Abstract
Background
Lindaspio polybranchiata, a member of the Spionidae family, has been reported at the Lingshui Cold Seep, where it formed a dense population around this nascent methane vent. We sequenced and assembled the genome of L. polybranchiata and performed comparative genomic analyses to investigate the genetic basis of adaptation to the deep sea. Supporting this, transcriptomic and fatty acid data further corroborate our findings.
Results
We report the first genome of a deep-sea spionid, L. polybranchiata. Over long-term adaptive evolution, genes associated with vision and biological rhythmicity were lost, which may indirectly benefit oligotrophy by eliminating energetically costly processes. Compared to its shallow-sea relatives, L. polybranchiata has a significantly higher proportion of polyunsaturated fatty acids (PUFAs) and expanded gene families involved in the biosynthesis of unsaturated fatty acids and chromatin stabilization, possibly in response to high hydrostatic pressure. Additionally, L. polybranchiata has broad digestive scope, allowing it to fully utilize the limited food resources in the deep sea to sustain a large population. As a pioneer species, L. polybranchiata has an expanded repertoire of genes encoding potential chemoreceptor proteins, including ionotropic receptors (IRs) and gustatory receptor-like receptors (GRLs). These proteins, characterized by their conserved 3D structures, may enhance the organism’s ability to detect chemical cues in chemosynthetic ecosystems, facilitating rapid settlement in suitable environments.
Conclusions
Our results shed light on the adaptation of Lindaspio to the darkness, high hydrostatic pressure, and food deprivation in the deep sea, providing insights into the molecular basis for L. polybranchiata becoming a pioneer species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-025-02112-2.
Keywords: Deep-sea Polychaeta, Adaptive evolution, High hydrostatic pressure, Visual degeneration, Digestive enzymes, Chemoreceptors
Background
Advances in ocean exploration have revealed that numerous animals, ranging from invertebrates (e.g., corals, sponges, worms, and mussels) to vertebrates (e.g., cartilaginous and bony fishes), are found in the deep sea—waters that constitute approximately 95% of the ocean’s volume [1, 2]. Spionidae is one of the largest and most diverse families of polychaetes, annelid worms found in almost all ocean habitats, from shallow waters to the deep sea [3, 4]. Spionids are considered opportunistic species characterized by early reproductive maturity, high fecundity, and short life spans, which enabled them to quickly colonize new habitats and emerge as dominant species in various benthic communities [5, 6]. Recently, many new species of the family Spionidae have been discovered in the deep sea [7–9]. The genus Lindaspio is an intriguing group of spionids endemic to deep-sea chemosynthetic ecosystems such as hydrothermal vents, methane seeps, and whale falls [10–12].
Lingshui Cold Seep was found to have erupted after 2019, located off Hainan Island in the South China Sea at a depth of 1700 m. This seep is characterized by typical deep-sea features, including complete darkness, high hydrostatic pressure, and high levels of reductive compounds like methane that characterize this seep. A continuous survey of the seep has revealed that the microbial communities have gradually changed over the past 5 years since its first recorded eruption [13]. Remarkably, rapid community succession has also been observed, with the cold seep sediment being colonized by L. polybranchiata. This species has emerged as the dominant species, developing a dense population with 30,000 individuals per square meter [14]. Within just 1 year, L. polybranchiata established a high-abundance population, making it a pioneer species in the early cold seep stage [14, 15]. Furthermore, L. polybranchiata is endemic to deep-sea chemosynthetic ecosystems and we discovered this species in Haima Cold Seep, around 150 km away from Lingshui Cold Seep. Its ability to become a pioneer species in deep-sea chemosynthetic ecosystems suggests the existence of unique adaptive mechanisms that allow it to thrive in extreme deep-sea environments and rapidly form large populations.
Morphological innovations of L. polybranchiata include enlarged gills (ostensibly an adaptation to hypoxia), an enlarged caruncle, and loss of eyes [14], but their genetic basis and molecular mechanisms are unknown. The enlarged caruncle, a sensory organ, in these worms may play an important role in detecting chemical clues and colonizing chemosynthetic ecosystems [14]. The loss of eyes and enlargement of sensory organs are common evolutionary responses to perpetual darkness in the deep sea [16–18]. Many deep-sea invertebrates and fish have evolved specialized chemosensory systems to detect and respond to chemical gradients in the water, ensuring survival in lightless conditions [19]. In contrast, some deep-sea organisms, including the silver spinyfin, have evolved a unique visual system with an expanded repertoire of rod opsin genes, enabling color vision in the dark and the ability to perceive bioluminescent signals [20]. Thus, this contrasting evolutionary path in deep-sea organisms warrants further investigation.
How largely undescribed deep-sea species evolved and adapted to their extreme environments can now be explored using genomic tools. Here, we assembled and annotated the genome of L. polybranchiata and juxtaposed it with its shallow-water Spionidae member Streblospio benedicti and other polychaete worm species to reveal candidate deep-sea adaptation genes.
Results and Discussion
Characteristics of the Lindaspio polybranchiata genome
We sequenced and assembled a 1.66 Gb L. polybranchiata genome with 8977 contigs and a contig N50 length of 303,282 bp using PacBio HiFi long reads with Hifiasm (Table 1). The assembly included 59.15% (0.98 Gb) repetitive elements, and 21,462 (77.3%) of the protein-coding gene models were functionally annotated (Additional file 1: Fig. S1). The BUSCO metazoan completeness score of the gene models was 90.1% (84.5% single copy and 5.6% duplicated genes) (Table 1). The average gene length was 12,902 bp, and the mean intron length was 2242 bp, similar to other polychaete species.
Table 1.
Statistics of the genome characteristics of Lindaspio polybranchiata
| Genome size | 1,664,211,210 bp |
| No. of contig | 8977 |
| N50 length | 303,282 bp |
| GC content | 38.73% |
| BUSCO score (genome) | C: 89.1% (S: 82.7%, D: 6.4%, F: 6.0%, M: 4.9%) |
| Repeat sequence | 59.15% |
| No. of protein-coding genes | 27,746 |
| BUSCO score (gene set) | C: 90.1% (S: 84.5%, D: 5.6%, F: 3.7%, M: 6.2%) |
| Average gene length | 12,902 bp |
| Average intron length | 2242 bp |
| Average exon length | 236 bp |
| Average intergenic length | 26,935 bp |
The L. polybranchiata assembly (1.66 Gb) was much larger than that of Streblospio benedicti (701.4 Mb) collected from the intertidal zone, which was also generated using long-read sequencing reads [21]. A proliferation of transposable elements often underlies large genome sizes across Metazoa [22–24]. There was a higher proportion (59.15% vs. 40.36%) of repetitive and other non-coding sequences in the L. polybranchiata assembly (Table 2). Many repetitive sequences (38.00%, average length 413 bp) were unclassified, hinting that further research is needed to classify annelid repetitive sequences.
Table 2.
Statistics of the repeat sequences in the genomes of Lindaspio polybranchiata and Streblospio benedicti
| L. polybranchiata | S. benedicti | |
|---|---|---|
| DNA | 6.58% | 5.02% |
| LINE | 5.70% | 4.44% |
| SINE | 2.21% | 0.99% |
| LTR | 1.23% | 2.68% |
| Unclassified | 38.00% | 26.16% |
| Satellites | 0.12% | 0.31% |
| Simple repeats | 4.36% | 1.12% |
| Low complexity | 0.17% | 0.12% |
Comparative genomics reveals L. polybranchiata deep-sea and pioneer species adaptations
Deep-sea conditions, including a reduced temperature and high hydrostatic pressure, may promote genome size increases that provide genetic material for evolutionary innovation [25, 26]. The genomes of 11 other polychaete worm species were obtained to provide a framework for comparative analyses [21, 27–35]. We identified 11,055 orthologous groups (OGs) with OrthoFinder, and a phylogenetic tree based on 134,940 amino acids of 528 single-copy OGs showed a topology consistent with a recent study that employed mitochondrial genomes in phylogenetic analyses [14]. L. polybranchiata showed a sister group relationship to S. benedicti with high support (bootstrap value: 100) (Fig. 1A). Although the clustering of two Spionidae species and Siboglinidae showed relatively low support (bootstrap value: 60), our results agree with the monophyly of clade Canalipalpata [36]. The divergence time between L. polybranchiata and S. benedicti was estimated at around 244.40 Ma (95% confidence interval of 150.16–346.29 Ma). During this time, magmatism of the Central Atlantic Magmatic Province (CAMP) and the Karoo-Ferrar Large Igneous Province (KFLIP) triggered mass extinctions [37]. Furthermore, the supercontinent Pangea had begun to rift, likely facilitating the colonization of the deep sea by coastal species [38]. These events may have promoted the divergence of deep-sea and shallow-water organisms.
