Abstract
Background
This study aimed to investigate the risk factors related to the failure of initial combined local methotrexate (MTX) treatment and minimally invasive surgery for late cesarean scar pregnancy (CSP).
Methods
This retrospective case-control study was conducted between January 2016 and December 2023, involving patients with late CSP (≥ 8 weeks) who received local MTX injection combined with either hysteroscopic or laparoscopic surgery. Cesarean scar pregnancy was classified as type I, II, or III based on the direction of growth of the gestational sac and the residual myometrial thickness as assessed by ultrasound. Binary logistic regression analysis was utilized to identify the risk factors associated with the failure of the initial combined treatment.
Results
Overall, 574 patients with late CSP were included in our study. Among them, 29 patients (5.1%) experienced treatment failure with the initial local MTX combined with minimally invasive surgery, while 545 patients (94.9%) achieved successful treatment outcomes. In the univariate analysis, several potential risk factors associated with the initial combined treatment failure were identified, including baseline serum β-human chorionic gonadotropin (β-hCG) levels, type of CSP, time interval between MTX and surgery, and positive fetal heart activity before surgery. Subsequent binary logistic regression analysis revealed the following independent risk factors linked to the failure of the initial combined treatment: baseline serum β-hCG levels exceeding 94,000 IU/L [odds ratio (OR) 3.060, 95% confidence interval (CI) 1.387–6.749, P = 0.006], type III CSP (OR 3.574, 95% CI 1.147–11.135, P = 0.028), a time interval greater than seven days between MTX and surgery (OR 3.847, 95% CI 1.725–8.581, P = 0.001), and the presence of a fetal heartbeat before surgery (OR 4.405, 95% CI 1.014–19.128, P = 0.048).
Conclusion
The findings indicate that higher baseline serum β-hCG levels, an extended time interval between MTX and surgery, type III CSP and a positive preoperative fetal heartbeat are significant risk factors for the failure of initial local MTX combined with minimally invasive surgery in patients with late CSP. Individualized treatment strategies are recommended for these high-risk patients with late CSP.
Keywords: Cesarean scar pregnancy, Methotrexate, Hysteroscopy, Laparoscopy, Risk factors
Introduction
Cesarean scar pregnancy (CSP) is a relatively rare form of ectopic pregnancy characterized by the implantation of a gestational sac (GS) within the scar tissue resulting from a previous cesarean delivery [1]. The exact prevalence of CSP remains uncertain, with literature indicating an incidence ranging from 1:1800 to 1:2500 pregnancies overall [2, 3]. In China, with the implementation of the two-child policy, cesarean delivery rates increased from 28.8% in 2004 to 36.7% in 2018, particularly in rural regions [4]. The incidence of CSP parallels the increasing rate of cesarean Sect. [5]. This trend is further supported by advancements in ultrasound imaging and increased awareness among physicians.
To date, more than 30 treatment protocols for CSP have been reported in the literature; however, there is no consensus on the optimal treatment approach [6]. The available treatment modalities include systemic or local administration of methotrexate (MTX) [7], dilation and curettage (including suction evacuation), uterine artery embolization (UAE), various surgical interventions (such as hysteroscopy, laparoscopy, laparotomy, and transvaginal surgery), high-intensity focused ultrasound, and combinations of these modalities [8]. In cases of late CSP (≥ 8 weeks), there is an increased risk of life-threatening conditions, such as uterine rupture and uncontrollable hemorrhage, and single treatment regimens are less effective [9]. Consequently, combining MTX or UAE with surgery is often considered a more desirable treatment modality [10, 11]. While UAE requires specialized equipment, trained radiologists, and incurs significant costs, it also carries risks of ovarian failure and endometrial damage [12, 13]. In contrast, MTX as a pretreatment approach is relatively easy to administer, has fewer side effects, and is less expensive. Thus, the combination of MTX with surgical intervention may represent a more suitable treatment approach for late CSP, particularly in district or county hospitals. Certain circumstances may indicate the failure of the initial combined treatment, such as the need for unexpected emergency surgery, alterations in surgical planning, significant vaginal bleeding following discharge, the presence of residual pregnancy tissue, or persistent abnormal serum β-hCG levels [14]. The aim of the present study was to investigate the risk factors associated with the failure of local MTX in conjunction with minimally invasive surgery for late CSP.
