Abstract
Approaches to mitigate the severity of infections and of immune responses are still needed for the treatment of cystic fibrosis (CF) even with the success of highly effective modulator therapies. Previous studies identified reduced levels of melatonin in a CF mouse model related to circadian rhythm dysregulation. Melatonin is known to have immunomodulatory properties and it was hypothesized that treatment with melatonin would improve responses to bacterial infection in CF mice. Data demonstrate that CF mice (G542X/G542X) treated with melatonin (10 µg/mL) in drinking water for 10 weeks had improved responses to airway infection with a clinical isolate of Pseudomonas aeruginosa. Melatonin-treated mice exhibited improved bacterial clearance, reduced inflammatory markers. Mice treated in drinking water for 1 week had improved bacterial clearance but no improvement in inflammation. Wild type (WT) control mice showed no response to melatonin treatment suggesting melatonin is eliciting a CF-specific response in this model. The efficacy of direct melatonin (1 µM) treatment to the airways was also tested and found to be ineffective. In conclusion, long-term systemic treatment with melatonin is an effective therapy in a CF mouse model that normalizes the response to airway infection to a WT pattern.
Subject terms: Immunology, Physiology
Introduction
In the era of highly effective modulator therapy (HEMT), there is still a need for supplemental therapies that further augment the control of inflammation. A small percentage of people with cystic fibrosis (pwCF) are ineligible for HEMT based on genotype while others cannot tolerate HEMT, necessitating the continued development of symptomatic therapies including immunomodulatory approaches.
Previous work in the laboratory has demonstrated changes to microtubule regulation including reduced acetylation and slower rates of formation in CF cells leads to pro-inflammatory signaling profiles1,2. Inhibition of the microtubule modifying protein histone deacetylase 6 (HDAC6) was shown to correct the acetylation levels of tubulin, improve intracellular transport, and reverse inflammatory signaling to more control levels in CF cells1. In vivo, depletion of HDAC6 expression in F508del CF mice corrected several CF-related phenotypes including growth, depression-like behavior, circadian timing regulation, and restored inflammation to wild type (WT) mouse control profiles after airway infection with a clinical strain of Pseudomonas aeruginosa (PA)3–5. These findings point to HDAC6-dependent mechanisms as key regulators of multiple CF phenotypes and we are interested in the potential relationship between circadian regulation and aggressive inflammation in CF.
We explored the role of microtubules in both circadian timing regulation and inflammation directly by examining both phenotypes in mice lacking expression of the tubulin polymerization promoting protein (Tppp -/-)6,7. Tppp -/- mice recapitulate both the circadian regulatory changes observed in CF mice and a CF-like inflammatory response after PA infection such as excessive neutrophil recruitment8. Tppp -/- have WT levels of CFTR function, so these phenotypes occur in a CFTR-independent manner7.
It is unclear how these HDAC6-sensitive pathways regulate inflammation. A common feature of both the CF mouse model and the Tppp -/- mouse model is that melatonin production is reduced compared to respective WT controls6. Likewise, HDAC6 depletion in the CF mouse model restored melatonin production, serving as a potential mechanism of circadian timing correction. Melatonin is well recognized as an important regulator of circadian timing and sleep9–11. However, melatonin is also a potent anti-inflammatory agent with efficacy shown in airway diseases12–16. Most recently, the efficacy of melatonin therapy in alleviating inflammation in COVID-19 has been demonstrated as well as benefit for the treatment of long-COVID14,15. Melatonin treatment has been shown to reduce proinflammatory markers in osteoarthritis, neurological diseases, and in response to LPS in various inflammatory model systems17–22. Its effects on reducing inflammatory signaling or responses in epithelial cells, macrophages, microglia, liver cells point to the broad impact of melatonin on the control of inflammation.
The goals of this study are to examine the efficacy of melatonin treatment on inflammation in response to airway infection in a CF mouse model. In this study, we utilize a CF mouse model homozygous for the G542X mutation. Previous studies of ours utilized a F608del/F508del mouse model. We switched models since the original F508del model was constructed with a neomycin resistance insert methodology. The G542X model was developed with CRISPR technology and contains no extraneous DNA inserts. Melatonin levels have not been measured directly in this mouse model, but our interest is in responsiveness to melatonin treatment. One previous study examined the efficacy of melatonin treatment on sleep in pwCF. De Castro-Silva et al. demonstrated that pwCF treated with 3 mg melatonin per day for 6 days showed significant improvement in sleep efficiency and benefitted sleep latency compared to a control group receiving placebo23. Exhaled nitrite and isoprostane were also measured in exhaled breath condensate. Nitrite levels were significantly reduced with melatonin treatment, but not isoprostane. Reduced nitrite levels could indicate a systemic improvement in inflammatory status and is consistent with the anti-oxidative stress impacts of melatonin. Indications of clinical efficacy at such a low dose of melatonin in a CF context suggest it may be a useful therapy to complement HEMT approaches. In this study, we examined the efficacy of melatonin delivered systemically via drinking water, both long-term treatment (post-weaning at 3 weeks for 70 days) and short-term treatment of 7-days at 8–10 weeks of age. Since previous studies demonstrated efficacy of melatonin in regulating inflammatory responses in epithelial cells24–28, we also examined melatonin delivered directly to the airways via bronchoscopy.
Results
Effect of long-term daily systemic treatment with melatonin on inflammation and bacterial clearance in an airway infection model
Effect of systemic melatonin treatment on weight loss in response to infection
WT and CF mice (Cftr (G542X/G542X)) were treated post-weaning (3-weeks of age) with melatonin in drinking water (10 µg/mL) or control water as described in Methods. At 10–12 weeks of age, mice were challenged with PA in the airways and harvested at 3-days post infection to assess the impact of melatonin treatment on inflammatory responses to infection. Weight loss is measured as a general assessment of health in response to infection. CF mice treated with melatonin lost significantly less weight at days 2 and 3 post infection than control mice (Fig. 1). These data suggest that melatonin treatment improved the resilience of CF mice in the presence of infection.