Fig. 1.
Comparative genomics analyses of Lindaspio polybranchiata and other annelids. A Phylogenetic tree and divergence times of annelids. Gray lines indicate the divergence time with the highest posterior density (HPD); red dots, fossil calibrations. B Venn diagram of spionid and siboglinid gene families. C The top 20 enriched gene ontology (GO) terms among expanded L. polybranchiata gene families
A total of 1398 gene families were unique to L. polybranchiata compared to the four other Canalipalpata species in our dataset (Fig. 1B). These gene families are involved in glycosaminoglycan (GAG) and glycosphingolipid (GSL) biosynthesis, and signal transduction (Additional file 1: Fig. S2). Deep-sea cold seeps are rich in hydrogen sulfide (H2S), a gas used by resident bacteria to fix carbon and provide essential sugars and amino acids, but this process also generates sulfur that is toxic at high levels [39, 40]. The unique L. polybranchiata GAG genes are associated with sulfur metabolism and include a homolog of chondroitin 4-sulfotransferase 11 (CHST11) hypothesized to play a role in the detoxification of sulfate to the cell matrix polysaccharide chondroitin 4′-sulfate by a population of the deep-sea vent-inhabiting lobster Shinkaia crosnieri [41]. The unique GSL genes (e.g., FUT1_2) could contribute to a more hydrostatic pressure-resistant cell membrane, a feature observed in studies of deep-sea organisms in the last 50 years [42] (and see “ High hydrostatic pressure tolerance”). Our analysis revealed that 95 shared gene families expanded in the L. polybranchiata genome, while 39 families contracted (Additional file 1: Fig. S3 and Additional file 2: Table S1). We performed GO and KEGG enrichment analyses to predict the functional outcomes of these events. The expanded gene families were primarily associated with processes such as unsaturated fatty acid metabolism, chitin binding, olfactory receptor activity, and chromatin stabilization (e.g., chromatin assembly or disassembly, DNA replication-dependent nucleosome assembly, and nucleosome assembly) (Fig. 1C, Additional file 2: Table S2). Contracted gene families included opsins and cryptochromes, genes associated with the sensory perception of light. Finally, we found that 135 genes with positive selection in L. polybranchiata showed enrichment for gene ontology terms associated with signal transduction, hormone secretion, and endocytosis (Additional file 1: Fig. S4). These genomic changes may reflect evolutionary innovations of L. polybranchiata, some of which are expanded upon below.
Evolution of eye loss
Waters below ~ 100 m lack sunlight and many sedentary deep-sea animals exhibit degenerated or modified eye structure [16, 43, 44]. Species within the Canalipalpata clade Sedentaria are no exception (Fig. 2A). Little research exists on how their eyes were lost, and the extent of genetic convergence that underlies such loss in marine worm lineages that diverged ~ 400 million years ago is unknown.
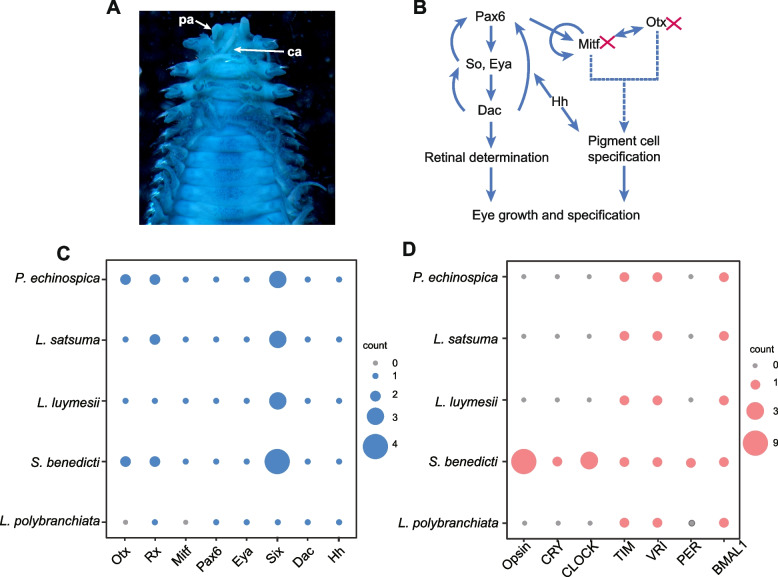
Fig. 2.
Gene families related to vision in marine sedentarians (spionids and siboglinids). A The anterior end of L. polybranchiata, dorsal view. ca, caruncle; pa, palp. B Diagram showing key transcription factors in eye development. Red crosses indicate gene loss in L. polybranchiata, disrupting Mitf and Otx pathways (dashed lines), essential for pigment cell specification. These interruptions may explain the lack of eyes. C The number of genes encoding eye transcription factors. D The number of genes involved in the vision and biological rhythm
All species in Siboglinidae, which inhabit ocean depths from 100 to 10,000 m, also lack eyes [45]. Previous studies have shown that transgenic expression of Ridgeia piscesae Pax6 (RpPax6; a transcription factor) in Drosophila leads to abnormal eye development, by repressing downstream transcription factors (e.g., sine oculis, so; a homolog of the vertebrate SIX homeobox genes) [46]. These findings suggest that similar disruptions in Pax6 function could underlie the loss of eyes in Siboglinidae. Our dataset revealed the amino acid changes in RpPax6 of the three other Siboglinidae species but not in Spionidae or Errantia (Additional file 1: Fig. S5). Next, we examined eye development transcription factors (the majority of which show functional conservation across vertebrates and invertebrates) of L. polybranchiata, the sighted S. benedicti, and the three blind siboglinids in our dataset. Two genes, Otx and Mitf, are only lost in L. polybranchiata (Fig. 2B, C). Additionally, a search for these genes in de novo-assembled transcriptome sequences and PacBio HiFi reads with tblastn yielded no hits, further supporting the loss of Otx and Mitf. Otx (orthodenticle-related homeobox) is essential for the formation of rhabdomeres, light-guiding rods in invertebrate microvillar photoreceptors, and is expressed earlier than any other eye field marker gene in the anterior neuroectoderm [47, 48]. Mitf (microphthalmia-associated transcription factor) acts in concert with Otx to regulate eye formation by supporting peripodial epithelium (PE), functionally analogous to vertebrate retinal pigmented epithelium [49, 50]. The lack of these transcription factor genes was likely essential for the loss of eyes by L. polybranchiata.
Adaptive convergent evolution between L. polybranchiata and Siboglinidae is therefore evident at a higher level, the eye development pathway: essential transcription factors are lost (L. polybranchiata Oxt and Mitf) or show loss-of-function (Siboglinidae Pax6) in distantly related, darkness-adapted species. We also identified a loss of all opsin and cryptochrome (CRY) photoreceptor genes in L. polybranchiata and the three Siboglinidae species in our dataset (Fig. 2D). The downstream photoreceptor genes likely became redundant in deep sea canalipalpates once their eyes were lost. Our results support the theory of convergent gene loss in species with similar ecological pressures (see [51] for review).
Biological rhythms in the deep sea
In L. polybranchiata and siboglinids, we observed loss of the genes encoding the photoreceptor CRY, which CHRONO regulates, and the essential master transcription factor CLOCK [52] (Fig. 2D). In agreement with the loss of their eyes, we speculate that blind, deep-sea canalipalpates do not show light-mediated biological rhythms. However, alternative biological activity mechanisms, including cues like tides and their transmitters, cannot be ruled out. In the amphipod Parhyale hawaiensis and the isopod Eurydice pulchra, the core circadian clock gene BMAL1 is essential for circatidal rhythms [53, 54]. The presence of BMAL1 in L. polybranchiata and three siboglinids suggests that these deep-sea worms may also retain a biological rhythm uncoupled from sunlight. Interestingly, in addition to eye loss, eliminating biological rhythm by the Mexican cavefish Astyanax mexicanus reduces energy consumption by a third compared to sighted surface fish [55]. Similarly, loss of visual and circadian system genes in blind canalipalpate worms could be an adaptation to a nutrient-poor environment.
