ABSTRACT
Purpose
Our study aimed to comprehensively describe the features of peripheral blood multiple immune cell phenotypes in solid tumor patients during pretreatment and after immunotherapy, providing a more convenient approach for studying the prognosis of immunotherapy in different solid tumor patients.
Methods
We prospectively recruited patients with advanced solid tumors from Peking Union Medical College Hospital (PUMCH) between February 2023 and April 2024. Using multicolor flow cytometry, our study comprehensively observed and described the signatures of peripheral blood lymphocyte subsets including activation, proliferation, function, naïve memory, and T cell exhaustion immune cell subsets in this population of pretreatment and after immunotherapy.
Results
Our study enrolled 59 advanced solid tumor patients with immunotherapy and 59 healthy controls were matched by age and gender. The results demonstrated a marked upregulation in the expression of lymphocyte activation markers CD38 and HLA‐DR, as well as exhaustion and proliferation markers PD‐1 and Ki67, in solid tumor patients compared to healthy controls. After immune checkpoint blockade (ICB) treatment, mainly the expression of Ki67CD4+T and HLA‐DRCD38CD4+T, was significantly upregulated compared to pretreatment levels (p = 0.017, p = 0.019, respectively). We further found that gynecological tumors with better prognoses had higher baseline activation levels of CD4+ T cells compared to other solid tumors with poorer prognoses.
Conclusion
Our study elucidated the characteristics of different lymphocyte subsets in the peripheral blood of solid tumor patients. Further research revealed changes in the phenotypes of different lymphocyte subsets after ICIs treatment, with the activated phenotype of CD4+ T cells playing a crucial role in the antitumor effect. This lays the groundwork for further exploration of prognostic biomarkers and predictive models for cancer patients with immunotherapy.
Keywords: immune checkpoint inhibitors, lymphocyte subsets, peripheral blood, solid tumor
This study prospectively enrolled patients with advanced or locally advanced unresectable solid tumors to investigate the characteristics of multiple lymphocyte phenotypes in peripheral blood following immunotherapy, using multicolor flow cytometry, for providing a foundation for more accurate identification of predictive biomarkers for immunotherapy efficacy and to support the development of predictive models for these patients. The findings indicate that compared to healthy adults, circulating lymphocytes in solid tumor patients exhibit higher levels of T cell exhaustion and proliferation, particularly in activated phenotypes. After immunotherapy, these T cells undergo further activation and proliferation. Additionally, patients with higher pre‐treatment levels of CD4+ T cell activation were found to have better clinical outcomes.
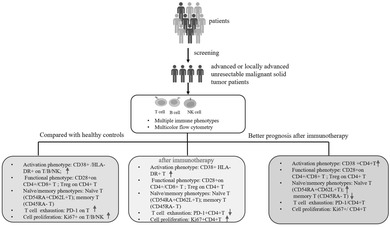
1. Introduction
Immunotherapies, particularly immune‐checkpoint inhibitors (ICIs) and adoptive cell transfer, have revolutionized the treatment of cancer since the US Food and Drug Administration approved the first anti‐CTLA‐4 antibodies (ipilimumab) for use in treating melanoma [1, 2]. The survival and prognosis of patients after immunotherapy have become focal points of interest for clinicians and researchers since then. Emerging evidence has shown that the tumor microenvironment (TME), which consists of tumor‐associated macrophages (TAMs), CD4+ and CD8+ T cells, dendritic cells (DCs), natural killer (NK) cells, tumor‐related endothelial cells (ECs), abnormal tumor vasculature, cancer‐associated fibroblasts (CAFs), and myeloid‐derived immunosuppressive cells (MDSCs) plays a pivotal role in driving cancer progression and governing the response to immune therapies [3, 4]. The association of infiltrating CD8+ cytotoxic T cells, as well as that of CD3+ T cells and CD45RO+ memory T cells, with longer disease‐free survival (DFS) and/or overall survival (OS) has been widely demonstrated in cancers with different histological features and anatomical location, in both primary and metastatic settings, including melanoma, most squamous cell carcinomas (SCCs), large cell lung cancer and several types of adenocarcinoma [5, 6, 7, 8, 9]. Other tumor‐infiltrating immune cells, such as T helper 1 (TH1) cells, T follicular helper (TFH), DCs, and NK cells, are associated with better prognosis after ICIs therapy. In contrast, M2 macrophages, Treg cells, and polymorphonuclear myeloid‐derived suppressor cells (PMN MDSCs) are associated with poor prognosis [10, 11, 12, 13, 14, 15]. Recent studies have shown that tumor mutational burden (TMB) can predict survival after immunotherapy across multiple cancer types [16]. However, all the studies mentioned above are based on the TME, requiring puncture or tissue biopsy to obtain samples. Therefore, it is urgently needed to find non‐invasive and repeatable methods to predict the effects of ICIs therapy.
Increasingly studies are attempting to explore prognostic biomarkers for tumor individuals from the peripheral blood. The number and percentage of lymphocytes in the body can reflect the current immune status of the body [17]. Substantial research indicates that peripheral blood inflammatory indexes, including neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), lymphocyte‐to‐monocyte ratio (LMR), systemic immune‐inflammation index (SII), and systemic inflammation response index (SIRI) have been used to evaluate the diagnosis and prognosis of malignant tumors including breast cancer and prostate cancer [18, 19, 20, 21, 22]. Although studies have also explored the effects of peripheral blood lymphocyte subsets on tumor prognosis, including CD3+, CD4+, CD8+, CD4+/CD8+ cell ratio, CD19+, CD56+, CD16+ monocytes, and CD127+ lymphocytes, the results remain inconclusive, which have focused solely on a single tumor type and not included immunotherapy or addressed a single lymphocyte phenotype [23, 24, 25].