Fig. 1.

Changes in animal weight post-infection. Animals were weighed immediately prior to infection and daily until harvest. Change was calculated as a percentage of initial weight. (A) Comparison of weight retention between melatonin-treated and control CF mice. Melatonin-treated animals had a statistically significant improvement over control. (B) Comparison of weight retention between melatonin-treated and control WT mice. Significance determined by t-test at each time point: *p < 0.05.
Bacterial recovery with and without long-term systemic melatonin treatment
The rate of bacterial clearance was assessed to determine if melatonin treatment had a positive impact on infection control. BAL fluid recovered CFU (Fig. 2A), lung homogenate CFU (Fig. 2B), and total CFU (BAL plus lung homogenate) were measured (Fig. 2C) in both WT and CF mice treated with vehicle or melatonin in water post-weaning. No significant difference in bacterial clearance at day 3 post-infection is observed between control and melatonin treated groups in BAL fluid alone, but when looking at total CFU, melatonin treated CF mice have significantly less recovered bacteria. In WT mice, however, there is no significant impact of treatment on bacterial clearance in either BAL fluid or total CFU. CF mice exhibit increased baseline CFU in both homogenate and total samples compared to WT mice.
Fig. 2.
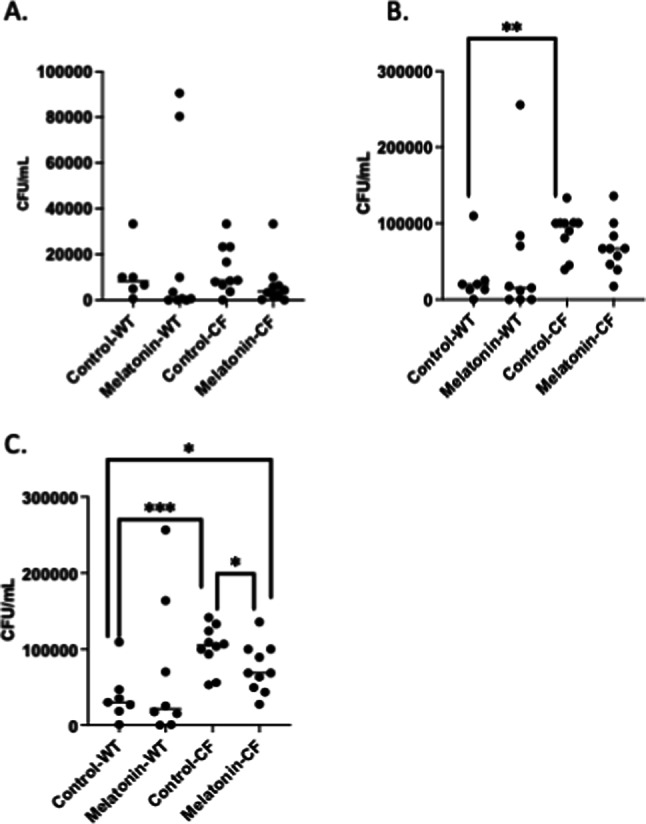
Airway clearance of P. aeruginosa post-infection. Colony-forming unit (CFU) counts per mL recovered from. (A) BAL fluid, (B) lung homogenates, and (C) total (BAL + lung homogenate) of control and post-weaning chronic melatonin-treated WT and CF mice. Significance determined by t-test, *p < 0.05, **p < 0.01, ***p, 0.001.
Systemic long-term melatonin reverts CF inflammatory cell recruitment to WT pattern
To further assess immune response, inflammatory cell recruitment to the airways was determined at day 3 post-infection from BAL fluid. In CF mice, percent distribution of both neutrophils and macrophages are significantly altered with melatonin treatment with neutrophils being significantly reduced while macrophage percent content being significantly increased (Fig. 3A,B), resulting in an inflammatory profile similar to WT. In WT mice there is no effect of melatonin treatment on inflammatory cell recruitment (Fig. 3A,B). CF mice display characteristic significant increases in % neutrophils and reduced % monocytes compared to WT mice. Total cells recovered for WT with and without melatonin, respectively are 350,250 +/− 74,208 and 851,940 +/− 31,035 cells. Total cells recovered for CF with and without melatonin, respectively are 641,263 +/− 114,127 and 776,785 +/− 104,180 cells. These data demonstrate that melatonin treatment alters the nature of the inflammatory response in CF mice, with likely CF-specific influence.
Fig. 3.

Immune cell composition post-infection. BAL fluid was used to determine cell differentials (Giesma/Miller). (A) Comparison of % neutrophils in BAL between control and post-weaning chronic melatonin-treated WT and CF mice. Melatonin treated mice had significantly lower neutrophil counts than controls. (B) Comparison of % monocytes in BAL between control and melatonin-treated WT and CF mice. Melatonin mice had significantly higher monocytes counts than controls. Significance determined by t-test, *p < 0.05, **p < 0.01, ***p, 0.001.
Effect of systemic melatonin treatment on cytokine levels
Based on previous results with HDAC6 depletion, it was hypothesized that melatonin would impact epithelial responses in the airway. To test this hypothesis, we examined the impact of melatonin treatment on epithelial derived cytokines to determine if treatment reduced their production thus altering inflammatory cell recruitment. CXCL1 (KC), TNF-a, and CXCL2 (MIP2) were measured from BAL fluid and lung homogenates collected at day 3 post-infection. Melatonin treatment had no impact on cytokine levels in BAL fluid in either WT or CF mice (Fig. 4A,C,E). However, melatonin significantly reduced all cytokine levels recovered from lung homogenates only in CF samples (Fig. 4B,D,F). IL-1β levels were below detectable limits. These data support that melatonin treatment is specifically favorable in the CF context.