High hydrostatic pressure tolerance
Hydrostatic pressure increases by ~ 100 kPa every 10 m, and L. polybranchiata is constantly exposed to ~ 17 MPa pressure. Such pressure should affect the cells of deep-sea organisms at all levels, including the cytoskeleton and cell membranes, protein stability, DNA structure, and the process of cell division [56, 57]. In addition to the unique GLS and GAG gene families described above, families associated with cytoskeleton maintenance (i.e., actin cytoskeleton, cortical cytoskeleton, and microtubule cytoskeleton) expanded in the L. polybranchiata genome (Additional file 2: Table S2). We also observed increased gene copy numbers of histone proteins (H1, H2B, H3, and H4), which may serve to sustain chromatin architecture at high pressure [58].
In deep-sea organisms—from bacteria to invertebrates and vertebrates—the composition of the cell membrane, in particular unsaturated fatty acids, appears to be critical to maintaining its structure and function at high hydrostatic pressure [59–61]. Compared to the shallow-water S. benedicti, L. polybranchiata has additional copies of fatty acid synthase (FASN) (three vs. seven copies). FASN plays a pivotal role in fatty acid biosynthesis and catalyzes a series of reactions, synthesizing long-chain fatty acids from acetyl-CoA and malonyl-CoA (Fig. 3A) [62]. The resulting products, myristate (C14:0) and palmitate (C16:0), serve as substrates for chain elongation and desaturation, ultimately producing unsaturated fatty acids [63, 64]. Intriguingly, the FASN copy number also increased in the genomes of other deep-sea inhabitants, including the Mariana Trench snailfish (Pseudoliparis swirei) and the hydrothermal sea anemone (Alvinactis idsseensis) [60, 65]. These findings suggest that extra copies of FASN represent a convergent evolutionary response in deep-sea organisms. Downstream genes, including SCD1 (two copies), FADS1 (one copy), and FADS2 (four copies), are essential for the synthesis of the omega-3 fatty acid eicosapentaenoic acid (EPA) and the balance between marine fatty acid intake and circulating levels of long-chain omega-3 fatty acids (Fig. 3A). Eicosapentaenoic acid (EPA) stabilizes deep-sea marine bacteria under high pressure [66]. Notably, all FADS genes and one SCD1 gene copy were highly expressed in the L. polybranchiata midgut (PB in Fig. 3B). The acyl-CoA oxidase (ACOX) gene family also expanded in L. polybranchiata (p = 0.030). ACOX1 and ACOX3 are involved in β-oxidation, the last step in the synthesis of the omega-3 fatty acid docosahexaenoic acid (DHA) downstream of EPA (Fig. 3A) [67].
Fig. 3.
Fatty acid analysis between deep-sea and shallow-water spionids. A Overview of unsaturated fatty acid synthesis. Expanded gene families of L. polybranchiata marked in pink. B Heatmap showing the expression of fatty acid synthesis genes in L. polybranchiata. CE denotes cephalosome (chaetigers 1–4); AB, anterior body without parapodia (chaetigers 5–20); PB, posterior body without parapodia (chaetigers 40 to end); VB, parapodia with both ventral and dorsal branchiae (chaetigers 40 to end); and DB, parapodia with only dorsal branchiae (chaetigers 5–20). C Fatty acid composition for two sea spionids. SFA, saturated fatty acids; MUFA, monosaturated fatty acids; PUFA, polysaturated fatty acids. D Polyunsaturated fatty acids with significantly different content in the two spionids in C. The data in C and D represent means ± standard deviations from six biological replicates, and asterisks indicate a significant difference (one-way ANOVA, p < 0.05) between the two species
Next, we compared the fatty acid composition of L. polybranchiata and the closely related shallow-sea species Rhynchospio aff. asiatica. The results aligned with the comparative genomic analyses. L. polybranchiata had a greater level of polyunsaturated fatty acids (PUFAs) (p = 0.004, one-way ANOVA; Fig. 3C), and EPA constituted the most abundant fatty acid in L. polybranchiata—accounting for 36.41–45.42% of the total fatty acid content (Fig. 3D). Our results support that PUFAs play a pivotal role in membrane function of deep-sea organisms and signify an evolutionary adaptation to high hydrostatic pressure in polychaetes.
Adaptions for deep sea oligotrophy
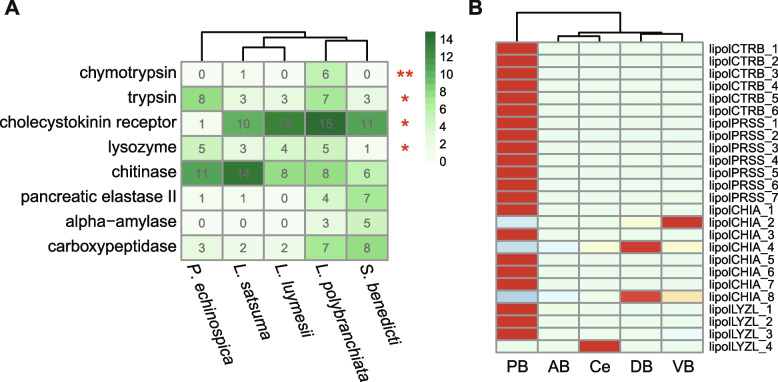
The organic input in pelagic oceans decreases by 90% at a depth of 1000 m [68]. The deep-sea heterotrophic macrofauna must adapt their nutrient absorption and utilization strategies to thrive in this environment [22, 69, 70]. L. polybranchiata is found in large numbers in the Lingshui Cold Seep, further limiting food availability. The peptide hormone cholecystokinin (CCK) stimulates the digestion of fat and protein and modulates appetite and energy balance via its receptor CCKR [71], and the expansion of the CCKR (15 gene copies) may optimize L. polybranchiata food utilization and energy storage. Several digestive enzyme gene families have undergone expansion in L. polybranchiata, including the pivotal proteolytic enzymes trypsin and chymotrypsin [72, 73] (Fig. 4A). These genes were predominantly expressed in the L. polybranchiata midgut (PB region) (Fig. 4B). Their expansion may allow efficient digestion of diverse food sources such as marine snow (biological debris from the top layers of the ocean that includes microorganisms and other nutrient-rich debris [74]) and resident chemosynthetic microorganisms [75, 76]. Bacteria and archaea are primary producers in deep-sea ecosystems and can serve as a crucial nutrient source for macrofauna [77, 78]. Compared with the shallow-water S. benedicti, cell wall-digesting lysozyme genes were expanded in L. polybranchiata (one vs. five gene copies) and deep-sea siboglinids (Fig. 4A). The number of chitinase genes—which digests hard-to-degrade macromolecules of bacteria, fungi, animals, and plants—was also increased in L. polybranchiata. In addition to maximizing nutrient uptake, the digestive enzyme gene duplications may also reflect compensation for reduced kinetic efficiency at low water temperatures (~ 2.5 °C in the case of L. polybranchiata), as proposed for Antarctic fish species [79, 80].
Fig. 4.
Expanded genes associated with digestion in L. polybranchiata. A Heatmap of the number of digestion genes in L. polybranchiata and four other sedentarians. Red stars indicate that the number of genes in L. polybranchiata significantly differs from the shallow-water S. benedicti (one-tailed Fisher’s exact tests, *p < 0.05, **p < 0.01). B Heatmap showing the expression of L. polybranchiata digestive enzyme genes. CRTB denotes chymotrypsin; PRSS, trypsin; CHIA, chitinase; LYZL, lysozyme. Body parts are designated CE (cephalosome: chaetigers 1–4), AB (anterior body without parapodia: chaetigers 5–20), PB (posterior body without parapodia: chaetigers 40 to end), VB (parapodia with both ventral and dorsal branchiae: chaetigers 40 to end), and DB (parapodia with only dorsal branchiae: chaetigers 5–20)
Genomic evidence for L. polybranchiata as a pioneer species
Pioneer species are the first to colonize a newly created or disturbed environment. Infaunal polychaetes are often the first colonizers in early stages of cold seep ecosystems. For instance, in the Hikurangi Margin cold seep, heterotrophic ampharetid polychaetes initially colonized white bacterial mats and sulfide-rich patches [81]. Similarly, in the Congo lobe complex, opportunistic, motile, and sulfide-tolerant taxa such as dorvilleid and hesionid polychaetes led the first wave of colonization. L. polybranchiata is highly abundant in its cold seep habitat and classified as the pioneer [14]. Other spionids were likely pioneers in nearshore ecosystems, including Streblospio gynobranchiata, closely related to S. benedicti in our dataset [82, 83].