Materials and methods
This retrospective case-control study was conducted at Shenyang Women’s and Children’s Hospital from January 2016 to December 2023. The study included all patients diagnosed with CSP at a gestational age of ≥ 8 weeks. The study protocol was approved by the Medical Ethics Committee of Shenyang Women’s and Children’s Hospital (ID: 201919). The inclusion criteria were as follows: (1) a history of cesarean delivery; (2) elevated serum β-hCG levels (≥ 5000 IU/L) [15]; (3) a gestational age of less than 12 weeks; (4) transvaginal ultrasound findings indicating that the GS was either partially or completely embedded within the cesarean scar, with an empty uterine cavity and endocervix, a thin or absent layer of healthy myometrium between the bladder and the GS, and evidence of sustained peri-trophoblastic circulation characterized by high blood flow velocity and low impedance [16]; and (5) normal vital signs and stable hemodynamics. The exclusion criteria included: (1) the presence of serious maternal cardiac, renal, hepatic, or hematological diseases; (2) gestational trophoblastic disease; (3) prior treatment for CSP; and (4) incomplete clinical data or loss to follow-up.
CSP is classified into three types according to the Chinese expert consensus on the diagnosis and management of CSP established in 2016. This classification is based on the direction of growth of the GS and the residual myometrial thickness (RMT) between the implanted GS and the bladder wall, as detected by ultrasound [1]. Each type of CSP was matched with a local MTX in conjunction with a minimally invasive surgical strategy, based on the clinical experience of our team (Table 1). The choice of treatment was made collaboratively by gynecologists and patients after a thorough discussion of the pros and cons about different treatments, and informed consent was signed.
Table 1.
Ultrasound classification of CSP and initial combined treatment strategy
| Type | Ultrasound diagnostic standards | Initial combined treatment strategy |
|---|---|---|
| I |
(1) The GS is partially implanted in the uterine scar and growing into the uterine cavity; (2) The GS is noticeably deformed, elongated, and sharply angled at its lower end; (3) The RMT > 3 mm. |
Local MTX + hysteroscopic CSP excision |
| II |
(1) The GS is partially implanted in the uterine scar and growing into the uterine cavity; (2) The GS was noticeably deformed, elongated, and sharply angled at its lower end; (3) The RMT ≤ 3 mm. |
(1) With the RMT ≥2 mm, local MTX + hysteroscopic CSP excision (2) With the RMT <2 mm, local MTX + laparoscopic CSP resection with subsequent repair of the uterine defect |
| III |
(1) The GS is completely embedded within the cesarean scar and protruded outward to the bladder; (2) Emptiness of the uterine cavity and cervical canal; (3) The RMT ≤ 3 mm. |
Local MTX + laparoscopic CSP resection with subsequent repair of the uterine defect |
CSP cesarean scar pregnancy; GS gestational sac; MTX methotrexate; RMT residual myometrial thickness
Combined treatment protocol
Local MTX
All patients were pretreated with a single dose of MTX (50 mg/m2) local injection. MTX was injected into the GS using a 20G needle under transabdominal ultrasound guidance. Serum β-hCG levels and ultrasound imaging were measured every other day following the administration of local MTX until a decrease of more than 15% in serum β-hCG levels was observed, or until the cessation of cardiac activity or a reduction in blood flow surrounding the CSP was noted. Surgical intervention, either hysteroscopic or laparoscopic, was performed the following day.
Hysteroscopy
The procedure for hysteroscopic excision of CSP was conducted as follows: hysteroscopic confirmation of the GS position, dissection of the GS from the uterine wall, suction evacuation under ultrasound surveillance, and hysteroscopy to check for or excise any residual gestational tissue. In instances of significant intraoperative hemorrhage and ineffective hysteroscopic electrocoagulation, a Foley catheter was applied with compression over the cesarean scar, guided by transabdominal ultrasound, for a duration of 24 h, alongside the administration of Oxytocin to promote hemostasis. If these interventions failed, patients received laparoscopy or laparotomy as salvage procedures.