Circulating immune cells, as indicators of systemic immune responses, hold the potential for predicting therapeutic outcomes and clinical benefits of immune checkpoint inhibitors (ICIs) in patients with advanced malignanciess [26, 27]. Recent technical advances in the detection of various immune cell subsets using multi‐color fluorescence flow cytometry, mass cytometry, and high‐throughput sequencing have enabled the identification and monitoring of different circulating immune cell subtypes in peripheral blood [28, 29, 30, 31]. Numerous studies indicate that subsets of peripheral blood lymphocytes are associated with the efficacy of ICIs treatment in solid tumors. These subsets include TIM‐3+ T cells, Ki67+ CD8 T cells, PD‐1+ CD8 T cells, and PD‐L1+ CD8 T cells, which are related to the prognosis of melanoma and non‐small cell lung cancer (NSCLC) [29, 32, 33, 34], but the tumor types and lymphocyte phenotype are relatively homogeneous. Despite the latest study depicts peripheral blood immunoprofiling revealing five immunotypes with immunotherapy response characteristics in patients with cancer by multiparameter flow cytometry and bulk RNA‐seq using peripheral blood [31] and T cell characteristics associated with toxicity to ICIs in patients with melanoma by mass cytometry by time of flight, single‐cell RNA sequencing, single‐cell V(D)J sequencing, bulk RNA sequencing and bulk T cell receptor (TCR) sequencing [35], these high‐throughput sequencing methods are complex and expensive, making them unsuitable for clinical application and widespread adoption. Recent studies have shown that circulating T cell subsets can serve as prognostic markers for various solid tumors after ICIs treatment, but the changes in different lymphocyte phenotypes have not been comprehensively explored [36, 37]. Here, this study seeks to employ a streamlined approach to elucidate the broader phenotypic characteristics of circulating lymphocyte subsets in various solid tumors patients, both before and after immune checkpoint inhibitor (ICI) therapy. The findings aim to establish a basis for identifying universal biomarkers that can predict clinical outcomes following ICIs treatment across different solid tumor types.
2. Materials and Methods
2.1. Study Subjects Selection and Experimental Procedures
The experimental procedure is shown in Figure 1. This study primarily enrolled adults with advanced or locally advanced unresectable malignant solid tumors who were planned to receive first‐line treatment or neoadjuvant therapy with immunotherapy regimens. Eligible patients would undergo a total of three immunotherapy sessions. Before each immunotherapy, peripheral blood was drawn for multicolor flow cytometry to detect lymphocyte subsets. Tumor patients based on the following exclusion criteria were excluded: (1) received adjuvant therapy; (2) underwent chemotherapy within 6 months before the start of immunotherapy; (3) received immunotherapy within the past year; (4) underwent radical chemoradiotherapy before the start of immunotherapy; (6) currently using any dose of hormones or immunosuppressants for disease treatment; (7) undergone organ or bone marrow transplantation; (8) currently suffering from active infectious diseases. Fifty‐nine Healthy adult (age ≥ 18 years) controls were also included, with any individuals showing signs of systemic infection, autoimmune disease, tumors, or abnormal clinical indicators being excluded.
FIGURE 1.
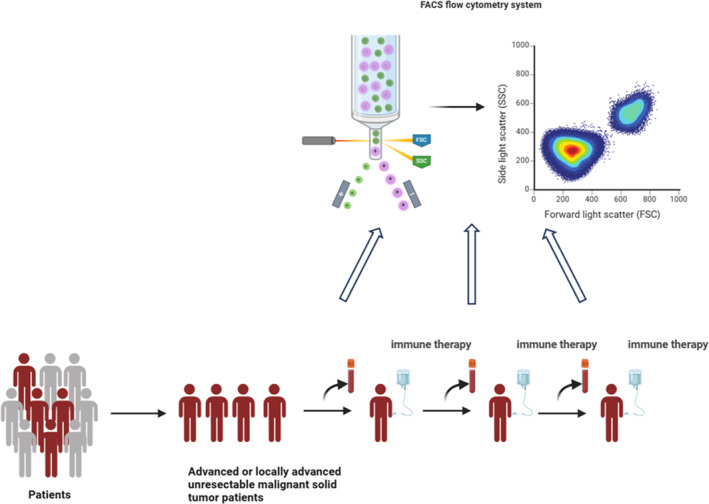
Experimental flow chart. This study primarily enrolled adults with advanced or locally advanced unresectable malignant solid tumors who were planned to receive first‐line treatment or neoadjuvant therapy with immunotherapy regimens. Eligible patients would undergo a total of three immunotherapy sessions. Before each immunotherapy, peripheral blood was drawn for multicolor flow cytometry to detect lymphocyte subsets.
2.2. Data Collection and Therapeutic Effect Evaluation
For eligible patients, we primarily collect the following clinical information including gender, age, diagnosis, pathological type, immune checkpoint blockade (ICB) drugs, Eastern Cooperative Oncology Group Performance Status (ECOG‐PS) score, tumor‐node‐metastasis (TNM) stages, PD‐L1 expression, tumor mutation burden (TMB), microsatellite instability (MSI), and therapeutic response. The evaluation of treatment efficacy is generally conducted after two immunotherapy cycles. Using computed tomography (CT) and magnetic resonance imaging (MRI), and based on RECIST 1.1 criteria [38]. The outcomes were categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The assessment of treatment‐related adverse events adhered to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [39].
2.3. Lymphocyte Immunophenotyping
Immunophenotyping of peripheral blood lymphocytes was analyzed by 18‐color flow cytometry (LSRFortessa & trade; BD Biosciences, USA) as previously described [40]. Freshly collected EDTA‐anticoagulated whole blood was incubated and tested with a panel of anti‐human monoclonal antibodies including ki67‐FITC, CD56‐PE, HLA‐DR‐PerCP‐Cy5.5, CD3‐PE‐Cy7, CD38‐APC, CD8‐APC‐Cy7, CD4‐Alexa Fluor 700, CD19‐V450, CD45‐V500‐C, PD‐1‐BV605, CD45‐RA‐FITC, CD62L‐PE, CD28‐PerCP‐Cy5.5, CD25‐APC, CD127‐BV605 (BD Biosciences, San Diego, CA, USA). Memory T cells were sorted by the CD45RA‐ population. Naïve T cells were sorted by the CD45RA+ CD62L+ population. CD25highCD127low population marked as Treg cells. The gating strategy is shown in Figure S1. Cell counts of lymphocyte subsets were calculated using a dual‐platform method with the white blood cell counts and lymphocyte differentials obtained from blood routine tests of the same specimen.
2.4. Statistical Analyses
The tabulated descriptive statistics are displayed as frequencies, median with interquartile range (IQR), and mean with standard deviation. To compare the clinical features of patients in various groups, we employed the t‐test for parametric continuous variables, and the Mann–Whitney U test for non‐parametric continuous data. Prism version 9 (GraphPad Inc., La Jolla, CA) and SPSS 26.0 (IBM Corporation, Armonk, New York, USA) were used for all statistical analyses. A significance level of p < 0.05 was applied to all tests.
