion
In this study, the combination of bortezomib and dexamethasone showed considerable efficacy in treating previously treated patients with CTCLs, including advanced-stage MF, achieving an ORR of 44.8%. While earlier research indicated some efficacy of bortezomib as a single agent in CTCL patients, many experienced disease progression soon after completing the standard bortezomib regimen [23]. To address this issue, we developed a treatment approach that extends exposure to the therapeutic agent by adding a maintenance phase with a dosing schedule of once every 4 weeks, following eight cycles of induction therapy. This approach notably extended the response duration, as evidenced by improvements in both ORR and ORR4. The median PFS among responders was 14.0 months, comparable to results seen with brentuximab vedotin [11]. Importantly, the duration and quality of response are more indicative of patient survival outcomes and quality of life than merely the response rate in the treatment of CTCLs.
The extended treatment strategy may inherently lead to increased toxicities. Neurotoxicity, particularly sensory and motor peripheral neuropathy, is a significant concern with bortezomib-based treatments. However, the shift to subcutaneous administration of bortezomib has significantly lowered the incidence of neurotoxicity [24]. In a previous phase II study, our group investigated bortezomib administered weekly in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for treating peripheral T-cell lymphomas [25]. Despite the concurrent use of vincristine, known to exacerbate neuropathy, peripheral neuropathy was less severe, prompting us to adopt this regimen in the current study. Indeed, peripheral neuropathy was reported in only 20.7% of participants, with all cases being either grade 1 or 2, which is much lower than the incidence reported in the previous phase II study using intravenous bortezomib [23]. Additionally, with weekly bortezomib administration, most hematologic toxicities were manageable.
Over the past decade, novel agents targeting surface molecules on CTCL tumor cells have been introduced and incorporated into routine care following their success in phase III studies [11,13]. Currently, other immunotherapeutic strategies, including agents targeting immune checkpoints, the killer-cell immunoglobulin-like receptor, and CAR-engineered T cells, are being explored and have shown promising results in early-stage clinical trials [14,26,27]. Despite these advancements, achieving long-term remission in CTCLs remains exceedingly rare with existing treatments [6]. Therefore, there is a pressing need to identify and validate a broader range of effective therapies. In this context, the demonstrated efficacy and safety of the bortezomib plus dexamethasone combination in the current study hold significant clinical importance. This treatment could serve as a reliable and safe element within prolonged treatment regimens for CTCLs.
In our exploratory analysis, we investigated the genomic characteristics of patients who provided tumor tissue samples for targeted sequencing. Our analysis revealed recurrent mutations in genes such as PLCO, TP53, CDKN2A, and CDKN2B among patients with relapsed or refractory CTCL. These findings are consistent with those reported in a prior study that performed targeted sequencing on 77 CTCL samples, encompassing early and advanced MF as well as other CTCL variants [28]. Notably, the prevalence of TP53 mutations in our study was 29% (n=6), which is significantly higher than the 10% to 15% mutation rates found in earlier studies [29]. Moreover, similar to observations in peripheral T-cell lymphoma (PTCL), a study reported high relapse rates in PTCL patients harboring TP53 mutations when treated with CHOP-based chemotherapy [29]. This elevated mutation frequency in our cohort may suggest a potential association between TP53 alterations and poorer outcomes following systemic cytotoxic chemotherapy.
Aligned with recent advancements in understanding the roles of genetic and epigenetic mutations, our study identified alterations in genes involved in the mitogen-activated protein kinase signaling pathway and chromatin remodeling, including BRAF, CREBBP, DNMT3A, and TET2 [28]. Despite these findings, the treatment outcomes, such as ORR and PFS, following therapy with bortezomib and dexamethasone were not directly correlated with these mutations. However, we observed a trend toward shorter PFS in patients with higher mutation loads (Fig. 3A). This suggests that mutation profiles might not consistently predict therapeutic outcomes in CTCL patients. Additionally, comparative genomic analysis performed before and after treatment did not show new mutations that could indicate disease progression, further questioning the prognostic utility of mutation profiling in CTCL. Nonetheless, the limited sample size of our study calls for further research with larger cohorts to assess the impact of genetic and epigenetic alterations more definitively.
In conclusion, the combination of bortezomib and dexamethasone has shown promising efficacy in treating relapsed and refractory CTCLs, with a durable response observed. This study supports the use of bortezomib and dexamethasone as an effective and safe treatment option for previously treated CTCLs.
Acknowledgments
This work was partly supported by the National Research Foundation grants (NRF-2018M3A9D3079499 to Y.S.C. and NRF-2022R1A2-C3012346 to Y.S.C.).
Footnotes
Ethical Statement
This study was approved by the Institutional Review Boards of Samsung Medical Center, Korea University Anam Hospital, Seoul National University Hospital, Inje University Haeundae Paik Hospital, Jeonbuk National University Hospital, Chungnam National University Hospital, Dong-A University Hospital, Pusan National University Hospital, National Cancer Center, Asan Medical Center, Konkuk University Hospital and Ajou University Hospital. It was conducted in accordance with the ethical principles of the Declaration of Helsinki and the Korea Good Clinical Practice guidelines. All participants were enrolled this study after written informed consents.
Author Contributions
Conceived and designed the analysis: Choi YS, Shim J, Kim SJ.
Collected the data: Choi YS, Kang KW, Yoon SE, Hong JS, Lim SN, Yhim HY, Kwon JH, Lee GW, Yang DH, Oh SY, Shin HJ, Eom HS, Yoon DH, Lee HG, Jeong SH, Kim WS, Kim SJ.