These polychaetes are capable of tolerating low-oxygen and high-hydrogen sulfide environments, which enables them to become dominant species. Although we lack genomic data for the mentioned polychaetes, all polychaetes in our data set possess genes related to hydrogen sulfide tolerance and detoxification (Additional file 2: Table S3). In addition to these shared characteristics, our comparative genomic analyses revealed a dynamic gene evolution by spionids (here: L. polybranchiata and S. benedicti), including 59 expanded and ten contracted gene families (Additional file 1: Fig. S4). The expanded gene families showed enrichment for sensory systems, protein digestion and absorption, and endocrine systems (e.g., thyroid hormone signaling pathway, oxytocin signaling pathway, and insulin secretion) gene ontology terms (Additional file 2: Table S4). Some of these are highlighted below.
Rapid growth and reproduction characterize pioneer species. Reproduction of all animals is mediated by steroid and peptide hormones and their receptors (i.e., the endocrine system). Little is known about endocrine signaling pathways in invertebrates beyond model species (e.g., Drosophila), but hormone receptors and their ligands associated with growth and development are considered a universal feature of this large and diverse group [84]. In the genomes of spionids, the gene families encoding hormone receptors associated with the regulation of development and reproduction have expanded, including the growth hormone secretagogue receptor (GHSR), somatostatin receptor (SSTR), and thyrotropin-releasing hormone receptor (TRHR) (Additional file 2: Table S4). Furthermore, endocrine signaling pathways for estrogen, gonadotropin-releasing hormone, and oxytocin are under positive selection in spionids (Additional file 2: Table S5). Because all extant spionids were likely pioneer species, these genomic changes may contribute to the success of these marine worms in their diverse habitats.
The settlement and recruitment of pioneer species of marine polychaetes rely on environmental sensing [85], particularly of microbial communities [86–88]. Chemoreception should be crucial for larval settlement and recruitment (in addition to reproduction and foraging) [89, 90]. Gustatory receptor-like receptors (GRLs) and ionotropic receptors (IRs) are important metazoan chemoreceptor proteins [91, 92]. Homologs of GRL (four genes) and IR (28 genes) were found in spionids genomes. Invertebrate GRLs contain seven transmembrane domains (TMDs), like G protein-coupled receptors but with an inverted membrane topology [93]. L. polybranchiata and S. benedicti harbor two GRL homologs, while none was identified in the three siboglinid genomes examined (Fig. 5A). The GRLs were expressed by the L. polybranchiata cephalosome (which includes its chemosensory antennae) (Fig. 5B). Spionid GRL genes form a single lineage, distributed across two subgroups along with the terrestrial polychaete Capitella teleta (Fig. 5A). Specifically, members of subgroup 1 exhibit a conservative motif order of 5–4–7–2–8–3-1, while subgroup 2 follows the motif order 5–4–6–2–8–3-1 (Fig. 5A). Further supporting their annotation, spionid GRLs harbor the gustatory receptor family signature motif (“TYhhhhhQF,” where “h” represents a hydrophobic amino acid) is present as well as tyrosine (Y) residues TM5–TM7 regions (Additional file 1: Fig. S6) crucial for olfactory receptor ion channel function [94]. The retainment of GRL genes could reflect a central chemoreceptive role by the pioneer species family Spionidae.
Fig. 5.
Chemoreceptors in marine sedentarians (spionids and siboglinids). A Phylogenetic tree of gustatory receptor-like receptors. Motifs were classified using MEME Suite. B Heatmap showing the expression of L. polybranchiata chemoreceptor genes. The prefix GRL denotes gustatory receptor-like receptors; IR, ionotropic receptors. C 3D structure-based tree of candidate IRs from spionids generated using AlphaFold2. Blue molecules indicate the ligand-binding domain of every type, with ligand-binding residues (R, T, D/E) in red. The accession numbers of the reference sequences are in Table S2
Most invertebrates rely more on IRs than GRLs to sense chemicals [95]. The IR gene family exhibited significant expansion in spionids compared with siboglinids (p = 0.022). The IR-like sequences grouped into two clades. Clade I proteins have three domains: an amino-terminal domain (ATD), a ligand-binding domain (LBD), and a transmembrane domain (TMD). Clade II proteins lack an ATD. A phylogenetic tree branched the spionid IRs into three main groups (Additional file 1: Fig. S7). To gain deeper insight into the structure and function of these IR candidates, we employed AlphaFold2 [96] to predict their tertiary structures and generate a structure tree (Fig. 5C). The proteins were classified into six types: types I and IV are specific to the spionids (L. polybranchiata + S. benedicti), and type II is abundant in S. benedicti. The ligand-binding pockets are primarily formed by β-sheets and α-helices, creating a cavity for signal molecule binding. The configurations of these pockets differ in pocket size, shape, and ligand-binding residues (i.e., different ligands) (Fig. 5C). Notably, type VI is a conserved IR25a subclade in the five canalipalpates in our dataset (Fig. 5C). The predicted 3D structures of lipolIR25a align well with canonical models, featuring three conserved domains (Fig. 6A–C). The structure of the LBD in lipolIR25a was highly similar to that of Drosophila melanogaster (root mean square deviation, RMSD, of 0.899 Å) (Fig. 6D). In D. melanogaster, IR25a mediates multiple sensory modalities, including chemical sensing, thermal sensing, and humidity sensing [97, 98]. These similarities suggest that IR25a is essential for the sensory behavior of L. polybranchiata and other marine worms. L. polybranchiata IR genes show distinct expression (Fig. 5B), suggesting they mediate different signals and functions. We propose that the diverse IRs in spionids facilitate the detection of a broad range of chemical signals to trigger responses from settlement to metamorphosis, a prerequisite for spionids to be pioneer species in either deep-sea or shallow ecosystems. The exclusive distribution of L. polybranchiata in chemosynthetic ecosystems hints at a preference for specific microbial communities or bacterial metabolites within sediments. These chemoreceptors may compensate for degraded vision and aid L. polybranchiata in perceiving external chemical stimuli essential for early-stage colonization and survival at the Lingshui Cold Seep.
Fig. 6.
The chemosensory ionotropic receptor IR25a is conserved in marine sedentarians (spionids and siboglinids). A Alignment of the S1 and S2 ligand-binding domains from seven candidate IR25a proteins. Three key ligand-binding residues (R, T, and D/E) are marked with an asterisk; S1 and S2 domains by horizontal orange lines. B Schematic representation of IR25a protein is shown. C AlphaFold2 prediction of L. polybranchiata IR25a. The structure shows distinct domains, including the amino-terminal domain (ATD), the ligand-binding domain (LBD), and the transmembrane domain (TMD). D Alignment of LBD structure of IR25a of L. polybranchiata (red) and Drosophila melanogaster (turquoise). Accession number: phauc_IR25a (XP_059149035), drmel_IR25a (NP_001260049)
Conclusions
The Lindaspio genus represents a fascinating group of spionids endemic to chemosynthetic ecosystems such as hydrothermal vents, methane seeps, and whale falls. Here, we assembled and annotated the genome of Lindaspio polybranchiata from the Lingshui Cold Seep, marking the first genome of a deep-sea Spionidae. Our study also offers a valuable genomic resource and new insights into the ecology and evolution of L. polybranchiata and other deep-sea spionids. However, it is important to acknowledge that the genome assembly quality of L. polybranchiata is not optimal. These limitations can be attributed to several challenges commonly faced in assembling genomes of deep-sea organisms. The extreme conditions of the deep sea, such as high hydrostatic pressure and low temperature, often result in DNA degradation during sampling and transport [99, 100]. Moreover, the inherent genomic complexity of L. polybranchiata, including higher heterozygosity (2.12%), elevated repetitive sequence proportion (59.15%), and expanded gene families, adds to the difficulty of achieving a high-quality assembly [101]. Future efforts are needed to further improve the quality of the genome.