3. Results
3.1. Baseline Characteristics of the Study Population
Our study prospectively recruited a total of 59 eligible patients with solid tumors from Peking Union Medical College Hospital (PUMCH) between February 2023 and April 2024. The baseline characteristics of the cohort are summarized in Table 1. The majority of the patients were male, with a median age of 60 years. Diagnoses included nasopharyngeal carcinoma, hypopharyngeal carcinoma, NSCLC, esophageal cancer, gastric cancer, gastroesophageal cancer, cervical cancer, ovarian cancer, and malignant mesothelioma. The predominant pathological types from biopsy were squamous carcinoma and adenocarcinoma, comprising 47.5% and 28.8% of cases, respectively. A small number were high‐grade serous carcinoma (HGSC), while four patients had other pathological types. Almost all subjects had lymph node involvement or distant organ metastases, with the exception of four individuals for whom disease stage was undefined. All participators received PD‐1 inhibitors approved for the treatment of specific malignancies, including Camrelizumab, Nivolumab, Toripalimab, Tislelizumab, and Sintilimab. Tumor response was assessed after two cycles of immunotherapy using RECIST version 1.1 criteria. Six individuals (10.2%) achieved CR, 27 (45.8%) achieved PR, 19 individuals (32.2%) had SD, and 1 individual (1.7%) experienced PD. Six patients (10.2%) did not undergo efficacy evaluation. The overall objective response rate (ORR) was 56%, with the median time to objective response being 7 weeks after initiation of therapy. Nine patients experienced immune‐related adverse events (irAEs), most commonly presenting as rash and liver abnormalities. Only one patient discontinued immunotherapy due to treatment‐related side effects. During the course of the study, Two patients died, one due to cerebral hemorrhage and the other from disease progression.
TABLE 1.
Baseline characteristics of the study population.
| Variates | Study population (N = 59) |
|---|---|
| Male, n (%) | 34 (58) |
| Age, median (IQR) | 60 (51, 65) |
| Diagnosis, n (%) | |
| Nasopharyngeal carcinoma | 1 (1.7) |
| Hypopharyngeal carcinoma | 9 (15.3) |
| Gastroesophageal cancer | 2 (3.4) |
| Esophageal carcinoma | 6 (10.2) |
| Gastric carcinoma | 12 (20.3) |
| Malignant mesothelioma | 1 (1.7) |
| Non‐small cell lung cancer | 9 (15.3) |
| Cervical cancer | 9 (15.3) |
| Ovarian cancer | 10 (16.9) |
| Pathological type, n (%) | |
| Squamous cell carcinoma | 28 (47.5) |
| Adenocarcinoma | 17 (28.8) |
| High‐grade serous carcinoma (HGSC) | 10 (16.9) |
| Others | 4 (6.8) |
| Tumor‐node‐metastasis stages (TNM), n (%) | |
| Ib | 1 (1.7) |
| IIa2 | 7 (11.9) |
| III | 18 (30.5) |
| IV | 29 (49.2) |
| Unstaged | 4 (6.8) |
| PD‐1 inhibitors, n (%) | |
| Camrelizumab | 3 (5.1) |
| Nivolumab | 4 (6.8) |
| Toripalimab | 10 (16.9) |
| Tislelizumab | 23 (39.0) |
| Sintilimab | 13 (22.0) |
| Others | 6 (10.2) |
| Response evaluation, n (%) | |
| Complete response (CR) | 6 (10.2) |
| Partial response (PR) | 27 (45.8) |
| Stable disease (SD) | 19 (32.2) |
| Progressive disease (PD) | 1 (1.7) |
| Unavailable | 6 (10.2) |
| Time of objective response occurrence (weeks), median (IQR) | 7 (6,8) |
| irAE, n (%) | 9 (15.3) |
| Dead, n (%) | 2 (3.4) |
Abbreviations: IQR, interquartile ranges; irAE, immune‐related adverse events.
3.2. The Signatures of Peripheral Lymphocyte Subsets in Solid Tumor Patients
We matched 59 healthy individuals by age and gender to compare the changes in peripheral blood lymphocyte subsets in patients with solid tumors, as prior studies have shown that lymphocyte subset variations are influenced by both age and gender [41]. The results were shown in Table 2. Among the study participants, 34 were male and 25 were female. The average age was 57.7 years in the healthy control group and 58.1 years in the solid tumor group. Although the proportion of peripheral blood lymphocytes in the tumor group was significantly lower compared to in the healthy control group (24.2 ± 8.4 vs. 29.4 ± 10.8, p = 0.005), there were no significant differences in the proportions of CD19+B cells, CD56+NK cells, CD3+ T cells, CD4+ T cells, and CD8+ T cells between the two groups. The immunosuppressive subset CD127lowCD25high CD4+ T cells (Treg cells) exhibited an increasing trend in the tumor group, though this increase was not statistically significant (7.2 ± 3.4 vs.6.3 ± 2.1, p = 0.066). Tumor occurrence did not significantly alter the proportions of naïve and memory T cell subsets (CD4+CD45RA‐/CD4+ (memory T cells): 68.1 ± 14.9 vs. 69.6 ± 13.8, p = 0.581; CD4+CD45RA+/CD4+: 32.0 ± 15.0 vs. 30.6 ± 13.8, p = 0.584; CD4+CD45RA+62L+/CD4+ (Naïve T cells): 28.0 ± 13.7 vs.28.4 ± 13.1, p = 0.889, respectively.) or functional T cell subsets (CD4+CD28+/CD4+: 88.5 ± 13.6 vs. 90.8 ± 8.0, p = 0.254; CD8+CD28+/CD8+: 50.0 ± 17.2 vs. 47.3 ± 20.5, p = 0.440, respectively.). However, all activation (CD38+HLA‐DR+ on CD4+/CD8+/CD19+/CD56+ subsets, p = 0.000) and proliferation markers (Ki67on CD4+/CD8+/CD19+/CD56+ subsets, p = 0.000) of peripheral blood lymphocytes were significantly upregulated in tumor patients compared to the healthy controls. Furthermore, PD‐1 expression was significantly elevated in the T cells of tumor patients (CD4+PD1+/CD4+: 26.2 ± 11.9 vs. 14.8 ± 5.7, p = 0.000; CD8+PD1+/CD8+: 26.5 ± 13.2 vs.14.7 ± 5.7, p = 0.000, respectively), whereas there was no remarkably increase in the CD19+ B cells (CD19+PD1+/CD19+: 3.0 ± 4.4 vs.2.1 ± 1.8, p = 0.168) or CD56+NK cells (CD56+PD1+/CD56+: 2.0 ± 2.4 vs.1.5 ± 2.6, p = 0.338).
TABLE 2.