Laparoscopy
During laparoscopic surgical procedures, the bulging portion of the gestational mass was exposed after dissection of the vesicouterine peritoneum. Three units of diluted vasopressin were injected into the lower uterine segment, after which the cesarean scar was incised, and the gestational mass was mostly removed using grasping forceps. Suction evacuation was conducted under laparoscopic surveillance to remove the remaining conceptus tissue. The cesarean scar was subsequently trimmed, and the uterus was sutured in two layers. In cases of excessive intraoperative bleeding, the subsequent intervention involved bilateral uterine artery ligation or bilateral internal iliac artery ligation, which could be performed via laparoscopy or laparotomy.
The failure of the initial combined treatment strategy was defined as the occurrence of unexpected emergency surgery; alterations in surgical planning (indicating a failure of the recommended first-line surgical approach); significant vaginal bleeding post-discharge (≥ 500 ml, inferred from a decrease in laboratory hemoglobin levels of 10 g/l prior to discharge) [17]; the presence of residual pregnancy tissue (identified as a persistent mass in the caesarean scar detected by ultrasound for more than one month); or persistent abnormal serum β-hCG levels (where serum β-hCG levels, measured weekly post-discharge, did not normalize within four weeks). Patients meeting any of these criteria were classified into the failure group, while those who did not were categorized as belonging to the success group.
Data collection
The clinical and ultrasonographic characteristics were extracted from electronic medical records, including age, body mass index (BMI), gravidity, parity, the number of previous cesarean sections (CS), time interval since the last CS, gestational age, baseline serum β-hCG level, serum β-hCG level and percentage change in β-hCG one day before surgery, time interval between MTX and surgery, presence or absence of a fetal heartbeat at baseline and before surgery, type of CSP, maximum diameter of GS and RMT.
Statistical analysis
All data were statistically analyzed by SPSS version 23 (IBM Corp., Armonk, NY, USA). Continuous data that followed a normal distribution were presented as the mean ± SD and tested by the Student’s t-test. Non-normally distributed variables were presented as medians (interquartile ranges, IQRs) and assessed using the Mann-Whitney U test. Categorical data were expressed as percentages and compared via the χ2 test. Binary logistic regression was used to identify independent risk factors associated with the failure of local MTX combined with surgery, with results presented as odds ratios (ORs) and 95% confidence intervals (CIs). All statistical tests were two-tailed, and P < 0.05 was considered statistically significant.
Results
During the study period, 1142 patients were admitted to our hospital for CSP management. Among them, 623 were diagnosed with CSP at a later gestational age (≥ 8 weeks). Following the application of exclusion criteria, 574 patients were included in our study. Of these, 545 patients who received successful treatment via an initial combined regimen of local MTX and minimally invasive surgery were classified into the success group, while the remaining 29 patients who failed the initial combined treatment were categorized into the failure group. The overall success rate of the initial combined treatment was 94.9%. Adverse effects related to MTX were observed in 51 patients (8.9%), including symptoms such as fever, elevated liver enzymes, oral ulceration, and bone marrow suppression, but no severe side effects were reported.
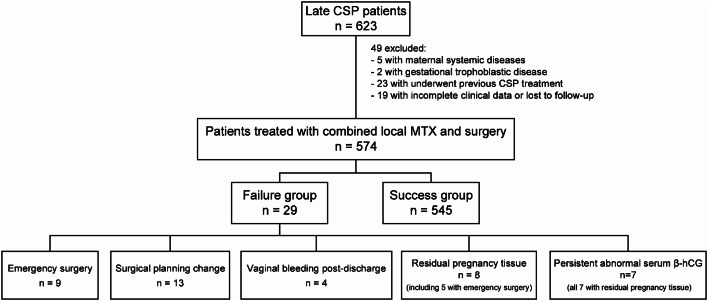
In the cohort identified as the failure group, nine patients underwent unexpected emergency suction evacuation, followed by hemostatic compression using a Foley catheter balloon due to incomplete abortion, prior to the advised first-line surgical intervention. Thirteen patients experienced a failure of the first-line surgical strategy, resulting in a change in surgical planning during the procedure. Specifically, three patients required conversion from hysteroscopic to laparoscopic surgery due to severe hemorrhage that did not respond to Foley catheter balloon compression for hemostatic control. In six patients, the gestational sac was noted to be protruding toward the bladder during hysteroscopy, leading to a diagnosis of type III CSP, which needed a transition to laparoscopic surgery. Furthermore, four patients had their laparoscopic surgeries converted to laparotomic procedures upon the identification of a highly vascularized surface of the GS. Four patients were readmitted due to significant vaginal bleeding post-discharge and were subsequently diagnosed with uterine arteriovenous fistula, a condition confirmed via uterine arteriography. Hemostatic resolution was achieved through UAE. Eight patients, including five who had previously undergone emergency suction evacuation, were readmitted due to the presence of residual pregnancy tissue. In seven cases, serum β-hCG levels remained elevated (> 5.3 IU/L), while one patient had normalized levels. The management of these cases involved either hysteroscopic excision or laparoscopic wedge resection of the remaining pregnancy tissue (Fig. 1). No patient required a hysterectomy.