Fig. 4.
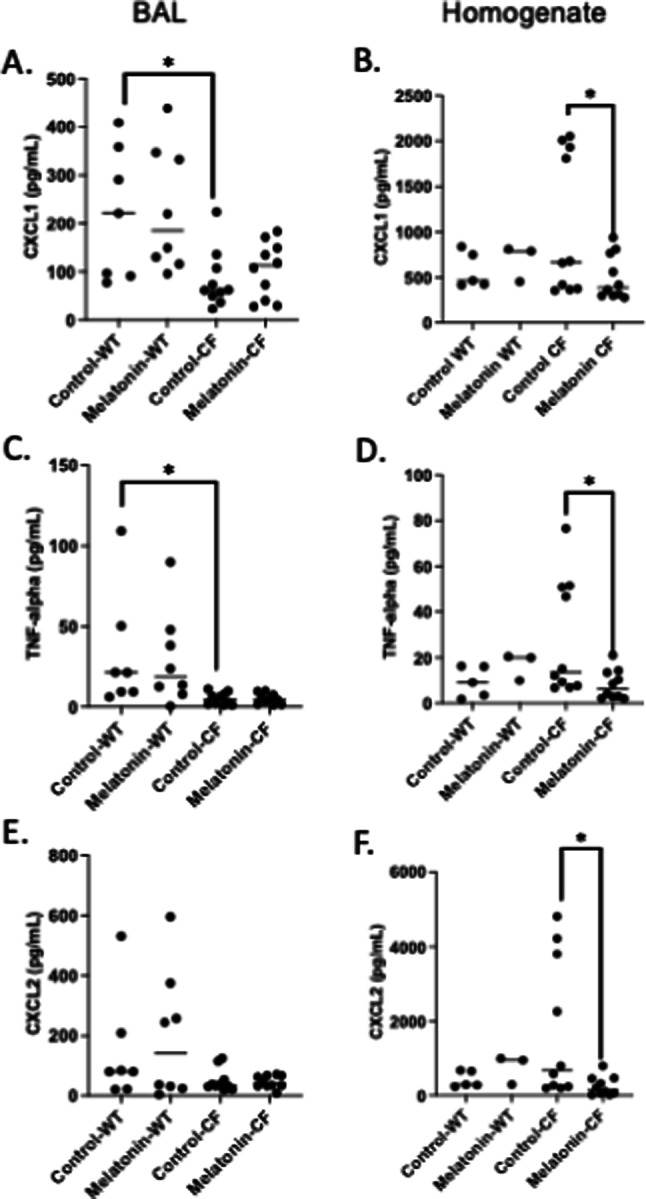
Pro-inflammatory cytokine levels post-infection. Cytokines were measured from BAL and lung homogenate supernatant using Luminex. Concentration (pg/mL) of CXCL1 in BAL (A) and homogenate (B), TNF-alpha in BAL (C) and homogenate (D), and CXCL2 in BAL (E) and homogenate (F) of control and post-weaning chronic melatonin-treated CF mice. Significance determined by t-test; *p < 0.05.
Effect of short-term daily systemic treatment with melatonin on inflammation and bacterial clearance in an airway infection model
1-Week systemic treatment improves bacterial clearance but no other outcome
CF mice were treated with melatonin in drinking water (10 µg/mL) or control water for 7 days prior to infection and through the 3-day infection protocol. There was no significant change in weight loss or recovery (Fig. 5A). BAL fluid recovered CFU, lung homogenate CFU, and total CFU (BAL plus lung homogenate) were in CF mice treated with vehicle or melatonin in water for 1 week. Significant reduction in recovered bacteria in both BAL and total was found at day 3 post-infection in response to melatonin treatment, however, few bacterial were observed in homogenates (Fig. 5B). Finally, % cell counts show no change in % neutrophils or % mononcytes in response to melatonin treatment (Fig. 5C). Cell type distribution remained characteristic of CF. Total cells recovered for CF with and without melatonin, respectively are 258,737 +/− 144,269 and 288,390 +/− 206,085 cells. Similarly, no change in cytokine production in either BAL or homogenate samples were observed (Fig. 6). Mice were also treated with melatonin without infection to determine the effects on inherent inflammation. No differences were observed (data not shown). These data demonstrate that systemic melatonin for a 1-week period effectively improves bacterial clearance, perhaps through augmenting inflammatory cell function, but does not influence measures of inflammation in any regard.
Fig. 5.
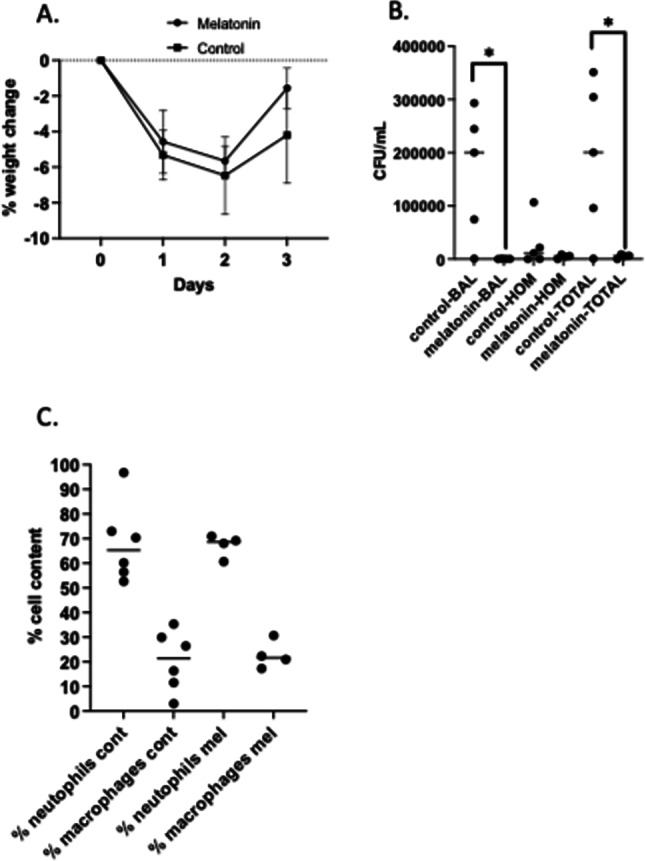
Efficacy of 1-week melatonin treatment on responses to infection. (A) Changes in animal weight post-infection. Animals were weighed immediately prior to infection and daily until harvest. Change was calculated as a percentage of initial weight. (B) Airway clearance of P. aeruginosa post-infection. Colony-forming unit (CFU) counts per mL recovered from. BAL fluid, lung homogenates (HOM), and TOTAL (BAL + lung homogenate) of control and post-weaning chronic melatonin-treated WT and CF mice. (C) Immune cell composition post-infection. BAL fluid was used to determine cell differentials of % neutrophils and % monocytes in BAL between control and 1-week melatonin-treated WT and CF mice. Significance determined by t-test, *p < 0.05.