Baseline changes in lymphocyte subsets in solid tumors population compared to healthy individuals.
| Parameters | Tumor group (N = 59) | Healthy control (N = 59) | p |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 34 (57.6) | 34 (57.6) | 0.852 |
| Female, n (%) | 25 (42.4) | 25 (42.4) | 0.852 |
| Ages(years), mean ± SD | 58.1 ± 10.5 | 57.7 ± 10.5 | 0.841 |
| Lymphocytes and subsets (%), mean ± SD | |||
| Lymphocyte | 24.2 ± 8.4 | 29.4 ± 10.8 | 0.005 |
| CD19+B cell | 9.9 ± 4.5 | 9.2 ± 4.3 | 0.455 |
| CD56+NK cell | 17.2 ± 10.2 | 15.8 ± 7.0 | 0.401 |
| CD3+ T cell | 68.7 ± 11.1 | 69.5 ± 9.0 | 0.643 |
| CD4+ T cell | 57.2 ± 12.0 | 54.6 ± 12.4 | 0.239 |
| CD8+ T cell | 34.4 ± 10.5 | 36.6 ± 10.9 | 0.271 |
| Treg cell | 7.2 ± 3.4 | 6.3 ± 2.1 | 0.066 |
| CD4+CD45RA‐/CD4+ | 68.1 ± 14.9 | 69.6 ± 13.8 | 0.581 |
| CD4+CD45RA+/CD4+ | 32.0 ± 15.0 | 30.6 ± 13.8 | 0.584 |
| CD4+CD45RA+62L+/CD4+ | 28.0 ± 13.7 | 28.4 ± 13.1 | 0.889 |
| CD4+CD28+/CD4+ | 88.5 ± 13.6 | 90.8 ± 8.0 | 0.254 |
| CD8+CD28+/CD8+ | 50.0 ± 17.2 | 47.3 ± 20.5 | 0.440 |
| CD4+CD38+/CD4+ | 22.0 ± 12.8 | 8.2 ± 4.9 | 0.000 |
| CD4+HLA‐DR+/CD4+ | 19.7 ± 12.6 | 7.3 ± 4.4 | 0.000 |
| CD4+HLA‐DR+CD38+/CD4+ | 4.3 ± 2.8 | 0.8 ± 0.6 | 0.000 |
| CD8+CD38+/CD8+ | 27.1 ± 22.8 | 4.3 ± 4.2 | 0.000 |
| CD8+HLA‐DR+/CD8+ | 43.4 ± 16.4 | 13.3 ± 9.1 | 0.000 |
| CD8+HLA‐DR+CD38+/CD8+ | 13.4 ± 9.5 | 1.9 ± 1.3 | 0.000 |
| CD19+CD38+/CD19+ | 43.9 ± 22.2 | 16.2 ± 11.4 | 0.000 |
| CD56+CD38+/CD56+ | 69.7 ± 20.3 | 41.7 ± 20.8 | 0.000 |
| CD56+HLA‐DR+/CD56+ | 36.8 ± 20.9 | 6.5 ± 5.2 | 0.000 |
| CD4+PD1+/CD4+ | 26.2 ± 11.9 | 14.8 ± 5.7 | 0.000 |
| CD8+PD1+/CD8+ | 26.5 ± 13.2 | 14.7 ± 5.7 | 0.000 |
| CD19+PD1+/CD19+ | 3.0 ± 4.4 | 2.1 ± 1.8 | 0.168 |
| CD56+PD1+/CD56+ | 2.0 ± 2.4 | 1.5 ± 2.6 | 0.338 |
| CD4+Ki67+/CD4+ | 2.7 ± 2.6 | 0.8 ± 0.5 | 0.000 |
| CD8+Ki67+/CD8+ | 2.8 ± 2.7 | 0.7 ± 0.4 | 0.000 |
| CD19+Ki67+/CD19+ | 4.3 ± 4.8 | 1.1 ± 0.8 | 0.000 |
| CD56+Ki67+/CD56+ | 5.0 ± 5.5 | 1.2 ± 0.9 | 0.000 |
Abbreviations: SD, standard deviation; HLA‐DR, human leukocyte antigen DR.
3.3. Changes in Peripheral Blood Lymphocyte Subsets in Tumor Patients After ICB Therapy
We further investigated whether PD‐1 inhibitors affected the peripheral blood lymphocyte subsets. The results revealed significant suppression of PD‐1 expression in CD4+ T cells, CD8+ T cells, and NK cells after ICB treatment, while PD‐1 expression in B cells remained unchanged compared to pre‐treatment levels (Figure 2A,B). Among various lymphocyte subsets, the most notable changes were observed in CD4+ T cells, where Ki67 and HLA‐DR/CD38 double‐positive activation markers were further upregulated after treatment. No significant changes were detected in the overall proportions of lymphocytes, B cells, NK cells, CD4+ T cells, CD8+ T cells, Treg cells, or in naïve (CD4+CD45RA+62L+/CD4+), memory (CD4+CD45RA‐/CD4+), and functional subpopulations (CD28+CD4+/CD8+), despite some fluctuations. Similarly, the activation and proliferation subpopulations of B cells and NK cells showed no noticeable differences post‐treatment (Figure 2C).
FIGURE 2.
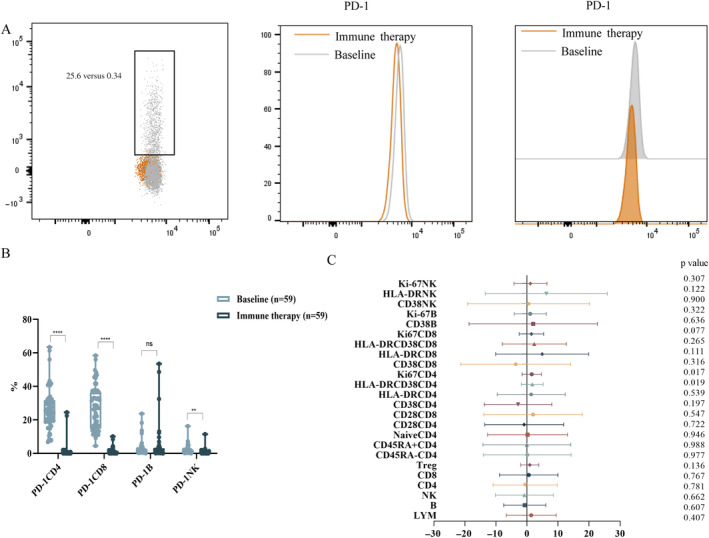
(A) Gating strategy for detecting PD‐1 expression on lymphocytes by flow cytometry. (B) PD‐1 expression in different lymphocyte subsets between baseline and after immune therapy. ****p < 0.0001, **p < 0.01. (C) Changes in lymphocyte subsets after immune therapy compared to baseline. The position of the dot or triangle: To the left of the X‐axis 0 indicates a decrease in lymphocyte subsets expression after PD‐1 inhibitor compared to baseline, while to the right of the X‐axis 0 indicates an increase in lymphocyte subsets expression after PD‐1 inhibitor compared to baseline. p < 0.05 indicates a significant difference.