Fig. 1.
Patient flowchart. CSP cesarean scar pregnancy; MTX methotrexate
In the analysis of single-variable statistics, the failure group exhibited higher baseline serum β-hCG levels compared to the success group [94034 (36536–134146) UI/L vs. 65955 (28068–88549) UI/L, P = 0.043] and a longer time interval between MTX and surgery [8 (5–10) d vs. 6 (5–8) d, P = 0.008]. Additionally, the incidence of type III CSP was higher in the failure group [P(I, II) = 0.485; P(II, III) = 0.011; P(I, III) = 0.006], along with a higher percentage of positive fetal heartbeats before surgery (10.3% vs. 2.4%, P = 0.042). However, no statistically significant differences were observed between the two groups in terms of maternal age, BMI, gravidity, parity, number of previous CS, time interval since the last CS, gestational age, maximum diameter of the GS, baseline fetal heartbeat, RMT, or serum β-hCG level and percentage change in β-hCG one day before surgery, with all P > 0.05 (Table 2).
Table 2.
Univariate analysis of clinical and ultrasonographic characteristics in two groups
| Characteristic | Failure group (n = 29) | Success group (n = 545) | P value |
|---|---|---|---|
| Age (year) | 30.0 (27.5–35.0) | 30.0 (26.5–33.5) | 0.590 |
| BMI (kg/m2) | 24.01 (22.56–26.35) | 25.19 (22.22–28.50) | 0.196 |
| Gravidity | 3 (2–4) | 3 (2–4) | 0.153 |
| Parity | 1 (1–2) | 1 (1–2) | 0.452 |
| No. of prior CS | 1 (1–2) | 1 (1–2) | 0.532 |
| Interval from last CS (year) | 5 (3-7.5) | 6 (3.5–8.5) | 0.610 |
| Gestational age (day) | 63 (57-64.5) | 63 (59–72) | 0.073 |
| Baseline serum β-hCG (UI/L) | 94,034 (36536–134146) | 65,955 (28068–88549) | 0.043 |
| Maximum diameter of GS (cm) | 6.3 (4.95–8.85) | 5.9 (4.5–7.85) | 0.368 |
| Baseline fetal heartbeat | 18 (62.1%) | 255 (46.8%) | 0.108 |
| Type of CSP | 0.008 | ||
| I a | 5 (17.2%) | 164 (30.1%) | |
| II b | 13 (44.8%) | 294 (53.9%) | |
| III c | 11 (38.0%) | 87 (16.0%) | |
| RMT (mm) | 1.9 (1.15–2.95) | 1.9 (1.4–2.5) | 0.599 |
| Interval between MTX and surgery (day) | 8 (5–10) | 6 (5–8) | 0.008 |
| Fetal heartbeat before surgery | 3 (10.3%) | 13 (2.4%) | 0.042 |
| Serum β-hCG before surgery (UI/L) | 19,788 (9461–69731) | 26,566 (14778–42253) | 0.489 |
| Percentage change of β-hCG (%) | 58.1 (30.5–88.9) | 47.4 (32.0-59.7) | 0.170 |
Data are presented as medians (IQRs), or n (%)
BMI body mass index; CS cesarean section; β-hCG β-human chorionic gonadotropin; GS gestational sac; RMT residual myometrial thickness
aP(I, II) = 0.485; bP(II, III) = 0.011; cP(I, III) = 0.006 (Bonferroni correction, α = 0.017)
In the binary logistic regression analysis, the baseline serum β-hCG level and the time interval between MTX and surgery were categorized into two groups using the receiver operating characteristic (ROC) curve. The determined cut-off values were 94,000 IU/L (AUC = 0.612, 95% CI 0.487–0.736, P = 0.043) and 7 days (AUC = 0.650, 95% CI 0.528–0.772, P = 0.007), respectively (Fig. 2). The independent risk factors associated with the failure of local MTX in conjunction with minimally invasive surgery were a baseline serum β-hCG level > 94,000 IU/L (OR 3.060, 95% CI 1.387–6.749, P = 0.006), type III CSP (OR 3.574, 95% CI 1.147–11.135, P = 0.028), a time interval between MTX and surgery > 7 days (OR 3.847, 95% CI 1.725–8.581, P = 0.001), and the presence of a positive fetal heartbeat before surgery (OR 4.405, 95% CI 1.014–19.128, P = 0.048) (Table 3).