Contributed data or analysis tools: Choi YS, Shim J, Kang KW, Yoon SE, Hong JS, Lim SN, Yhim HY, Kwon JH, Lee GW, Yang DH, Oh SY, Shin HJ, Eom HS, Yoon DH, Lee HG, Jeong SH, Kim WS, Kim SJ.
Performed the analysis: Choi YS, Shim J, Kim SJ.
Wrote the paper: Choi YS, Shim J, Kim SJ.
Conflicts of Interest
This study was supported by research grant and investigational products from Boryung Pharmacuetical Co. Ltd.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Lee H. Mycosis fungoides and Sezary syndrome. Blood Res. 2023;58:66–82. doi: 10.5045/br.2023.2023023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YP, Yoon SE, Song Y, Kim SJ, Yoon DH, Chen TY, et al. Cutaneous T-cell lymphoma in Asian patients: a multinational, multicenter, prospective registry study in Asia. Int J Hematol. 2021;114:355–62. doi: 10.1007/s12185-021-03179-7. [DOI] [PubMed] [Google Scholar]
- 3.Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:193–209. doi: 10.1002/ajh.26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–9. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 5.Brazel D, Pinter-Brown L. SOHO state-of-the-art updates and next questions: a modern approach to the systemic treatment of advanced CTCL. Clin Lymphoma Myeloma Leuk. 2023;23:401–9. doi: 10.1016/j.clml.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Bakr FS, Whittaker SJ. Advances in the understanding and treatment of cutaneous T-cell lymphoma. Front Oncol. 2022;12:1043254. doi: 10.3389/fonc.2022.1043254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinzani PL, Venturini F, Stefoni V, Fina M, Pellegrini C, Derenzini E, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2010;21:860–3. doi: 10.1093/annonc/mdp508. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–91. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 9.Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168:811–9. doi: 10.1111/bjh.13222. [DOI] [PubMed] [Google Scholar]
- 10.Witzig TE, Reeder C, Han JJ, LaPlant B, Stenson M, Tun HW, et al. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood. 2015;126:328–35. doi: 10.1182/blood-2015-02-629543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390:555–66. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Do YR, Lee HS, Lee WS, Kong JH, Kwak JY, et al. A multi-center and non-interventional registry of brentuximab vedotin in patients with relapsed or refractory CD30-positive lymphoma: the CISL1803/BRAVO study. Blood Res. 2023;58:194–200. doi: 10.5045/br.2023.2023206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Gomez AM, Kuramitsu S, Siurala M, Da T, Agarwal S, et al. Identifying highly active anti-CCR4 CAR T cells for the treatment of T-cell lymphoma. Blood Adv. 2023;7:3416–30. doi: 10.1182/bloodadvances.2022008327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodadoust MS, Rook AH, Porcu P, Foss F, Moskowitz AJ, Shustov A, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sezary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38:20–8. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phyo ZH, Shanbhag S, Rozati S. Update on biology of cutaneous T-cell lymphoma. Front Oncol. 2020;10:765. doi: 10.3389/fonc.2020.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungewickell A, Bhaduri A, Rios E, Reuter J, Lee CS, Mah A, et al. Genomic analysis of mycosis fungoides and Sezary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056–60. doi: 10.1038/ng.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolay JP, Melchers S, Albrecht JD, Assaf C, Dippel E, Stadler R, et al. Dimethyl fumarate treatment in relapsed and refractory cutaneous T-cell lymphoma: a multicenter phase 2 study. Blood. 2023;142:794–805. doi: 10.1182/blood.2022018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang TP, Poltoratsky V, Vancurova I. Bortezomib inhibits expression of TGF-beta1, IL-10, and CXCR4, resulting in decreased survival and migration of cutaneous T cell lymphoma cells. J Immunol. 2015;194:2942–53. doi: 10.4049/jimmunol.1402610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:479–84. doi: 10.1182/blood-2006-10-054601. [DOI] [PubMed] [Google Scholar]
- 21.Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyeon J, Lee B, Shin SH, Yoo HY, Kim SJ, Kim WS, et al. Targeted deep sequencing of gastric marginal zone lymphoma identified alterations of TRAF3 and TNFAIP3 that were mutually exclusive for MALT1 rearrangement. Mod Pathol. 2018;31:1418–28. doi: 10.1038/s41379-018-0064-0. [DOI] [PubMed] [Google Scholar]
- 23.Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:4293–7. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
- 24.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Yoon DH, Kang HJ, Kim JS, Park SK, Kim HJ, et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: a multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48:3223–31. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Bagot M, Porcu P, Marie-Cardine A, Battistella M, William BM, Vermeer M, et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: an international, first-in-human, open-label, phase 1 trial. Lancet Oncol. 2019;20:1160–70. doi: 10.1016/S1470-2045(19)30320-1. [DOI] [PubMed] [Google Scholar]
- 27.Khodadoust MS, Mou E, Kim YH. Integrating novel agents into the treatment of advanced mycosis fungoides and Sezary syndrome. Blood. 2023;141:695–703. doi: 10.1182/blood.2020008241. [DOI] [PubMed] [Google Scholar]
- 28.Tensen CP, Quint KD, Vermeer MH. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood. 2022;139:15–33. doi: 10.1182/blood.2019004256. [DOI] [PubMed] [Google Scholar]
- 29.Johnson WT, Ganesan N, Epstein-Peterson ZD, Moskowitz AJ, Stuver RN, Maccaro CR, et al. TP53 mutations identify high-risk events for peripheral T-cell lymphoma treated with CHOP-based chemotherapy. Blood Adv. 2023;7:5172–86. doi: 10.1182/bloodadvances.2023009953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.