Fig. 6.

Pro-inflammatory cytokine levels post-infection. Cytokines were measured from BAL and lung homogenate supernatant using Luminex. Concentration (pg/mL) of CXCL1 in BAL (A) and homogenate (B), TNF-alpha in BAL (C) and homogenate (D), and CXCL2 in BAL (E) and homogenate (F) of control and 1-weekmelatonin-treated CF mice.
Effect of direct airway treatment with melatonin on inflammation and bacterial clearance in an airway infection model
Direct melatonin treatment effect on bacterial clearance
BAL fluid recovered CFU (Fig. 7A), lung homogenate CFU (Fig. 7B), and total CFU (BAL plus lung homogenate) were measured (Fig. 7C) in both WT and CF mice treated with vehicle or melatonin directly appied intratracheally to the lungs. No significant difference in bacterial clearance at day 3 post-infection is observed between control and melatonin treated groups in any measure. CFU recovery was greater in BAL fluid in CF mice compared to WT controls. These data suggest that melatonin has no direct effect on bacteria viability and reduced clearance in systemic treated mice is likely due to altered inflammatory cell function.
Fig. 7.
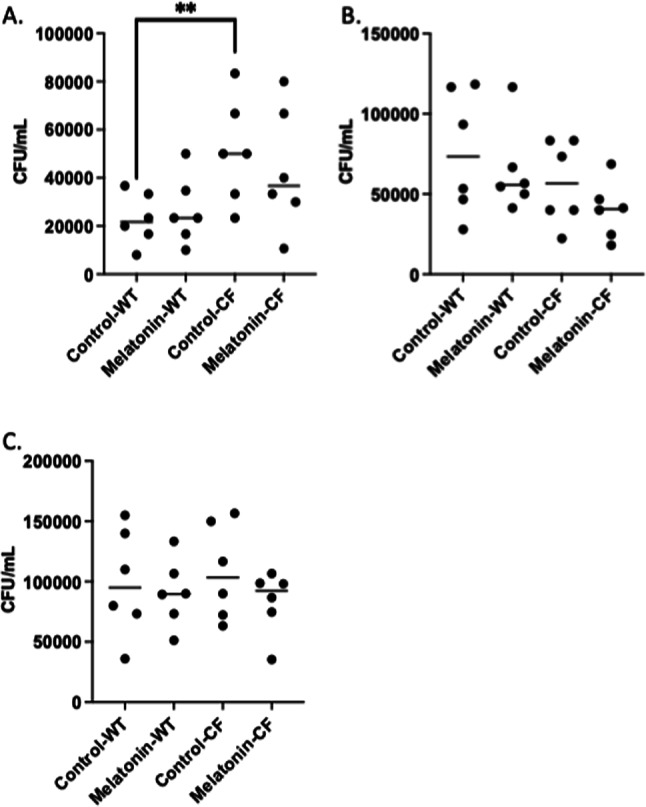
Airway clearance of P. aeruginosa post-infection. Colony-forming unit (CFU) counts per mL recovered from. (A) BAL fluid, (B) lung homogenates, and (C) total (BAL + lung homogenate) of control and acute intratracheal melatonin-treated WT and CF mice. Significance determined by t-test, **p < 0.01.
Direct melatonin treatment effect on weight loss in response to infection and inflammatory cell recruitment
Long-term systemic administration of melatonin proved effective in modulating bacterial clearance and restoring WT patterning of inflammatory cell recruitment, while short-term systemic treatment was effective in promoting bacterial clearance but had no impact on inflammatory measures. Melatonin has been shown to have anti-inflammatory properties when treating epithelial cells directly, so the direct administration of melatonin to the airways was examined as an anti-inflammatory therapy. Mice were treated one day prior to infection with 1 µM melatonin in saline directly to the lung using a bronchoscopic technique. Treatments were repeated daily through day 2 post-infection and mice were harvested on day 3 post-infection as with systemic treatment with melatonin. Direct treatment of the airways with melatonin resulted in no improvement in bacterial clearance (Fig. 7A–C), weight loss (Fig. 8A,B), or inflammatory cell composition (Fig. 8C,D). Total cells recovered for WT with and without melatonin, respectively are 801,700 +/− 154,368 and 488,000 +/− 133,133 cells. Total cells recovered for CF with and without melatonin, respectively are 745,500 +/− 170,741 and 518,667 +/− 95,740 cells. Inflammatory cell data are for the direct treatment are unusual in that WT mice have extensive neutrophil counts, similar to those observed in CF mice. It is not clear why this pattern occurs. It is speculated that the extra repeated bronchoscopy manipulation necessary for the daily intratracheal instillation of melatonin are causing an independent inflammatory response.