Fig. 2.

ROC curves of the baseline serum β-hCG level and the time interval between MTX and surgery. ROC curve Receiver operating characteristic curve
Table 3.
Multivariable logistic regression analysis of risk factors for the initial combined treatment failure
| OR | 95%CI | P value | |
|---|---|---|---|
| Baseline serum β-hCG > 94,000 IU/L | 3.060 | 1.387–6.749 | 0.006 |
| Type of CSP | 0.041 | ||
| I | Referent | - | |
| II | 1.358 | 0.466–3.952 | 0.575 |
| III | 3.574 | 1.147–11.135 | 0.028 |
| Interval between MTX and surgery > 7 days | 3.847 | 1.725–8.581 | 0.001 |
| Fetal heartbeat before surgery | 4.405 | 1.014–19.128 | 0.048 |
In the failure group, all four patients (100%) experienced significant vaginal bleeding post-discharge when their baseline serum β-hCG levels exceeded 94,000 IU/L. Among those diagnosed with type III CSP, ten patients (76.9%) underwent changes in their the recommended first-line surgical strategy during the procedure. Additionally, all nine patients (100%) who underwent unanticipated emergency surgery for incomplete abortion had a time interval of more than seven days between MTX pretreatment and surgery (Table 4).
Table 4.
Detailed information on the independent risk factors in the failure group
| Failure reasons | Baseline serum β-hCG, IU/L | Type of CSP | Interval between MTX and surgery, day |
Fetal heartbeat before surgery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| > 94,000 | ≤ 94,000 | I | II | III | > 7 | ≤ 7 | Yes | No | ||||
|
emergency surgery (n = 9) |
3 | 6 | 3 | 6 | 0 | 9 | 0 | 0 | 9 | |||
|
surgical planning change (n = 13) |
7 | 6 | 1 | 2 | 10 | 8 | 5 | 3 | 10 | |||
|
vaginal bleeding post-discharge (n = 4) |
4 | 0 | 1 | 3 | 0 | 2 | 2 | 0 | 4 | |||
|
residual pregnancy tissue (n = 8) |
3 | 5 | 3 | 4 | 1 | 5 | 3 | 0 | 8 | |||
|
persistent abnormal serum β-hCG (n = 7) |
3 | 4 | 3 | 3 | 1 | 4 | 3 | 0 | 7 | |||
Discussion
In the present study, 94.9% of patients diagnosed with late CSP were successfully treated with an initial therapeutic regimen that combined local MTX with minimally invasive surgical intervention. This treatment strategy did not result in serious side effects from MTX or the need for hysterectomy. A higher baseline serum β-hCG level, a longer time interval between MTX and surgery, Type III CSP, and a positive preoperative fetal heartbeat were associated with the failure in the initial combined treatment approach for late CSP.
Our data indicated that a baseline serum β-hCG level greater than 94,000 IU/L is one of the risk factors for the failure of local MTX combined with minimally invasive surgery. All four patients (100%) experienced significant vaginal bleeding following discharge and were subsequently diagnosed with uterine arteriovenous fistulas. During pregnancy, the trophoblast invades the myometrium and remodels the vascular structure. The presence of a thin or absent decidua basalis at the site of the cesarean scar facilitates the unopposed invasion of trophoblastic cells, thereby increasing myometrial vascularity/arteriovenous malformations in cesarean scar pregnancies [18].Elevated serum β-hCG levels indicate increased gestational trophoblastic activity, which is associated with a higher degree of invasiveness and an increased likelihood of developing uterine arteriovenous fistulas. In such high-risk cases, it is crucial to maintain heightened awareness of the potential existence of pathological uterine vascular malformations.