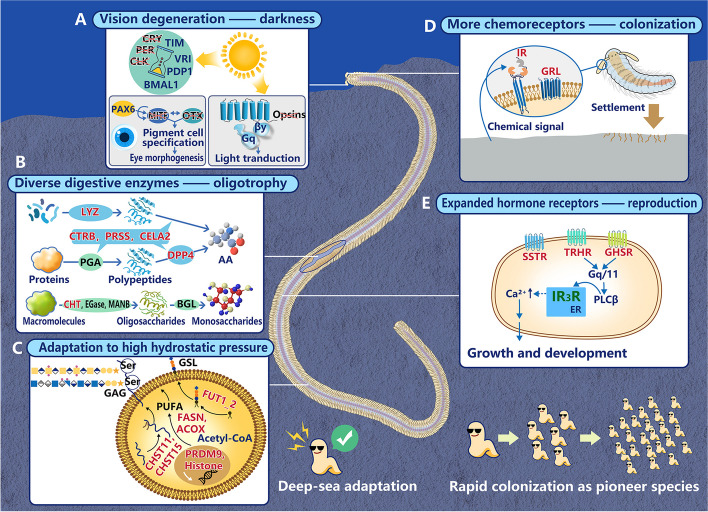
Based on our comparative genomic analyses, we propose a conceptual model illustrating how L. polybranchiata evolved as a pioneer species in the deep sea (Fig. 7). Compared to their shallow-water counterparts, L. polybranchiata shows evidence of adaptation tailored to a deep-sea environment. This includes a reduction in genes related to vision and biological rhythms likely driven by life in perfect darkness but possibly also reducing energy requirements, similar to the Mexican cavefish [55, 102]. In this context, such gene loss may be considered an adaptation to nutrient-poor environments (oligotrophy). Future studies should consider the adaptive benefits of losing ostensibly disused genes in deep-sea animals. L. polybranchiata also shows evidence of an expanded digestive capability, possibly to utilize a broader range of substrates as food, advantageous for survival in a nutrient-limited environment. There is also a modification in membrane lipid composition, specifically an increase in PUFAs, fatty acids that enhance membrane fluidity under high hydrostatic pressure.
Fig. 7.
A conceptual model illustrating how L. polybranchiata evolved as a pioneer species in the deep sea. The red crosses indicate gene loss, while red highlights denote expanded gene families. A The sun with arrows illustrates light activating circadian rhythm genes and triggering light transduction via opsins, while darkness leads to vision degeneration. The pie with an hourglass symbolizes circadian rhythm and the core circadian clock genes were lost. B Diverse digestive enzymes break down macromolecules into absorbable nutrients, supporting adaptation to oligotrophic conditions. C The synthesis pathways of unsaturated fatty acids, GSLs, and GAGs, involving unique and expanded gene families, enhance membrane fluidity in response to high hydrostatic pressure. D The worm model represents the larval stage, where expanded IRs and GRLs facilitate the perception of chemical cues for colonization in the sediment. E The gene families encoding hormone receptors, regulating development and reproduction have expanded
L. polybranchiata is a pioneer species in its nascent cold seep, but most spionids are considered pioneer species. Their pioneer status is strengthened by shared genomic features, such as an expanded chemoreceptor repertoire that likely aids in detecting various chemical cues, facilitating the colonization of new habitats. Additionally, an increase in spionid hormone receptors may regulate development and reproduction, supporting population propagation. Our findings provide a molecular foundation for understanding how spionids perceive chemical signals in their environment, settle in suitable habitats, and establish abundant populations.
Methods
Sampling and sequencing
Lindaspio polybranchiata were collected using a remotely operated vehicle (ROV) in the Lingshui Cold Seep at 1700 m depth (Additional file 1: Fig. S8). The specimen (n = 1) was rinsed with sterile water before being flash-frozen in liquid nitrogen and then stored at − 80 °C for subsequent analysis. For whole-genome resequencing, genomic DNA was extracted by phenol–chloroform extraction, which was used to construct DNA fragment libraries with a TruSeq DNA PCR-Free library Prep kit and then sequenced on the Illumina NovaSeq 6000 platforms. For PacBio HiFi sequencing, DNA was extracted with DNeasy Blood & Tissue Kit (QIAGEN). After obtaining high-quality genomic DNA, a PCR-free SMRT bell library was constructed, and sequencing was performed using the PacBio Sequel II sequencing platform by Annoroad Gene Technology, Beijing, to generate 97.4 Gb raw bases and 869.7 Gb subread bases.
Genome assembly
We used FastQC v0.11.5 [103] to assess the quality of raw Illumina data and Trimmomatic v0.36 [104] to filter the raw Illumina data and obtain clean data with the parameter as “LEADING:5 TRAILING:5 SLIDINGWINDOW:5:20 MINLEN:50 TOPHRED33.” K-mer frequency-based method was used to determine genome size, heterozygosity ratio, and repeat content. In detail, the K-mers were counted using Jellyfish v2.2.3 [105] with the parameters “-m 17,” “-m 19,” and “-m 21.” The resulting k-mer frequency histogram was then used to estimate genome size in GenomeScope [106] with the parameters “-k 17 -p 2,” “-k 19 -p 2,” and “-k 21 -p 2.” The results from all three evaluations were similar (Additional file 1: Fig. S9). Next, CCS v. 4.2.0 (https://github.com/PacificBiosciences/ccs) was used to filter subreads data, retaining 46.92 gigabase pairs (Gbp) PacBio HiFi reads. We assembled the L. polybranchiata using Hifiasm v0.19.4 with HiFi reads [107] and used Purge Haplotigs v1.1.2 [108] to remove redundant sequences and generate a draft genome. Benchmarking Universal Single-Copy Orthologs (BUSCO) v5.2.2 with the metazoan_odb10 dataset was used to assess the completeness of the genome draft [109].
Genome prediction and functional annotation
Before gene model prediction, repetitive sequences were identified by RepeatModeler v2.0.1 and RepeatMasker v4.1.2 [110] based on the Repbase v21.01 and Dfam v3.5 database [111, 112]. The repetitive regions were soft-masked for later gene model prediction. For ab initio gene prediction, the clean transcriptome (RNA-seq) reads were mapped to the genomes with HISAT2 v2.2.1 [113], and the mapping results and homologous protein sequences were used to train Augustus v3.4.0 in Braker2 v3.0.3 [114, 115]. Moreover, the EST evidence assembled with Trinity v2.8.5 [116] with default parameters and protein homology evidence were used for genome model prediction with MAKER v3.1.4 [117]. For transcript-based prediction, we used Stringtie v2.1.4 [118] to obtain the gene annotation files based on RNA-seq alignments generated by HISAT2 v2.2.1 and then used Transdecoder v5.5.0 to predict gene structure information [113]. The final version of protein-coding genes was generated by integrating all evidence in EVidenceModeler v2.1.0 [119]. Gene functional annotation was performed by searching the following databases: National Center for Biotechnology Information (NCBI) NR v202304, Swiss-Prot v202304, eggNOG v5.0, Kyoto Encyclopedia of Genes and Genomes (KEGG) v87.0, and HMMER v3.3.1 (Additional file 1: Fig. S1).
Molecular phylogeny and gene family analysis
To determine the phylogenetic position of L. polybranchiata, we downloaded the genomes of 11 annelids from NCBI (Additional file 2: Table S6). We used OrthoFinder v2.5.4 to identify orthologs, using BLASTP program with a threshold value of e-value ≤ 1e − 9 and query-coverage ≥ 0.5 [120]. According to the results of gene family clustering, 528 single-copy orthologous genes were used for phylogenetic analyses. For each ortholog group, the amino acid sequences were aligned with “linsi” of MAFFT v7.520 [121]. Then, we used Gblocks 0.91b to extract conserved blocks with default settings [122]. These conserved blocks were concatenated to construct a phylogenetic tree with the maximum likelihood method under the PROTGAMMAGTRX model using RAxML v8.2.12 applying 1000 bootstrap replicates [123]. Furthermore, the divergence time was calculated with the MCMCtree program in PAML, with settings nburn-in = 10,000,000, nsamfreq = 100, and nsample = 500,000 [124]. Four fossil correction points were used: Oweniidae appeared about 514 million years ago (Ma); Eisenia andrei and Helobdella robusta diverged at 295.9–345.0 Ma; Sedentaria and Errantia diverged 207.5–513.5 Ma; Siboglinidae appeared about 50–126 Ma (http://www.timetree.org/). Fossil calibrations are marked in Fig. 1A.
CAFE (Computational Analysis of gene Family Evolution) v4.2.1 was used for gene family evolution analysis based on the OrtherFinder results, and only orthologous groups with a p value < 0.05 were considered significantly expanded or contracted compared to the ancestor in each node [125]. Expanded and contracted gene families in L. polybranchiata compared to the shallow-water Streblospio benedicti were tested for gene ontology (GO) and KEGG enrichment with OmicShare tools (https://www.omicshare.com/tools).
Positive selection analysis
Positive selection analysis was conducted using the codeml package in PAML v4.9 [124]. The phylogenomic results were used as the species tree to guide the analysis. To identify candidate positively selected genes (PSGs), we compared the likelihood of selected genes using the YN00 model and the branch-site model, with L. polybranchiata designated as the foreground and other annelids as the background. To pinpoint potential positive selection on the foreground branch, a likelihood ratio test (LRT) was performed to compare two proposed positive selection sites. p values were calculated using chi-square (χ2) statistics (df = 1; FDR corrected) and genes with a p value < 0.05 and a Bayesian probability > 90% were classified as PSGs. GO and KEGG enrichment analyses were performed following the same protocols as those used for the expansion of gene families.