Fig. 8.

Changes in weight post-infection in animals treated with intratracheal melatonin. Animals were weighed immediately prior to infection and daily until harvest. Change was calculated as a percentage of initial weight. (A) Comparison of weight retention between melatonin-treated and control CF mice. (C,D) Immune cell composition with intratracheal melatonin application. BAL fluid was used to determine (C) % neutrophils and (D) % monocytes between control and intratracheal melatonin-treated CF mice.
Discussion
The purpose of this study is to determine the viability of melatonin therapy as an approach to improve responses to airway infection in a mouse model of CF. Like sleep, anxiety, and depression, inflammation is a phenotype that is not completely reversed by HEMT, though studies vary in relative efficacy29–31. Supplemental treatments to further target inflammatory and immune pathways are still needed to effectively treat CF patients. We have demonstrated that CF mice produce less melatonin compared to WT controls and CF mice also lack circadian regulation of production7. It is hypothesized that this lack of melatonin production is one potential contributor to increased inflammatory signaling in CF. The goal of this work is to determine the differential effects of systemic and direct lung delivery of melatonin on CF lung inflammation and bacterial clearance using a mouse model of Pseudomonas aeruginosa infection.
It was originally hypothesized that direct administration to the airways may be a more effective mode of treatment with melatonin since it has been reported that melatonin can act directly on the epithelium by reducing oxidative stress. It was thought that direct administration to the airways would more aggressively dampen the impact of localized airway infection. This hypothesis clearly tested negatively. It is possible that an insufficient dose or insufficient treatment time is responsible for the lack of efficacy, but data demonstrating the lack of melatonin receptor expression in mouse nasal epithelium strongly suggests that lack of receptor expression is the most likely reason for lack of efficacy.
Systemic treatment with melatonin, however, was very effective in mediating a WT-like inflammatory response in CF mice in response to airway PA infection. Bacterial clearance was improved, and most notably, inflammatory cell recruitment was no longer neutrophil dominant and converted to a monocyte dominant response in the CF mice. We examined epithelial derived cytokines predominantly based on our initial hypothesis, but consistent with the lack of direct airway treatment, there is no change in epithelial cytokine production. Both IFN-γ and IL-1β levels were also measured, but both cytokines were below detection limits, preventing any determination of the involvement of these pathways.
The most significant impact of melatonin treatment was the reversal of CF inflammatory cell recruitment from being neutrophil dominant to a more WT pattern. Melatonin has been shown to influence the function of both neutrophils and monocytes. Ren et al. have demonstrated that exogenous melatonin suppresses neutrophil migration through the inhibition of ERK activity in zebrafish32. This finding is consistent with the inhibition of neutrophil recruitment to the airways after infection in CF mice reported here. Likewise, exogenous melatonin treatment has been shown to enhance monocyte infiltration in a liver-graft mouse model33. Again, these findings are consistent with increased monocyte recruitment in the CF mouse airways after infection. Data support that exogenous melatonin is not acting at the level of epithelial cytokine production, but more study is needed to determine the mechanisms influencing inflammatory cell recruitment. It is not yet clear if exogenous melatonin is acting on neutrophils and monocytes individually to influence infiltration profiles or if a more central signaling mechanisms is being modulated.
Melatonin is known to influence several pathways and outcomes that could be responsible for the inflammatory cell responses observed in this study. One possibility is the anti-oxidative stress properties of melatonin. The antioxidant properties of melatonin are well described and have been purported to involve direct scavenging of free radicals, increasing mitochondrial efficiency, and activation of antioxidant enzymes34. Specifically, melatonin has been shown to increase the function of the antioxidant signaling protein Nrf235,36. Loss of Nrf2 function has been widely reported in CF and we have shown that interventions in the cAMP pathway can restore Nrf2 regulation in CF cells37. It is possible that melatonin restores WT-like responses to bacterial challenge in CF mice through improvement of oxidative stress by restoration of the Nrf2 pathway. Another potential mechanism is the control of circadian timing by melatonin. We have demonstrated that circadian timing is altered in CF mice and is accompanied by reduced melatonin levels7,38. Altered circadian regulation can also be a potent proinflammatory stimulus39–41. Restoration of circadian timing by systemic melatonin therapy is currently being evaluated.
Melatonin may represent a safe and easily accessible approach to augment therapy with HEMT to help control infection and immune response. Though the original hypothesis that melatonin treatment would be effective in reversing epithelial proinflammatory signaling tested negatively, systemic therapy proved very effective in facilitating bacterial clearance and reducing the neutrophil dominated inflammatory response characteristic of CF airway disease. These studies are conducted using a standard protocol utilizing a clinical isolate of a common CF pathogen, Pseudomonas aeruginosa. Given that only systemic treatments of melatonin proved to be effective, it is proposed that melatonin is improving overall immune function in the CF mice. Given the systemic nature of the response, it is speculated that this treatment would improve responses to other pathogens as well. However, further study to assess the efficacy on other pathogens or mixed pathogens which would be more physiologically relevant need to be completed.
The goal of this study was to examine the efficacy of melatonin treatment on modulating characteristic CF responses to bacterial infection and to examine the effect of timing of melatonin intervention on that efficacy. It was found that chronic treatment initiated right after weaning nearly completely reversed characteristic CF responses to infection. Bacterial clearance, inflammatory cell recruitment levels and patterns, and cytokine production were more like WT mice treated chronically with melatonin compared to vehicle controls. Short-term systemic treatment (1-week) improved bacterial clearance but had no other impact. Mechanistically, long-term treatment may alter inflammatory cell classification that shorter term treatments fail to achieve. Previous work has demonstrated that monocytes from pwCF fail to polarize into M2 or anti-inflammatory phenotypes42. Liu et al. reported that melatonin treated mesenchymal stem cells increased M2 polarization of macrophages and improved diabetic wound healing43. The polarization of macrophages in response to melatonin treatment will be evaluated in future studies. Additionally, we have demonstrated that circadian timing is disrupted in CF mouse models7. It is well-established that disrupted circadian regulation can be inflammatory. Chronic melatonin treatment may help restore circadian timing in CF models and help normalize inflammatory signaling. The effect of melatonin treatment on circadian regulation is under investigation. These findings suggest that melatonin may have benefits as an anti-inflammatory therapy for pwCF.