Serum β-hCG is secreted by syncytiotrophoblasts. The concentration of serum β-hCG in a CSP increases rapidly from gestational weeks five to nine and fluctuates thereafter [19]. Previous studies have shown that in the combined treatment for CSP, it is essential to wait for the serum β-hCG level to decrease to a very low level (1000–3000 IU/L) following systemic MTX pretreatment before proceeding with surgery [20, 21]. In contrast, our investigation revealed that neither the serum β-hCG level nor the percentage change in serum β-hCG prior to surgery was correlated with the failure of local MTX in conjunction with minimally invasive surgery for late CSP. This discrepancy may be attributed to the observation that serum β-hCG level tend to plateau nearly five days after local MTX injection and decline steadily after seven days [22]. Local intra-gestational injection of MTX results in a higher concentration of MTX with a prompt response, which could expedite the process of embryo death.
The present study revealed that performing more than seven days after local MTX pretreatment was also a significant risk factor associated with the initial failure of combined treatment for late CSP. When the interval between MTX and surgery exceeded seven days, all nine patients (100%) experienced unanticipated emergency surgeries due to incomplete abortion. Cytotrophoblasts and syncytiotrophoblasts undergo successive lysis following local MTX injection after a period of more than seven days [23]. This process results in the partial detachment of the gestational sac from the cesarean scar, leading to incomplete abortion and unplanned emergency surgery. Therefore, it may be advisable to consider a surgical timeframe of within seven days following local MTX pretreatment as more optimal.
Tang Q et al. [24] reported a low success rate among patients with type III CSP who underwent systemic MTX pretreatment followed by hysteroscopy under laparoscopic monitoring. Consistent with these findings, our data indicated that type III CSP is one of the risk factors related to initial combined treatment failure in late CSP, with ten patients (76.9%) who underwent intraoperative surgical planning change. In CSP patients with a gestational age of 8 weeks or more, there is a pathologically implanted placenta [25]. In type III CSP, the gestational sac is entirely embedded within the cesarean scar and protrudes toward the bladder. An RMT of less than 3 mm has been linked to unfavorable treatment outcomes [26]. For patients with type III late CSP, it may be advisable to consider prophylactic bilateral uterine artery or bilateral internal iliac artery ligation during laparoscopic procedures, or to opt for laparotomy. Furthermore, referral to a tertiary care center is recommended for the management of such cases.
The present study found that a positive preoperative fetal heartbeat serves as an independent risk factor for the failure of local MTX in conjunction with minimally invasive surgery. This finding aligns with the results of a study conducted by Yang M et al., which reported that a positive fetal heartbeat prior to surgery is a risk factor for initial treatment failure in UAE combined with hysteroscopy [17]. Di Spienzo Sardo A et al. demonstrated that the success rate of systemic MTX combined with hysteroscopic surgery for CSP is 100% when the fetal heartbeat is absent before surgery [27]. A positive preoperative fetal heartbeat indicates the presence of a live embryo. Consequently, patients with a positive fetal heartbeat before surgery may require additional local MTX or may be pretreated with UAE coupled with MTX. Surgery should be performed after confirming the absence of a fetal heartbeat.
To date, few studies have focused on the risk factors associated with the failure of local MTX combined with minimally invasive surgery for late CSP. Notably, the present study is a large-scale retrospective case-control study. For the first time, we reported that performing surgery more than seven days after local MTX pretreatment is an independent risk factor for the initial combined treatment failure in late CSP. However, this study has several limitations. The absence of ultrasonographic blood flow patterns around the GS or within the cesarean scar area precluded an assessment of their potential impact on the outcomes of the combined treatment. Previous research has indicated that the blood flow pattern surrounding the GS may be a risk factor associated with the failure of combined MTX or UAE and dilation and curettage for CSP [28, 29]. This aspect is planned for evaluation in future studies. Additionally, it is important to acknowledge that this investigation was a single-center retrospective study, which may introduce selection bias that cannot be entirely eliminated.