Chemoreceptor and photoreceptor gene annotation
The chemoreceptor sequences of the superphylum Lophotrochozoa (including Annelida) were downloaded from NCBI and UniProtKB databases. All sequences were aligned to the genomes using tblastn v2.14.1 [126] with an e-value of 1e − 8. We extracted the candidate gene regions and their 20-kb flanking sequences and used GeneWise v2.4.1 [127] to predict the complete gene structure. HMMSCAN (HMMER v3.3.2) was applied to identify Pfam domains in protein-coding genes. Finally, all candidate protein sequences were aligned with MAFFT v7.505 [121], and the maximum likelihood tree was constructed with FastTree v2.1.11 with default setting [128]. The information of the reference sequences used for tree construction is provided in Additional file 2: Table S7. The motifs of these candidate proteins were identified using meme in MEME Suite v5.5.7 [129]. The gene family expansion or contraction analyses in the chemoreceptor gene family were conducted using Fisher’s exact test (one-tailed) in R 3.6.2 with the stats package [130]. To better understand the structure and function of these candidate proteins, we used AlphaFold2 v2.0.1 to predict their 3D structures and constructed a structural tree [96].
The photoreceptor gene family was searched following the same pipeline. Moreover, we search the lost photoreceptor genes in de novo-assembled transcriptome sequences and PacBio HiFi reads using tblastn v2.14.1 [126] at the thresholds of ≥ 30% amino acid identity, ≤ 1e − 5 e-value, and ≥ 60% query cover per subject. Candidate transcript ORFs were predicted and translated into amino acid sequences using TBtools [131]. For candidate HiFi reads, GeneWise v2.4.1 [122] was used to predict complete gene structures. Then, all candidate proteins were analyzed for Pfam domains using HMMSCAN (HMMER v3.3.2) and aligned with MAFFT v7.505 [116] to examine key residues.
Transcriptome sequencing and analysis
The body of Spionidae is elongated, with no distinct segmentation, composed of a trunk and parapodial appendages. L. polybranchiata is characterized by a distinct caruncle (a sensory organ extending to chaetiger 2), modified notopodial spines (chaetigers 2–4), and more neuropodial branchiae in the parapodia (from chaetiger 20) (Additional file 1: Fig. S10) [15]. To facilitate genome annotation and provide a transcriptional profile along the body axis of L. polybranchiata, worms (n = 6) were sectioned into five parts [15]: CE (cephalosome, chaetigers 1–4), AB (anterior body without parapodia, chaetigers 5–20), PB (posterior body without parapodia, chaetigers 40 to end), VB (parapodia with both ventral and dorsal branchiae, chaetigers 40 to end), and DB (parapodia with only dorsal branchiae, chaetigers 5–20). According to the standard manufacturer’s protocol, total RNA was extracted using TRIzol reagent (Invitrogen). RNA quality was determined by 1% agarose gel electrophoresis and Agilent 5400 (Agilent, USA), while RNA concentration was measured with a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, USA). Transcriptome libraries were generated using NEBNext Ultra RNA Library Prep Kit (NEB, USA) and then sequenced on the Illumina platform by Novogene Bioinformatics Technology Company (Beijing, China) to produce 150 bp reads, yielding approximately 10 Gb of raw data per sample. The RNA-seq data was filtered with fastp v0.23.4 with the following parameters: “–detect_adapter_for_pe –cut_mean_quality 20 –length_required 50 −5 −3 -W 4 -e 20” [132] and aligned to the reference genome with HISAT2 v2.2.1 with default parameters [113]. Then, transcripts per kilobase million (TPM) were calculated using Cufflinks v2.1.1 [133]. The heatmap of expression levels across different tissues was generated with the pheatmap package in R 3.6.2 [134].
Fatty acid analysis
To compare the difference in fatty acid (FA) composition between L. polybranchiata and shallow-water spionids, we collected a closely related species, Rhynchospio aff. asiatica, in the intertidal region of Qingdao Bay. The specimens of L. polybranchiata (n = 6) and R. aff. asiatica (n = 6) were dried in a freeze dryer for 24 h. We weighed 2–5 mg of dried tissue for FAs analysis. FAs were extracted at 20 °C for 12 h in 3 ml extraction solution with mixed solution of dichloromethane, chloroform, and methanol in equal volumes. At the beginning of the extraction, 100 μl of C19:0 and C21:0 was added to each sample as an internal standard. We washed the extract with a 2.25 ml 1 M solution of potassium chloride, collected the lower liquid phase, and blew out the residual water using a nitrogen blower. Next, 100 μl toluene and 200 µl methanol (supplemented with 1% concentrated sulfuric acid) were added to every sample. The esterification reaction was continued at 50 °C for 12 h. The fatty acid methyl esters (FAMEs) were then washed with 600 µl 5% NaCl solution and 200 µl hexane. The upper layer of clarified liquid was collected into a new glass centrifuge tube and 100 μl of hexane was added to redissolve the FAMEs. Finally, the FAMEs were analyzed by gas chromatography on a mass spectroscopy ISQ 7000 instrument (Thermo Fischer Scientific, Waltham, USA) using hydrogen as carrier gas.
Supplementary Information
Additional file 1: Fig. S1. The gene numbers annotated by multiple databases. Fig. S2. The KEGG enrichment of unique gene families in L. polybranchiata. Fig. S3. Phylogenetic tree and gene family analysis of L. polybranchiata and other annelids. Fig. S4. KEGG enrichment of genes under positive selection in L. polybranchiata. Fig. S5. Alignment of paired domain of Pax6. Fig. S6. Alignment of TM5–7 domains of GRLs from L. polybranchiata, S. benedicti, and C. teleta. Fig. S7. Phylogenetic tree of candidate IRs across Canalipalpata. Fig. S8. Location of sampling stations. Fig. S9. K-mer distribution of the Lindaspio polybranchiata genome sequences. Fig. S10. Anatomical regions of L. polybranchiata used for transcriptomic analysis.
Additional file 2: Table S1. The gene family analysis results with CAFE. Table S2. The GO enrichment of expanded gene families in L. polybranchiata. Table S3. Gene counts related to hydrogen sulfide tolerance and detoxification. Table S4. The KEGG enrichment of expanded gene families in spionids. Table S5. The KEGG enrichment of positively selected genes in spionids. Table S6. Taxonomic information and genome sizes for the annelids used in this study. Table S7. The reference sequences of chemoreceptor.
Acknowledgements
We appreciate the assistance provided by the crews on Research Vessel ‘Kexue’. We are also grateful to Xinjiang Wan for his help with software installation.
Abbreviations
- CRY
Cryptochrome
- PER
Period
- CLK
CLOCK
- TIM
Timeless
- VRI
Vrille
- PDP1
Par domain protein 1
- OTX
Orthodenticle-related homeobox
- MITF
Microphthalmia-associated transcription factor
- CRTB
Chymotrypsin
- PRSS
Trypsin
- CHIA
Chitinase
- LYZL
Lysozyme
- DPP4
Dipeptidyl-peptidase 4
- EGase
β-1,4-Endoglucanase
- MANB
Mannosidase
- BGL
β-Glucosidase
- CELA2
Pancreatic elastase II
- GAG
Glycosaminoglycan
- GSL
Glycosphingolipid
- CHST11
Chondroitin 4-sulfotransferase 1
- CHST15
N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase
- FASN
Fatty acid synthase
- ACOX
Acyl-CoA oxidase
- FUT1_2
Galactoside 2-L-fucosyltransferase
- PRDM9
PR/SET domain containing protein 9
- IR
Ionotropic receptors
- GRL
Gustatory receptor-like receptors
- SSTR
Somatostatin receptor
- TRHR
Thyrotropin-releasing hormone receptor
- GHSR
Growth hormone secretagogue receptor
Authors' contributions
Y. Y., M. W., and C. L. conceived the idea. Y. Y., H. W., M. L., Z. Z., and H. Z. collected the sample. Y. Y. prepared DNA sequencing and performed genomic and transcriptomic analyses. Y. G. performed genome assembly. D. W. performed genome structure analyses. Y. Y wrote the manuscript and additional supplementary files. C. L., M. W., I. S., and Y. G. revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (42030407 and 42076091), the NSFC Innovative Group Grant (42221005), the National Key R&D Program of China (2022YFC2804003), the National Key R&D Program of China (2023YFC2811501), Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2022QNLM030004), and State Key Laboratory of Microbial Technology Open Projects Fund (Project NO. M2023-10).