Methods
Mice
Cftr(G542X/G542X) and age-matched WT controls were obtained from the Cystic Fibrosis Mouse Model Core at CWRU. To decrease the incidence of intestinal obstruction that is common in CF mice, mice were allowed access to sterile water with osmotic laxative, PEG-3350 with electrolytes (Kremers Urban Pharmaceuticals). All mice were maintained on a 12 h light, 12 h dark cycle at a mean ambient temperature of 22 °C. The Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University approved all animal protocols. All methods were conducted according to necessary guidelines and established regulations.
Preparation of Pseudomonas aeruginosa-laden agarose beads
PA labeled with mCherry (mCH PA M57-15) was streaked on a 0.3 mg/ml gentamycin/LB agar plate and incubated at 37 °C overnight. Five colonies of mCH PA M57-15 were collected and added to 25 mL of LB broth and incubated at 37 °C, 200 rpm, for 18–20 h. After 20 h, the optical density at 600 nm of the mCH PA M57-15 was measured to obtain an absorbance of ~ 0.3 for a 10-fold dilution. Bacteria were stored on ice until ready to use.
Two 600-mL beakers containing 250 mL sterile mineral oil and stir bars and a flask of sterile 2% agarose in PBS were warmed in a 50 °C water bath for 30 min. The two beakers of mineral oil were placed in ~ 6 × 10 inch plastic container and then placed on magnetic stir plates and set at a medium-high stir to form a vortex. 23 mL of warm, dissolved agarose/PBS was pipetted into one of the beaker of mineral oil to form sterile beads. 5 mL of mCH PAM57-15 was pipetted into the remaining agarose/PBS and mixed and then 23 mL of the mCH PAM57-15/agarose/PBS mixture was pipetted into the other beaker of mineral oil. After 6 min of stirring, 20 g of ice was added to the plastic container every minute for 10 min while monitoring the stirring to ensure a constant vortex. After 10 min, the bead/oil mixture was poured into 50 mL conical tubes containing 15 mL of pre-warmed 0.5% SDC/PBS and spun at 4 °C, 3000 g, for 15 min to separate the beads on the bottom from the top layer of oil. The oil was removed and the top half layer of beads were pipetted into 50 ml conical tubes containing 20 mL of pre-warmed 0.25% SDC/PBS and spun under the same conditions. After the second wash, any oil/PBS was removed to ~ 15mL and the beads were pipetted into 50 ml conical tubes containing 20 mL of PBS and washed 4×. After the final wash, the PBS was removed to a 3:4 volume of beads to PBS. A 100 µL aliquot of the bead mixture was used to image and measure the size of beads, which should be ~ 20–600 μm. The beads were viewed under the mCherry channel to ensure sterile beads are sterile and bacterial-laden beads have mCH PA M57-15. A 1:50 dilution of the beads was homogenized at the highest speed for one minute and serially diluted 10-fold 10 times. The dilutions were plated on a 0.3 mg/mL gentamycin/LB agar plate and incubated at 37 °C overnight. The following day, the CFU/mL was calculated and the bead/PBS mixture was diluted to a concentration of 25,000 CFU/50 µL.
Intratracheal instillation of mouse airway with PolyDiagnost bronchoscope
The mice receiving sterile bead treatment were used first to avoid cross contamination. The mouse was anesthetized with isoflurane via inhalation with a nosecone. Ophthalmic ointment was applied the eyes. Forceps were used to gently pull the tongue while the optic modular (with 20G x 1” flexible catheter attached) was guided past the larynx into the trachea. Once desired location in trachea/lungs was reached, the optic modular was carefully removed, leaving the 20Gx1” flexible catheter in place. A 1 ml syringe fitted with a Polyshaft was used to mix and draw up desired substance, leaving no dead space in catheter. The Polyshaft was immediately inserted into the flexible catheter and desired substance administered. The mouse was placed into a clean, sterile cage on top of a warming pad and monitored until out of anesthesia. The weight and condition of mice were monitored until the day of harvest.
Harvest
Sterile bead inoculated mice were harvested first to avoid cross contamination. MIce were euthanized by CO2 inhalation, placed in the supine position, and disinfected with 70% ethanol. A midline, median incision was made to expose the thoracic cage and the diaphragm cut to expose the lungs. A 22G x 1” flexible catheter was inserted between the tracheal cartilage just before the bronchi. Surgical thread was tied around the trachea and catheter. BAL fluid was collected using a syringe filled with 1 ml PBS inserted into the catheter, injected into and withdrawn from the lungs. 10 µL of BAL fluid was used to determine total white blood cell count. Another 15 µL was plated on 0.3 mg/mL gentamycin/LB agar for CFU/µL count, and 200 µL of BAL fluid was used to determine cell differentials using Giemsa/Miller staining. The BAL fluid was then spun at 4 °C, 500 g, for 10 min. The supernatant was used for cytokine analysis (CXCL1, TNF-a, CXCL2) using Luminex. The lungs were removed, rinsed with PBS and placed in 2mL tubes containing zirconium homogenization beads and homogenized and 15 µL plated on 0.3 mg/mL gentamycin/LB agar for CFU/µL count. The remaining lung homogenate was spun at 4 °C, 5000 g, 10 min and the supernatant removed. The remaining supernatant was stored at − 80 °C. For staining of lung tissue, lungs were fixed in formalin, embedded in paraffin, deparaffinized in xylene and ethanol, and stained with hematoxylin and eosin by the histology core facility at CWRU.