Patients were categorized into the OR group and the NOR group based on whether their treatment achieved an ORR. The OR group included patients with either a PR or CR, while the NOR group consisted of those with PD or SD. Baseline characteristics for both groups were presented in Table 3. Among the 15 patients with gynecological tumors, who were evaluable, all achieved ORR following immunotherapy, with a median age of 51 years. In contrast, 20 patients experienced poor prognosis post‐treatment, with a median age of 63 years. A significant gender difference was observed between the two groups.
TABLE 3.
Baseline characteristics of the OR group and NOR group.
| Variates | Gynecological tumors (n = 15) | Other solid tumors (n = 20) | p |
|---|---|---|---|
| Male, n (%) | 0 | 16 (80) | < 0.0001 |
| Female, n (%) | 15 (100) | 4 (20) | < 0.0001 |
| Ages, median (IQR) | 51 (44,59) | 63 (49,69) | 0.099 |
| Response evaluation, n (%) | |||
| OR (PR + CR) | 15 (100) | 0 | < 0.0001 |
| NOR (SD + PD) | 0 | 20 (100) | < 0.0001 |
| irAE, n (%) | 2 (13.3) | 3 (15.0) | 0.727 |
Abbreviations: CR, complete response; IQR, interquartile ranges; irAE, immune‐related adverse events; OR, objective response; PD, progressive disease; PR, partial response; SD, stable disease.
Our statistical analysis revealed that the baseline levels of CD38+CD4+ T cells and CD38+B cells were significantly higher in gynecologic tumor patients compared to those with other types of tumors associated with poor prognosis (p = 0.009 and p = 0.029, respectively). In contrast, the proportion of CD45RA‐CD4+T cells was lower in the gynecologic tumor group than in the other group (p = 0.041), while the proportion of CD45RA+CD4+ T cells showed an opposite trend (p = 0.043). Other lymphocyte subsets, including HLA‐DR on CD4+/CD8+/NK cells, and CD38/Ki67/PD‐1on CD4+/CD8+/B/NK cells, showed no significant distinguishes between the groups (Figure 3A). We further compared changes in lymphocyte subsets between the two groups after immunotherapy. The results indicated that the proportion of PD‐1+NK cells was higher in the OR group compared to the NOR group. However, the lymphocyte subsets that differed between the two groups at baseline did not show significant changes following treatment (Figure 3B).
FIGURE 3.
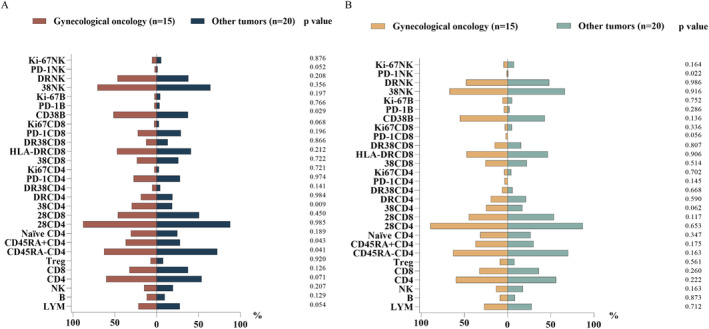
(A) Comparison of baseline expression levels of different lymphocyte subsets in peripheral blood between gynecological tumors and other solid tumors. (B) Changes of peripheral lymphocyte subsets during two groups after immune therapy.
4. Discussion
Our study comprehensively delineated the signatures of different functional immune cell phenotypes in the peripheral blood of solid tumor patients. The results indicated that activation subsets, proliferation subsets, and T cell exhaustion markers in solid tumor patients pre‐treatment were significantly higher compared to those in healthy individuals. These results were also consistent with previous studies. In tumor patients, tumor cells often overexpress PD‐L1, primarily evading cellular immunity by binding to the co‐inhibitory molecule PD‐1 on the surface of T cells [42]. Consequently, the expression of PD‐1 in T cells was elevated, while B cells and NK cells showed no difference compared to healthy individuals (Table 2). At the same time, the immune system activates defense mechanisms against tumors, which include innate immunity, and cellular immunity, primarily mediated by NK cells, and T cells, respectively [43, 44]. These cells activated and proliferated, leading to increased expression of CD38, HLA‐DR, and Ki67 of NK/T cells compared to healthy individuals, thereby exerting antitumor effects (Table 2). Studies thought that B cells promote tumor growth [45, 46]. The activation and proliferation of B cells were also significantly increased (Table 2). This finding did not contradict these studies, as we primarily included patients with advanced tumors. Besides, the percentages of functional subsets, naïve/memory subsets, and various lymphocyte populations including CD4+ T cells, Treg cells, CD8+ T cells, CD19+ B cells, and CD56+ NK cells showed no significant changes in our study. Although numerous studies have demonstrated the critical roles of T cell exhaustion, Treg cells, and other immune cells in the progression and prognosis of tumors, they have primarily focused on tumor‐infiltrating immune cells in the TME [47, 48, 49]. Recent studies have also attempted to explore the correlation between peripheral blood immune cells and tumor‐infiltrating lymphocytes (TIL), which indicated that the percentage of peripheral blood Treg cells is similar to that of TIL in patients with colorectal cancer liver metastasis and ovarian cancer [50]. Previous studies have also shown that peripheral blood NK cell absolute count and CD4/CD8 ratio can predict long‐term prognosis in lung cancer patients treated with radiotherapy combined with ICIs therapy [51]. These results indicate that our panel could reflect the immune status in tumor patients to some extent, rather than obtaining lymphocytes from the TME through biopsy or puncture. Compared to previous studies that analyzed immune changes in tumor patients from the perspective of a single lymphocyte phenotype, our research provided a more comprehensive view of the multiple phenotypic changes in different subsets of circulating lymphocytes, which better provided a data foundation for further application in clinical settings to assist in tumor diagnosis and explore biomarkers for tumor prognosis after ICIs therapy.
We further investigated whether ICIs affect the immune system in solid tumor patients. Our research found that all patients with ICB therapy didn't experience severe irAE. Their safety profile was consistent with previous reports [52, 53]. Further study showed that the PD‐1 expressions of T and NK cells could be significantly suppressed by PD‐1 inhibitors excluding the B cells (Figure 2). One possible reason why the expression of PD‐1 on the surface of B cells was not inhibited by PD‐1 inhibitors may be due to the heterogeneity of solid tumor patients, in which PD‐1 B expression remained high after ICIs in individual patients and PD‐1 B expression had no significant higher compared to healthy controls. Another possible reason was that PD‐1 inhibitors may inhibit different subtypes of B cells, primarily inhibiting IgM+ memory B Cells [54]. The most significant changes in peripheral blood lymphocyte subsets after ICIs were observed in Ki67+CD4+ T cells and HLA‐DRCD38+CD4+ T cells, which were significantly upregulated compared to baseline. This indicates that ICI treatment further promotes the activation and proliferation of CD4+ T cells, which was a novel discovery that was rarely reported before. The specific mechanism requires further validation. Additionally, there was no significant difference in the expression levels of naive/memory T cells following ICIs treatment compared to before therapy, which may be attributed to the antitumor effects primarily exerted by effector T cells.