Data availability
The assemblies and protein sequences of published genome used in this study were from the NCBI repository: Streblospio benedicti (GCA_019095985.1) [21], Paraescarpia echinospica (GCA_020002185.1) [28], Lamellibrachia luymesi (GCA_009193005.1) [29], Lamellibrachia satsuma (GCA_022478865.1) [33], Branchipolynoe longqiensis (GCA_030323885.1) [34], Harmothoe impar (GCA_947462335.1) [35], Capitella teleta (GCA_000328365.1) [31], Helobdella robusta (GCA_000326865.1) [31], Owenia fusiformis (GCA_903813345.2) [27], Dimorphilus gyrociliatus (GCA_904063045.1) [27], and from the National Genomics Data Center: Eisenia andrei (GWHACBE00000000) [32]. Genome assembly and annotation of the Lindaspio polybranchiata are available at Figshare [135]. All sequencing data for the genome and transcriptome are also available at the CNGB Sequence Archive (CNSA) of the China National GeneBank DataBase (CNGBdb) under project CNP0006250 [136] and National Center for Biotechnology Information (NCBI) under project PRJNA1188207 [137]. The computer commands were shared on GitHub [138].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Minxiao Wang, Email: wangminxiao@qdio.ac.cn.
Chaolun Li, Email: lcl@qdio.ac.cn.
References
- 1.Danovaro R, Corinaldesi C, Dell’Anno A, Snelgrove PVR. The deep-sea under global change. Curr Biol. 2017;27(11):R461–5. [DOI] [PubMed] [Google Scholar]
- 2.Rabone M, Wiethase JH, Simon-Lledó E, Emery AM, Jones DOB, Dahlgren TG, Bribiesca-Contreras G, Wiklund H, Horton T, Glover AG. How many metazoan species live in the world’s largest mineral exploration region? Curr Biol. 2023;33(12):2383–96. [DOI] [PubMed] [Google Scholar]
- 3.Guggolz T, Meißner K, Schwentner M, Dahlgren TG, Wiklund H, Bonifácio P, Brandt A. High diversity and pan-oceanic distribution of deep-sea polychaetes: Prionospio and Aurospio (Annelida: Spionidae) in the Atlantic and Pacific Ocean. Org Divers Evol. 2020;20:171–87. [Google Scholar]
- 4.Walker LM. Polydora and Dipolydora (Polychaeta Spionidae) of estuaries and bays of subtropical eastern Australia: a review and morphometric investigation of their taxonomy and distribution. Lismore: Southern Cross University; 2008. [Google Scholar]
- 5.David AA, Williams JD. Asexual reproduction and anterior regeneration under high and low temperatures in the sponge associate Polydora colonia (Polychaeta: Spionidae). Invertebr Reprod Dev. 2012;56(4):315–24. [Google Scholar]
- 6.Birch GF, O’Donnell MA, McCready S. Complex relationships between shallow muddy benthic assemblages, sediment chemistry and toxicity in estuaries in southern New South Wales, Australia. Mar Pollut Bull. 2018;129(2):573–91. [DOI] [PubMed] [Google Scholar]
- 7.Graff JR, Blake JA, Wishner KF. A new species of Malacoceros (Polychaeta: Spionidae) from Kick’em Jenny, a hydrothermally active submarine volcano in the Lesser Antilles Arc. J Mar Biol Assoc U K. 2008;88(5):925–30. [Google Scholar]
- 8.Guggolz T, Meißner K, Schwentner M, Brandt A. Diversity and distribution of Laonice species (Annelida: Spionidae) in the tropical North Atlantic and Puerto Rico Trench. Sci Rep. 2019;9(1):9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hourdez S, Desbruyères D, Laubier L, Gardiner SL. Malacoceros samurai, a new species of Spionidae (Annelida: Polychaeta) from hydrothermal vent chimney walls on the south East Pacific Rise. Proc Biol Soc Wash. 2006;119(4):592–9. [Google Scholar]
- 10.Bellan G, Dauvin JC, Laubier L. The genus Lindaspio (Annelida: Polychaeta: Spionidae), and a new species from an oil field off Congo, western Africa. J Nat Hist. 2003;37(20):2413–24. [Google Scholar]
- 11.Blake JA, Maciolek NJ. Polychaeta from deep-sea hydrothermal vents in the Eastern Pacific. III: a new genus and two new species of Spionidae from the Guaymas Basin and Juan de Fuca ridge with comments on a related species from the western North Atlantic. Proc Biol Soc Wash. 1992;105(4):723–32. [Google Scholar]
- 12.Sumida PY, Alfaro-Lucas JM, Shimabukuro M, Kitazato H, Perez JA, Soares-Gomes A, Toyofuku T, Lima AO, Ara K, Fujiwara Y. Deep-sea whale fall fauna from the Atlantic resembles that of the Pacific Ocean. Sci Rep. 2016;6:22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Q, Liu X, Wang J, Chen T, Han Y, Zhang L, Li S, Zhao J, Dong Y, Guo B. Microbial succession, community assembly and adaptation over five years in a newly discovered deep-sea cold seep. bioRxiv. 2024.10.31.619006. 10.1101/2024.10.31.619006.
- 14.Yan Y, Wang M, Wu X, Wang H, Zhong Z, Li C. Mitochondrial and morphological adaptions of Lindaspio polybranchiata (Annelida: Spionidae) in the South China Sea. Mar Ecol Prog Ser. 2024;730:43–58. [Google Scholar]
- 15.Sui J, Dong D, Wu X, Li X. A new species of the genus Lindaspio Blake & Maciolek, 1992 (Annelida, Spionidae) from a cold seep near Hainan Island, China. Zookeys. 2023;1153:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez BC, Martínez A, Worsaae K, Osborn KJ. Morphological convergence and adaptation in cave and pelagic scale worms (Polynoidae, Annelida). Sci Rep. 2021;11(1):10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Dev Biol. 2001;231(1):1–12. [DOI] [PubMed] [Google Scholar]
- 18.Soares D, Niemiller ML. Sensory adaptations of fishes to subterranean environments. Biosci. 2013;63(4):274–83. [Google Scholar]
- 19.Liu Z, Huang Y, Chen H, Liu C, Wang M, Bian C, Wang L, Song L. Chromosome-level genome assembly of the deep-sea snail Phymorhynchus buccinoides provides insights into the adaptation to the cold seep habitat. BMC Genomics. 2023;24(1):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musilova Z, Cortesi F, Matschiner M, Davies WIL, Patel JS, Stieb SM, de Busserolles F, Malmstrøm M, Tørresen OK, Brown CJ, et al. Vision using multiple distinct rod opsins in deep-sea fishes. Science. 2019;364(6440):588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakas C, Harry ND, Scholl EH, Rockman MV, Lavrov D. The genome of the poecilogonous annelid Streblospio benedicti. Genome Biol Evol. 2022;14(2):evac008. [DOI] [PMC free article] [PubMed]
- 22.Yuan J, Zhang X, Kou Q, Sun Y, Liu C, Li S, Yu Y, Zhang C, Jin S, Xiang J, et al. Genome of a giant isopod, Bathynomus jamesi, provides insights into body size evolution and adaptation to deep-sea environment. BMC Biol. 2022;20(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Meng L, Wang M, Zhong Z, Li D, Zhang Y, Li H, Zhang H, Seim I, Li Y, et al. Hologenome analysis reveals independent evolution to chemosymbiosis by deep-sea bivalves. BMC Biol. 2023;21(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Zhang Y, Meng L, Fan G, Bai J, Chen J, Song Y, Seim I, Wang C, Shao Z, et al. Genome sequencing of deep-sea hydrothermal vent snails reveals adaptions to extreme environments. Gigascience. 2020;9(12):giaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie H, Jamieson AJ, Piertney SB. Genome size variation in deep-sea amphipods. R Soc Open Sci. 2017;4(9):170862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chénais B, Caruso A, Hiard S, Casse N. The impact of transposable elements on eukaryotic genomes: from genome size increase to genetic adaptation to stressful environments. Gene. 2012;509(1):7–15. [DOI] [PubMed] [Google Scholar]