Systemic melatonin treatment
Beginning at 3 weeks of age, mice were given free access to drinking water in graduated sipper tubes, containing either melatonin or control for up to seventy days for long-term-treatment. For short-term treatment, mice were treated at an age 8 to 10 weeks old for 7-days prior to and through infection. Water was prepared by dissolving powdered melatonin (Sigma-Aldrich) in a minimal volume of 100% ethanol, diluting in distilled water to create a stock solution (200 µg/mL), then diluting in sterile drinking water to treatment concentration (10 µg/mL). Drinking water for CF mice was prepared containing osmotic laxative, as described previously. Water intake was measured daily. Animal body weight and chow intake (by weight) were recorded twice weekly.
Direct airway melatonin treatment
Mice were treated for three consecutive days (day 0, day 1, and day2 of infection protocol) with melatonin (1 µM) via the PolyDiagnost bronchoscope described above. Melatonin is delivered in 25 µL total volume was delivered 10 min before infection on day 0 and then at the same time each day for days 1 and 2 post-infection.
Acknowledgements
We acknowledge the use of the CF Mouse Resource Center at Case Western Reserve University (CWRU). The authors thank P. Bead for technical assistance.
Author contributions
KS performed infection studies, acquiring and analyzing data, and manuscript preparation and review. SR performed infection studies and analyzed inflammatory markers, ZF assisted with infection studies. TK was responsible for study design, data analysis, and manuscript preparation.
Funding
This work is supported by R01 HL156928-01A1, CFF RDP R447-CR11, CFF KELLEY21I0, and CFF HODGES19R1.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rymut, S. M. et al. Reduced microtubule acetylation in cystic fibrosis epithelial cells. Am. J. Physiol. Lung cell. Mol. Physiol.305, L419-431. 10.1152/ajplung.00411.2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rymut, S. M. et al. Role of exchange protein activated by cAMP 1 in regulating rates of microtubule formation in cystic fibrosis epithelial cells. Am. J. Respir. Cell Mol. Biol.53, 853–862. 10.1165/rcmb.2014-0462OC (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey, D. A., Rymut, S. M. & Kelley, T. J. Alleviation of depression-like behavior in a cystic fibrosis mouse model by Hdac6 depletion. Sci. Rep.10, 16278. 10.1038/s41598-020-73298-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenjack, J., Hodges, C. A., Darrah, R. J. & Kelley, T. J. HDAC6 depletion improves cystic fibrosis mouse airway responses to bacterial challenge. Sci. Rep.9, 10282. 10.1038/s41598-019-46555-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rymut, S. M. et al. Improved growth patterns in cystic fibrosis mice after loss of histone deacetylase 6. Sci. Rep.7, 3676. 10.1038/s41598-017-03931-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbato, E., Darrah, R. & Kelley, T. J. Tubulin polymerization promoting protein affects the circadian timing system in C57Bl/6 mice. J. Circadian Rhythms19, 5. 10.5334/jcr.207 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbato, E., Darrah, R. & Kelley, T. J. The circadian system in cystic fibrosis mice is regulated by histone deacetylase 6. Am. J. Physiol. Cell Physiol.323, C1112-c1120. 10.1152/ajpcell.00248.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endres, T., Duesler, L., Corey, D. A. & Kelley, T. J. In vivo impact of tubulin polymerization promoting protein (Tppp) knockout to the airway inflammatory response. Sci. Rep.13, 12272. 10.1038/s41598-023-39443-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly, P. J., Quigg, M. & Davis, E. M. Improvement in non-24-h sleep-wake rhythm disorder in a sighted individual treated with a melatonin receptor agonist. Sleep Med.116, 41–42. 10.1016/j.sleep.2023.10.041 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Del Casale, A. et al. The use of prolonged-release melatonin in circadian medicine: A systematic review. Minerva Med.10.23736/s0026-4806.24.09303-0 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Megha, K. B. et al. Significance of melatonin in the regulation of circadian rhythms and disease management. Mol. Neurobiol.10.1007/s12035-024-03915-0 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Aydin, P. et al. The melatonin agonist ramelteon attenuates bleomycin-induced lung fibrosis by suppressing the NLRP3/TGF-Β1/HMGB1 signaling pathway. Adv. Med. Sci.68, 322–331. 10.1016/j.advms.2023.09.004 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Guo, S. N. et al. Melatonin regulates circadian clock proteins expression in allergic airway inflammation. Heliyon10, e27471. 10.1016/j.heliyon.2024.e27471 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lempesis, I. G., Georgakopoulou, V. E., Reiter, R. J. & Spandidos, D. A. A mid-pandemic night’s dream: Melatonin, from harbinger of anti-inflammation to mitochondrial savior in acute and long COVID-19 (review). Int. J. Mol. Med.53, 28. 10.3892/ijmm.2024.5352 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed Taha, A. et al. Safety and efficacy of melatonin as an adjuvant therapy in COVID-19 patients: Systematic review and meta-analysis. Adv. Med. Sci.68, 341–352. 10.1016/j.advms.2023.09.007 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Xu, M. M. et al. Melatonin improves influenza virus infection-induced acute exacerbation of COPD by suppressing macrophage M1 polarization and apoptosis. Respir. Res.25, 186 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedeto-Stojanov, D. et al. Melatonin as a promising anti-inflammatory agent in an in vivo animal model of sepsis-induced rat liver damage. Int. J. Mol. Sci.25, 455. 10.3390/ijms25010455 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Carvalho, J. F. & Skare, T. L. Melatonin supplementation improves rheumatological disease activity: A systematic review. Clin. Nutr. ESPEN55, 414–419. 10.1016/j.clnesp.2023.04.011 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., Ma, B., Wang, Z., Chen, Y. & Dong, Y. The effect mechanism of N6-adenosine methylation (m6A) in melatonin regulated LPS-induced colon inflammation. Int. J. Biol. Sci.20, 2491–2506. 10.7150/ijbs.95316 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan, H. C. et al. Melatonin enhanced microglia M2 polarization in rat model of neuro-inflammation via regulating ER stress/PPARδ/SIRT1 signaling axis. J. Neuroimmune Pharmacol.19, 11. 10.1007/s11481-024-10108-y (2024). [DOI] [PubMed] [Google Scholar]