The effects of ICB therapy in tumor patients were of concern to researchers. Our study found that all patients with gynecological tumors including cervical cancer and ovarian cancer, which conducted effect evaluations, achieved either PR or CR after immunotherapy. These patients acquired OR showed higher ratios of CD38+CD4+ T cells and CD38+B cells compared to NOR patients at baseline, which still kept an upward trend after ICIs (Figure 3). Although our previous studies suggested that gender caused fluctuations in peripheral blood lymphocyte subsets, the level of T cell activation did not show significant differences between men and female [41]. Further explanation revealed that the activation levels of CD4+ T cells and CD19+ B cells played a crucial role in the prognosis of ICI therapy for solid tumors. However, our results didn't show significant discrepancy in the expression of Treg between the OR group and NOR group, despite previous research suggesting Treg in down‐regulating anti‐tumor immune responses has become an accepted paradigm [55]. The possible explanation was the presence of different Treg subtypes, with effector Treg cells primarily exerting antitumor effects in tumors [55]. Overall, circulating CD4+ T cells presented a novel treatment strategy in solid tumor patients with ICB therapy, especially the gynecological tumors, which was consistent with other studies [56, 57]. We need to further increase the sample size to clarify the changes in CD4+ T cell phenotypes and strengthen our results.
There are some limitations in our study. First, we could not get the results after three cycles of PD‐1 inhibitors because of the inconvenient follow‐up of the participants. Second, the results of this study could not eliminate the influences of other chemotherapy drugs, which exerted antitumor effects by promoting immune activation [58]. Third, we could not distinguish the changes in different lymphocyte subsets within various solid tumors due to the difficulty of patient enrollment. Finally, We are uncertain if different types of ICIs will result in alterations to the phenotypes of circulating lymphocytes, but it is currently difficult to assess the specific effects of different ICIs on circulating lymphocyte subsets due to the challenges in patient enrollment. Meanwhile, we cannot also further stratify and discuss the changes in peripheral blood immune subsets among patients with different tumor stages because of small samples. In our future research, we will continue to include eligible patients to increase the sample size and further explore the changes in circulating lymphocyte subsets among different ICIs, tumor types, and stages. We also try to identify common biomarkers that predict clinical outcomes in different types of solid tumors and to construct predictive models.
5. Conclusions
Our study demonstrated alterations in multiple immune cell phenotypes in solid tumor patients including the activation subpopulation, proliferative subpopulation, and T‐cell exhaustion. Further elucidating the impact of ICIs therapy on circulating lymphocyte subsets, with a particular emphasis on the pivotal role of CD4+ T cell activation. The related exploration of predictive effect thresholds is expected to be more extensively explored in the future and the establishment of an efficacy prediction model requires further investigation.
Author Contributions
Ling Chen and Hourui Tan: acquired, analyzed all the data, interpreted the data, and drafted the manuscript. Ruixuan Geng and Yifan Li: collected patient samples and organized clinical data. Yingyi Wang and Taisheng Li: designed the study, obtained funding, and evaluated data. All authors read and approved the final manuscript.
Ethics Statement
The study obtained ethical review and approval from the institutional review board of Peking Union Medical College Hospital, Beijing, China (S‐K3429), and performed in line with the principles of the Declaration of Helsinki. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1.
Acknowledgments
We thank the patients and their families for their understanding and support of the research and thank the staff of the PUMCH Medical Oncology Clinical Center for their contribution to this work.
Funding: This work was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences, 2021‐I2M‐1‐037. Special Research Fund for the Central High‐level Hospitals of Peking Union Medical College Hospital, 2022‐PUMCH‐B‐117, 2022‐PUMCH‐A‐126, 2022‐PUMCH‐D‐008, 2022‐PUMCHA‐213.
Ling Chen and Hourui Tan contributed equally to this study.
Contributor Information
Yingyi Wang, Email: wangyingyi@pumch.cn.
Taisheng Li, Email: litsh@263.net.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the first author on reasonable request.
References
- 1. Yang Y., “Cancer Immunotherapy: Harnessing the Immune System to Battle cancer,” Journal of Clinical Investigation 125, no. 9 (2015): 3335–3337, 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodi F. S., O'Day S. J., McDermott D. F., et al., “Improved Survival With Ipilimumab in Patients With Metastatic Melanoma,” New England Journal of Medicine 363, no. 8 (2010): 711–723, 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiam‐Galvez K. J., Allen B. M., and Spitzer M. H., “Systemic Immunity in Cancer,” Nature Reviews. Cancer 21, no. 6 (2021): 345–359, 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng K., Cai N., Zhu J., Yang X., Liang H., and Zhang W., “Tumor‐Associated Macrophages in Liver cancer: From Mechanisms to Therapy,” Cancer Commun (Lond) 42, no. 11 (2022): 1112–1140, 10.1002/cac2.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galon J., Costes A., Sanchez‐Cabo F., et al., “Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome,” Science 313, no. 5795 (2006): 1960–1964, 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6. Fridman W. H., Pages F., Sautes‐Fridman C., and Galon J., “The Immune Contexture in Human Tumours: Impact on Clinical Outcome,” Nature Reviews. Cancer 12, no. 4 (2012): 298–306, 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 7. Fridman W. H., Zitvogel L., Sautes‐Fridman C., and Kroemer G., “The Immune Contexture in cancer Prognosis and Treatment,” Nature Reviews. Clinical Oncology 14, no. 12 (2017): 717–734, 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 8. Hu Z., Gu X., Zhong R., and Zhong H., “Tumor‐Infiltrating CD45RO(+) Memory Cells Correlate With Favorable Prognosis in Patients With Lung Adenocarcinoma,” Journal of Thoracic Disease 10, no. 4 (2018): 2089–2099, 10.21037/jtd.2018.03.