Conclusion
In summary, local MTX combined with minimally invasive surgery is a safe and effective treatment modality for patients with late CSP and can be performed in district or county hospitals. Independent risk factors associated with initial combined treatment failure include a baseline serum β-hCG level exceeding 94,000 IU/L, type III CSP, a time interval greater than seven days between MTX and surgery, and the presence of a positive fetal heartbeat before surgery. High-risk patients with late CSP are recommended to be referred to a tertiary care center that utilizes an individualized treatment strategy.
Acknowledgements
We thank the Department of Ultrasound for providing the ultrasound data of late CSP patients.
Abbreviations
- CSP
Cesarean scar pregnancy
- MTX
Methotrexate
- UAE
Uterine artery embolization
- GS
Gestational sac
- β-hCG
β-human chorionic gonadotropin
- RMT
Residual myometrial thickness
- BMI
Body mass index
- CS
Cesarean section
Author contributions
Xiaotong Cheng contributed to the design, implementation, methodology, and manuscript preparation. Jumin Niu was involved in the conceptualization, design, oversight, review, and approval of the final manuscript. Danyang Song and Yushi Xiang engaged in data curation, analysis, and interpretation. Yansong Liu and Xiaocui Nie participated in the editing and review of the manuscript. All authors reviewed the manuscript.
Funding
This research was fully sponsored by the Science and Technology Program of Shenyang with grant number 20-205-4-036.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethics Committee of Shenyang Women’s and Children’s Hospital (ID: 201919). The patients provided written informed consent in accordance with the hospital’s guidelines, as is the standard procedure prior to surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Family Planning Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Expert opinion of diagnosis and treatment of cesarean scar pregnancy (2016). Chin J Obstet Gynecol. 2016;51:568–72. [DOI] [PubMed] [Google Scholar]
- 2.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol. 2003;21:220–7. [DOI] [PubMed] [Google Scholar]
- 3.Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23:247–53. [DOI] [PubMed] [Google Scholar]
- 4.Li HT, Hellerstein S, Zhou YB, Liu JM, Blustein J. Trends in Cesarean Delivery Rates in China, 2008–2018. JAMA. 2020;323:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of caesarean deliveries: early placenta accreta and caesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207:14–29. [DOI] [PubMed] [Google Scholar]
- 6.Fu P, Sun H, Zhang L, Liu R. Efficacy and safety of treatment modalities for cesarean scar pregnancy: a systematic review and network meta-analysis. Am J Obstet Gynecol MFM. 2024;6:101328. [DOI] [PubMed] [Google Scholar]
- 7.Levin G, Zigron R, Dior UP, Gilad R, Shushan A, Benshushan A, et al. Conservative management of caesarean scar pregnancies with systemic multidose methotrexate: predictors of treatment failure and reproductive outcomes. Reprod Biomed Online. 2019;39:827–34. [DOI] [PubMed] [Google Scholar]
- 8.Maheux-Lacroix S, Li F, Bujold E, Nesbitt-Hawes E, Deans R, Abbott J. Cesarean scar pregnancies: a systematic review of Treatment options. J Minim Invasive Gynecol. 2017;24:915–25. [DOI] [PubMed] [Google Scholar]
- 9.Kaelin Agten A, Jurkovic D, Timor-Tritsch I, Jones N, Johnson S, Monteagudo A, et al. First-trimester cesarean scar pregnancy: a comparative analysis of treatment options from the international registry. Am J Obstet Gynecol. 2023;21:S0002–5. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Ross WT, Chu AL, Deimling TA. An updated guide to the diagnosis and management of cesarean scar pregnancies. Curr Opin Obstet Gynecol. 2020;32:255–62. [DOI] [PubMed] [Google Scholar]
- 11.Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) consult Series #49: cesarean scar pregnancy. Am J Obstet Gynecol. 2020;222:B2–14. [DOI] [PubMed] [Google Scholar]