- 21.Zhao, Z. et al. Melatonin ameliorates osteoarthritis rat cartilage injury by inhibiting matrix metalloproteinases and JAK2/STAT3 signaling pathway. Inflammopharmacology31, 359–368. 10.1007/s10787-022-01102-y (2023). [DOI] [PubMed] [Google Scholar]
- 22.Zhu, H. et al. Melatonin-driven NLRP3 inflammation inhibition via regulation of NF-κB nucleocytoplasmic transport: Implications for postoperative cognitive dysfunction. Inflammation46, 1471–1492. 10.1007/s10753-023-01822-5 (2023). [DOI] [PubMed] [Google Scholar]
- 23.de Castro-Silva, C. et al. Melatonin improves sleep and reduces nitrite in the exhaled breath condensate in cystic fibrosis—A randomized, double-blind placebo-controlled study. J. Pineal Res.48, 65–71. 10.1111/j.1600-079X.2009.00726.x (2010). [DOI] [PubMed] [Google Scholar]
- 24.Ge, W. et al. Melatonin protects sheep endometrial epithelial cells against lipopolysaccharide-induced inflammation in vitro. Reprod. Domest. Anim.57, 1602–1614. 10.1111/rda.14237 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Gong, M. J. et al. Melatonin reduces IL-33 and TSLP expression in human nasal epithelial cells by scavenging ROS directly. Immun. Inflamm. Dis.11, e788. 10.1002/iid3.788 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ning, L. et al. A novel mechanism for the protection against acute lung injury by melatonin: Mitochondrial quality control of lung epithelial cells is preserved through SIRT3-dependent deacetylation of SOD2. Cell. Mol. Life Sci. CMLS79, 610. 10.1007/s00018-022-04628-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su, X., Li, W., Zhang, D. & Zhu, H. Melatonin regulates lncRNA NEAT1/miR-138–5p/HIF-1α Axis through MOV10 to affect acid-related esophageal epithelial cell pyroptosis. Pharmacology108, 344–358. 10.1159/000530090 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Zhang, M., Li, L., Fang, D., Kelley, T. J. & Burgess, J. D. Single cell titration-type assay for plasma membrane cholesterol chemical potential. Anal. Chem.90, 5903–5908. 10.1021/acs.analchem.8b00736 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Harris, J. K. et al. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann. Am. Thorac. Soc.17, 212–220. 10.1513/AnnalsATS.201907-493OC (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hisert, K. B. et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respir. Crit. Care Med.195, 1617–1628. 10.1164/rccm.201609-1954OC (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNally, P. et al. Ivacaftor and airway inflammation in preschool children with cystic fibrosis. Am. J. Respir. Crit. Care Med.204, 605–608. 10.1164/rccm.202012-4332LE (2021). [DOI] [PubMed] [Google Scholar]
- 32.Ren, D. L. et al. Exogenous melatonin inhibits neutrophil migration through suppression of ERK activation. J. Endocrinol.227, 49–60. 10.1530/joe-15-0329 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Song, Z. et al. Exogenous melatonin protects small-for-size liver grafts by promoting monocyte infiltration and releases interleukin-6. J. Pineal Res.65, e12486. 10.1111/jpi.12486 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Reiter, R. J. Antioxidant actions of melatonin. Adv. Pharmacol.38, 103–117. 10.1016/s1054-3589(08)60981-3 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Alruhaimi, R. S. et al. The melatonin receptor agonist agomelatine protects against acute pancreatitis induced by cadmium by attenuating inflammation and oxidative stress and modulating Nrf2/HO-1 pathway. Int. Immunopharmacol.124, 110833. 10.1016/j.intimp.2023.110833 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Janjetovic, Z. et al. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep.7, 1274. 10.1038/s41598-017-01305-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziady, A. G. et al. Interaction with CREB binding protein modulates the activities of Nrf2 and NF-kappaB in cystic fibrosis airway epithelial cells. Am. J. Physiol. Lung cell. Mol. Physiol.302, L1221-1231. 10.1152/ajplung.00156.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbato, E., Mianzo, H., Litman, P. & Darrah, R. Dysregulation of circadian rhythm gene expression in cystic fibrosis mice. J. Circadian Rhythms17, 2. 10.5334/jcr.175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho Cabral, P., Weinerman, J., Olivier, M. & Cermakian, N. Time of day and circadian disruption influence host response and parasite growth in a mouse model of cerebral malaria. iScience27, 109684. 10.1016/j.isci.2024.109684 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casanova, N. G. et al. Circadian disruption dysregulates lung gene expression associated with inflammatory lung injury. Front. Immunol.15, 1348181. 10.3389/fimmu.2024.1348181 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.March, S. et al. Autonomous circadian rhythms in the human hepatocyte regulate hepatic drug metabolism and inflammatory responses. Sci. Adv.10, eadm9281. 10.1126/sciadv.adm9281 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarique, A. A. et al. CFTR-dependent defect in alternatively-activated macrophages in cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc.16, 475–482. 10.1016/j.jcf.2017.03.011 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Liu, W. et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther.11, 259. 10.1186/s13287-020-01756-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