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu C. L., Ou D. L., Bai L. Y., et al., “Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma,” Liver Cancer 10, no. 4 (2021): 346–359, 10.1159/000515305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takashima Y., Kawaguchi A., Kanayama T., Hayano A., and Yamanaka R., “Correlation Between Lower Balance of Th2 Helper T‐Cells and Expression of PD‐L1/PD‐1 axis Genes Enables Prognostic Prediction in Patients With Glioblastoma,” Oncotarget 9, no. 27 (2018): 19065–19078, 10.18632/oncotarget.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu‐Trantien C. and Willard‐Gallo K., “PD‐1(Hi)CXCR5(−)CD4(+) T(FH) Cells Play Defense in Cancer and Offense in Arthritis,” Trends in Immunology 38, no. 12 (2017): 875–878, 10.1016/j.it.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 12. Shang B., Liu Y., Jiang S. J., and Liu Y., “Prognostic Value of Tumor‐Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and meta‐Analysis,” Scientific Reports 5 (2015): 15179, 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khan M., Arooj S., and Wang H., “NK Cell‐Based Immune Checkpoint Inhibition,” Frontiers in Immunology 11 (2020): 167, 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Routy B., Jackson T., Mahlmann L., et al., “Melanoma and Microbiota: Current Understanding and Future Directions,” Cancer Cell 42, no. 1 (2024): 16–34, 10.1016/j.ccell.2023.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruni D., Angell H. K., and Galon J., “The Immune Contexture and Immunoscore in cancer Prognosis and Therapeutic Efficacy,” Nature Reviews. Cancer 20, no. 11 (2020): 662–680, 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 16. Samstein R. M., Lee C. H., Shoushtari A. N., et al., “Tumor Mutational Load Predicts Survival After Immunotherapy Across Multiple cancer Types,” Nature Genetics 51, no. 2 (2019): 202–206, 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie J., Guo Z., Zhu Y., Ma M., and Jia G., “Peripheral Blood Inflammatory Indexes in Breast cancer: A Review,” Medicine (Baltimore) 102, no. 48 (2023): e36315, 10.1097/MD.0000000000036315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rimini M., Casadei‐Gardini A., Ravaioli A., et al., “Could Inflammatory Indices and Metabolic Syndrome Predict the Risk of Cancer Development? Analysis From the Bagnacavallo Population Study,” Journal of Clinical Medicine 9, no. 4 (2020): 1177, 10.3390/jcm9041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L., Kong X., Yan C., Fang Y., and Wang J., “The Research Progress on the Prognostic Value of the Common Hematological Parameters in Peripheral Venous Blood in Breast Cancer,” Oncotargets and Therapy 13 (2020): 1397–1412, 10.2147/OTT.S227171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S. Y., Lee S. I., Min B. W., and Oh S. C., “Prognostic Implication of Systemic Inflammatory Markers in Young Patients With Resectable Colorectal cancer,” Annals of Surgical Treatment and Research 100, no. 1 (2021): 25–32, 10.4174/astr.2021.100.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuca G., Guarini V., Antoniotti C., et al., “The Pan‐Immune‐Inflammation Value Is a New Prognostic Biomarker in Metastatic Colorectal cancer: Results From a Pooled‐Analysis of the Valentino and TRIBE First‐Line Trials,” British Journal of Cancer 123, no. 3 (2020): 403–409, 10.1038/s41416-020-0894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiang Q., Liu Y., Xiao J., Ou L., and Du J., “Prognostic Value of Lymphocyte‐To‐Monocyte Ratio (LMR) in Patients With Prostate Cancer: A Systematic Review and Meta‐Analysis,” American Journal of Men's Health 18, no. 2 (2024): 15579883241234747, 10.1177/15579883241234747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X. and Jing J., “Effect of Peripheral Blood Lymphocytes on Prognosis of Multiple Cancers,” Cancer Control 30 (2023): 10732748231202921, 10.1177/10732748231202921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu Y., Ye S., Goswami S., et al., “Clinical Significance of Peripheral Blood and Tumor Tissue Lymphocyte Subsets in Cervical cancer Patients,” BMC Cancer 20, no. 1 (2020): 173, 10.1186/s12885-020-6633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mao F., Yang C., Luo W., Wang Y., Xie J., and Wang H., “Peripheral Blood Lymphocyte Subsets Are Associated With the Clinical Outcomes of Prostate cancer Patients,” International Immunopharmacology 113, no. Pt A (2022): 109287, 10.1016/j.intimp.2022.109287. [DOI] [PubMed] [Google Scholar]
- 26. Kim K. H., Kim C. G., and Shin E. C., “Peripheral Blood Immune Cell‐Based Biomarkers in Anti‐PD‐1/PD‐L1 Therapy,” Immune Network 20, no. 1 (2020): e8, 10.4110/in.2020.20.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Griffiths J. I., Wallet P., Pflieger L. T., et al., “Circulating Immune Cell Phenotype Dynamics Reflect the Strength of Tumor‐Immune Cell Interactions in Patients During Immunotherapy,” Proceedings of the National Academy of Sciences of the United States of America 117, no. 27 (2020): 16072–16082, 10.1073/pnas.1918937117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nixon A. B., Schalper K. A., Jacobs I., Potluri S., Wang I. M., and Fleener C., “Peripheral Immune‐Based Biomarkers in cancer Immunotherapy: Can We Realize Their Predictive Potential?,” Journal for Immunotherapy of Cancer 7, no. 1 (2019): 325, 10.1186/s40425-019-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nabet B. Y., Esfahani M. S., Moding E. J., et al., “Noninvasive Early Identification of Therapeutic Benefit From Immune Checkpoint Inhibition,” Cell 183, no. 2 (2020): 363, 10.1016/j.cell.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duchemann B., Remon J., Naigeon M., et al., “Integrating Circulating Biomarkers in the Immune Checkpoint Inhibitor Treatment in Lung Cancer,” Cancers (Basel) 12, no. 12 (2020): 3625, 10.3390/cancers12123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dyikanov D., Zaitsev A., Vasileva T., et al., “Comprehensive Peripheral Blood Immunoprofiling Reveals Five Immunotypes With Immunotherapy Response Characteristics in Patients With cancer,” Cancer Cell 42, no. 5 (2024): 759, 10.1016/j.ccell.2024.04.008. [DOI] [PubMed] [Google Scholar]
- 32. Kato R., Yamasaki M., Urakawa S., et al., “Increased Tim‐3(+) T Cells in PBMCs During Nivolumab Therapy Correlate With Responses and Prognosis of Advanced Esophageal Squamous Cell Carcinoma Patients,” Cancer Immunology, Immunotherapy 67, no. 11 (2018): 1673–1683, 10.1007/s00262-018-2225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacquelot N., Roberti M. P., Enot D. P., et al., “Predictors of Responses to Immune Checkpoint Blockade in Advanced Melanoma,” Nature Communications 8, no. 1 (2017): 592, 10.1038/s41467-017-00608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwon M., An M., Klempner S. J., et al., “Determinants of Response and Intrinsic Resistance to PD‐1 Blockade in Microsatellite Instability‐High Gastric Cancer,” Cancer Discovery 11, no. 9 (2021): 2168–2185, 10.1158/2159-8290.CD-21-0219. [DOI] [PubMed] [Google Scholar]