- 12.Arthur R, Kachura J, Liu G, Chan C, Shapiro H. Laparoscopic myomectomy versus uterine artery embolization: long-term impact on markers of ovarian reserve. J Obstet Gynaecol Can. 2014;36:240–7. [DOI] [PubMed] [Google Scholar]
- 13.Chang WH, Chou FW, Wang PH. Cesarean scar pregnancy. Taiwan J Obstet Gynecol. 2022;61:923–4. [DOI] [PubMed] [Google Scholar]
- 14.Ban Y, Shen J, Wang X, Zhang T, Lu X, Qu W, et al. Cesarean scar ectopic pregnancy clinical classification System with recommended Surgical Strategy. Obstet Gynecol. 2023;141:927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016;105:958–67. [DOI] [PubMed] [Google Scholar]
- 16.Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207:e441–13. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Cao L, Yan J, Tang Y, Cao N, Huang L. Risk factors associated with the failure of initial treatment for cesarean scar pregnancy. Int J Gynaecol Obstet. 2023;162:937–44. [DOI] [PubMed] [Google Scholar]
- 18.Timor-Tritsch IE, Monteagudo A, Calì G, D’Antonio F, Agten AK. Cesarean scar pregnancy: patient counseling and management. Obstet Gynecol Clin North Am. 2019;46:813–28. [DOI] [PubMed] [Google Scholar]
- 19.Tsai NC, Cheng LY, Yang TH, Hsu TY, Kung FT. Serum β-human chorionic gonadotropin profile and its correlations with ultrasound parameters in low-lying-implantation ectopic pregnancy in the first trimester. J Obstet Gynaecol Res. 2020;46:844–50. [DOI] [PubMed] [Google Scholar]
- 20.Shao MJ, Hu MX, Xu XJ, Zhang L, Hu M. Management of caesarean scar pregnancies using an intrauterine or abdominal approach based on the myometrial thickness between the gestational mass and the bladder wall. Gynecol Obstet Invest. 2013;76:151–7. [DOI] [PubMed] [Google Scholar]
- 21.Sun XY, Zhang XG. Clinical effect of ultrasound-guided methotrexate administration assisted hysteroscopy in the treatment of cesarean scar pregnancy. Chin J Family Plann Gynecotokology. 2020;12:38–40. [Google Scholar]
- 22.Uludag SZ, Kutuk MS, Ak M, Ozgun MT, Dolanbay M, Aygen EM, et al. Comparison of systemic and local methotrexate treatments in cesarean scar pregnancies: time to change conventional treatment and follow-up protocols. Eur J Obstet Gynecol Reprod Biol. 2016;206:131–5. [DOI] [PubMed] [Google Scholar]
- 23.Natale A, Candiani M, Barbieri M, Calia C, Odorizzi MP, Busacca M. Pre- and post-treatment patterns of human chorionic gonadotropin for early detection of persistence after a single dose of methotrexate for ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2004;117:87–92. [DOI] [PubMed] [Google Scholar]
- 24.Tang Q, Qin Y, Zhou Q, Tang J, Zhou Q, Qiao J, et al. Hysteroscopic treatment and reproductive outcomes in cesarean scar pregnancy: experience at a single institution. Fertil Steril. 2021;116:1559–66. [DOI] [PubMed] [Google Scholar]
- 25.Timor-Tritsch IE, Monteagudo A, Calì G, D’Antonio F, Kaelin Agten A. Cesarean scar pregnancy: diagnosis and Pathogenesis. Obstet Gynecol Clin North Am. 2019;46:797–811. [DOI] [PubMed] [Google Scholar]
- 26.Kaelin Agten A, Cali G, Monteagudo A, Oviedo J, Ramos J, Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted on the scar versus in the niche. Am J Obstet Gynecol. 2017;216:e5101–6. [DOI] [PubMed] [Google Scholar]
- 27.Di Spiezio Sardo A, Zizolfi B, Saccone G, Ferrara C, Sglavo G, De Angelis MC, et al. Hysteroscopic resection vs ultrasound-guided dilation and evacuation for treatment of cesarean scar ectopic pregnancy: a randomized clinical trial. Am J Obstet Gynecol. 2023;229:e4371–7. [DOI] [PubMed] [Google Scholar]
- 28.Fang Q, Sun L, Tang Y, Qian C, Yao X. Quantitative risk assessment to guide the treatment of cesarean scar pregnancy. Int J Gynaecol Obstet. 2017;139:78–83. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Ma H, Peng H, He L, Bian C, Zhao X. Risk factors for intra-operative haemorrhage and bleeding risk scoring system for caesarean scar pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2015;195:141–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.