- 35. Lozano A. X., Chaudhuri A. A., Nene A., et al., “T Cell Characteristics Associated With Toxicity to Immune Checkpoint Blockade in Patients With Melanoma,” Nature Medicine 28, no. 2 (2022): 353–362, 10.1038/s41591-021-01623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gorgulho J., Roderburg C., Beier F., et al., “Soluble Lymphocyte Activation Gene‐3 (sLAG3) and CD4/CD8 Ratio Dynamics as Predictive Biomarkers in Patients Undergoing Immune Checkpoint Blockade for Solid Malignancies,” British Journal of Cancer 130, no. 6 (2024): 1013–1022, 10.1038/s41416-023-02558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gorgulho J., Roderburg C., Heymann F., et al., “Serum Levels of Soluble B and T Lymphocyte Attenuator Predict Overall Survival in Patients Undergoing Immune Checkpoint Inhibitor Therapy for Solid Malignancies,” International Journal of Cancer 149, no. 5 (2021): 1189–1198, 10.1002/ijc.33610. [DOI] [PubMed] [Google Scholar]
- 38. Eisenhauer E. A., Therasse P., Bogaerts J., et al., “New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1),” European Journal of Cancer 45, no. 2 (2009): 228–247, 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 39. Lin Y. S., Li S., Yang X., et al., “First‐Line Hepatic Arterial Infusion Chemotherapy Plus Lenvatinib and PD‐(L)1 Inhibitors Versus Systemic Chemotherapy Alone or With PD‐(L)1 Inhibitors in Unresectable Intrahepatic Cholangiocarcinoma,” Journal of Cancer Research and Clinical Oncology 150, no. 6 (2024): 309, 10.1007/s00432-024-05795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin L., Duan X., Dong J. Z., et al., “The Unreversible Reduced but Persistent Activated NK and CD8(+) T Cells in Severe/Critical COVID‐19 During Omicron Pandemic in China,” Emerging Microbes & Infections 12, no. 1 (2023): 2208679, 10.1080/22221751.2023.2208679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qin L., Jing X., Qiu Z., et al., “Aging of Immune System: Immune Signature From Peripheral Blood Lymphocyte Subsets in 1068 Healthy Adults,” Aging (Albany NY) 8, no. 5 (2016): 848–859, 10.18632/aging.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Francisco L. M., Sage P. T., and Sharpe A. H., “The PD‐1 Pathway in Tolerance and Autoimmunity,” Immunological Reviews 236 (2010): 219–242, 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rui R., Zhou L., and He S., “Cancer Immunotherapies: Advances and Bottlenecks,” Frontiers in Immunology 14 (2023): 1212476, 10.3389/fimmu.2023.1212476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonzalez H., Hagerling C., and Werb Z., “Roles of the Immune System in cancer: From Tumor Initiation to Metastatic Progression,” Genes & Development 32, no. 19–20 (2018): 1267–1284, 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schioppa T., Moore R., Thompson R. G., et al., “B Regulatory Cells and the Tumor‐Promoting Actions of TNF‐Alpha During Squamous Carcinogenesis,” Proceedings of the National Academy of Sciences of the United States of America 108, no. 26 (2011): 10662–10667, 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Visser K. E., Korets L. V., and Coussens L. M., “De Novo Carcinogenesis Promoted by Chronic Inflammation Is B Lymphocyte Dependent,” Cancer Cell 7, no. 5 (2005): 411–423, 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 47. Chow A., Perica K., Klebanoff C. A., and Wolchok J. D., “Clinical Implications of T Cell Exhaustion for cancer Immunotherapy,” Nature Reviews. Clinical Oncology 19, no. 12 (2022): 775–790, 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Curiel T. J., Coukos G., Zou L., et al., “Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival,” Nature Medicine 10, no. 9 (2004): 942–949, 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 49. Ishigami S., Natsugoe S., Tokuda K., et al., “Prognostic Value of Intratumoral Natural Killer Cells in Gastric Carcinoma,” Cancer 88, no. 3 (2000): 577–583. [PubMed] [Google Scholar]
- 50. Kovacsovics‐Bankowski M., Chisholm L., Vercellini J., et al., “Detailed Characterization of Tumor Infiltrating Lymphocytes in Two Distinct Human Solid Malignancies Show Phenotypic Similarities,” Journal for Immunotherapy of Cancer 2, no. 1 (2014): 38, 10.1186/s40425-014-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li P., Qin P., Fu X., et al., “Associations Between Peripheral Blood Lymphocyte Subsets and Clinical Outcomes in Patients With Lung cancer Treated With Immune Checkpoint Inhibitor,” Annals of Palliative Medicine 10, no. 3 (2021): 3039–3049, 10.21037/apm-21-163. [DOI] [PubMed] [Google Scholar]
- 52. Xu C., Chen Y. P., Du X. J., et al., “Comparative Safety of Immune Checkpoint Inhibitors in cancer: Systematic Review and Network meta‐Analysis,” BMJ 363 (2018): k4226, 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okiyama N. and Tanaka R., “Immune‐Related Adverse Events in Various Organs Caused by Immune Checkpoint Inhibitors,” Allergology International 71, no. 2 (2022): 169–178, 10.1016/j.alit.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 54. Xia L., Guo L., Kang J., et al., “Predictable Roles of Peripheral IgM Memory B Cells for the Responses to Anti‐PD‐1 Monotherapy Against Advanced Non‐Small Cell Lung Cancer,” Frontiers in Immunology 12 (2021): 759217, 10.3389/fimmu.2021.759217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takeuchi Y. and Nishikawa H., “Roles of Regulatory T Cells in cancer Immunity,” International Immunology 28, no. 8 (2016): 401–409, 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kagamu H., Kitano S., Yamaguchi O., et al., “CD4(+) T‐Cell Immunity in the Peripheral Blood Correlates With Response to Anti‐PD‐1 Therapy,” Cancer Immunology Research 8, no. 3 (2020): 334–344, 10.1158/2326-6066.CIR-19-0574. [DOI] [PubMed] [Google Scholar]
- 57. Zuazo M., Arasanz H., Fernandez‐Hinojal G., et al., “Functional Systemic CD4 Immunity Is Required for Clinical Responses to PD‐L1/PD‐1 Blockade Therapy,” EMBO Molecular Medicine 11, no. 7 (2019): e10293, 10.15252/emmm.201910293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghiringhelli F. and Apetoh L., “The Interplay Between the Immune System and 622 Chemotherapy: Emerging Methods for Optimizing Therapy,” Expert Review of Clinical Immunology 10, no. 1 (2014): 19–30, 10.1586/1744666X.2014.865520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the first author on reasonable request.