- 27.Martín-Zamora FM, Liang Y, Guynes K, Carrillo-Baltodano AM, Davies BE, Donnellan RD, Tan Y, Moggioli G, Seudre O, Tran M. Annelid functional genomics reveal the origins of bilaterian life cycles. Nature. 2023;615(7950):105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Sun J, Yang Y, Lan Y, Ip JC, Wong WC, Kwan YH, Zhang Y, Han Z, Qiu JW, et al. Genomic signatures supporting the symbiosis and formation of chitinous tube in the deep-sea tubeworm Paraescarpia echinospica. Mol Biol Evol. 2021;38(10):4116–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dattagupta S, Miles LL, Barnabei MS, Fisher CR. The hydrocarbon seep tubeworm Lamellibrachia luymesi primarily eliminates sulfate and hydrogen ions across its roots to conserve energy and ensure sulfide supply. J Exp Biol. 2006;209(Pt 19):3795–805. [DOI] [PubMed] [Google Scholar]
- 30.Martín-Durán JM, Vellutini BC, Marlétaz F, Cetrangolo V, Cvetesic N, Thiel D, Henriet S, Grau-Bové X, Carrillo-Baltodano AM, Gu W. Conservative route to genome compaction in a miniature annelid. Nat Ecol Evol. 2021;5(2):231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simakov O, Marletaz F, Cho S-J, Edsinger-Gonzales E, Havlak P, Hellsten U, Kuo D-H, Larsson T, Lv J, Arendt D. Insights into bilaterian evolution from three spiralian genomes. Nature. 2013;493(7433):526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao Y, Wang X-B, Zhang J-J, Li M-L, Wu S-S, Ma X-Y, Wang X, Zhao H-F, Li Y, Zhu HH. Genome and single-cell RNA-sequencing of the earthworm Eisenia andrei identifies cellular mechanisms underlying regeneration. Nat Commun. 2020;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida T, Yoshioka Y, Yoshida Y, Fujie M, Yamaki A, Sasaki A, Inoue K, Shinzato C. Genomic and transcriptomic analyses illuminate the molecular basis of the unique lifestyle of a tubeworm, Lamellibrachia satsuma. DNA Res. 2023;30(4):dsad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X, Wang H, Xu T, Zhang Y, Chen C, Sun Y, Qiu JW, Zhou Y, Sun J. Genomic analysis of a scale worm provides insights into its adaptation to deep-sea hydrothermal vents. Genome Biol Evol. 2023;15(7):evad125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adkins P, Mrowicki R, Harley J, Lab MBAGA, of Life WSIT, Consortium DToL. The genome sequence of a scale worm, Harmothoe impar (Johnston, 1839). Wellcome Open Res. 2023;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weigert A, Bleidorn C. Current status of annelid phylogeny. Org Divers Evol. 2016;16(2):345–62. [Google Scholar]
- 37.Schoepfer SD, Algeo TJ, van de Schootbrugge B, Whiteside JH. The Triassic–Jurassic transition – a review of environmental change at the dawn of modern life. Earth Sci Rev. 2022;232:104099.
- 38.Bardet N, Falconnet J, Fischer V, Houssaye A, Jouve S, Pereda Suberbiola X, Pérez-García A, Rage JC, Vincent P. Mesozoic marine reptile palaeobiogeography in response to drifting plates. Gondwana Res. 2014;26(3–4):869–87. [Google Scholar]
- 39.Li WL, Dong X, Lu R, Zhou YL, Zheng PF, Feng D, Wang Y. Microbial ecology of sulfur cycling near the sulfate–methane transition of deep-sea cold seep sediments. Environ Microbiol. 2021;23(11):6844–58. [DOI] [PubMed] [Google Scholar]
- 40.Cao L, Lian C, Zhang X, Zhang H, Wang H, Zhou L, Wang M, Chen H, Luan Z, Li C. In situ detection of the fine scale heterogeneity of active cold seep environment of the Formosa Ridge, the South China Sea. J Mar Syst. 2021;218:103530. [Google Scholar]
- 41.Xiao Y, Xu T, Sun J, Wang Y, Wong WC, Kwan YH, Chen C, Qiu J-W, Qian P-Y. Population genetic structure and gene expression plasticity of the deep-sea vent and seep squat lobster Shinkaia crosnieri. Front Mar Sci. 2020;7:7. [Google Scholar]
- 42.Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788(1):184–93. [DOI] [PubMed] [Google Scholar]
- 43.Williams ST, Noone ES, Smith LM, Sumner-Rooney L. Evolutionary loss of shell pigmentation, pattern, and eye structure in deep-sea snails in the dysphotic zone. Evolution. 2022;76:3026. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Yasuhara M, Wei CL, Tian SY, Aye KKT, Gemery L, Cronin TM, Frenzel P, Horne DJ. Sight and blindness: the relationship between ostracod eyes, water depth, and light availability in the Arctic Ocean. Limnol Oceanogr. 2024;69(6):1418–28. [Google Scholar]
- 45.Rouse G. A cladistic analysis of Siboglinidae Caullery, 1914 (Polychaeta, Annelida): formerly the phyla Pogonophora and Vestimentifera. Zool J Linn Soc. 2001;132(1):55–80. [Google Scholar]
- 46.Yuan H, Wang W, Hu B, Pan C, Chen M, Ke L, Yang L, Chen J. Cloning and functional analysis of Pax6 from the hydrothermal vent tubeworm Ridgeia piscesae. PLoS ONE. 2016;11(12): e0168579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diacou R, Nandigrami P, Fiser A, Liu W, Ashery-Padan R, Cvekl A. Cell fate decisions, transcription factors and signaling during early retinal development. Prog Retin Eye Res. 2022;91:101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandendries ER, Johnson D, Reinke R. OrthodenticleIs is required for photoreceptor cell development in the Drosophila eye. Dev Biol. 1996;173(1):243–55. [DOI] [PubMed] [Google Scholar]
- 49.Zhang T, Zhou Q, Jusić N, Lu W, Pignoni F, Neal SJ. Mitf, with Yki and STRIPAK-PP2A, is a key determinant of form and fate in the progenitor epithelium of the Drosophila eye. Eur J Cell Biol. 2024;103(2):151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallsson JH, Haflidadóttir BS, Stivers C, Odenwald W, Arnheiter H, Pignoni F, Steingrímsson E. The basic helix-loop-helix leucine zipper transcription factor Mitf is conserved in Drosophila and functions in eye development. Genetics. 2004;167(1):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albalat R, Cañestro C. Evolution by gene loss. Nat Rev Genet. 2016;17(7):379–91. [DOI] [PubMed] [Google Scholar]
- 52.McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107(5):567–78. [DOI] [PubMed] [Google Scholar]
- 53.Kwiatkowski ER, Schnytzer Y, Rosenthal JJC, Emery P. Behavioral circatidal rhythms require Bmal1 in Parhyale hawaiensis. Curr Biol. 2023;33(10):1867–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Copenhaver GP, Lin Z, Green EW, Webster SG, Hastings MH, Wilcockson DC, Kyriacou CP. The circadian clock gene bmal1 is necessary for co-ordinated circatidal rhythms in the marine isopod Eurydice pulchra (Leach). PLoS Genet. 2023;19(10):e1011011. [DOI] [PMC free article] [PubMed]
- 55.Moran D, Softley R, Warrant EJ. Eyeless Mexican cavefish save energy by eliminating the circadian rhythm in metabolism. PLoS ONE. 2014;9(9):e107877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meersman F, Heremans K. High hydrostatic pressure effects in the biosphere: from molecules to microbiology. In: Chris Michiels DHB, Abram Aertsen, editor. High‐pressure microbiology. 2008:1–17.
- 57.Mestre NC, Calado R, Soares AMVM. Exploitation of deep-sea resources: the urgent need to understand the role of high pressure in the toxicity of chemical pollutants to deep-sea organisms. Environ Pollut. 2014;185:369–71. [DOI] [PubMed] [Google Scholar]
- 58.Frey B, Janko C, Ebel N, Meister S, Schlucker E, Meyer-Pittroff R, Fietkau R, Herrmann M, Gaipl US. Cells under pressure-treatment of eukaryotic cells with high hydrostatic pressure, from physiologic aspects to pressure induced cell death. Curr Med Chem. 2008;15(23):2329–36. [DOI] [PubMed] [Google Scholar]
- 59.Tamby A, Sinninghe Damsté JS, Villanueva L. Microbial membrane lipid adaptations to high hydrostatic pressure in the marine environment. Front Mol Biosci. 2023;9:1058381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Liu H, Feng C, Lu Z, Liu J, Huang Y, Tang H, Xu Z, Pu Y, Zhang H. Genetic adaptations of sea anemone to hydrothermal environment. Sci Adv. 2023;9(42):eadh0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loor JJ, Parzanini C, Parrish CC, Hamel J-F, Mercier A. Functional diversity and nutritional content in a deep-sea faunal assemblage through total lipid, lipid class, and fatty acid analyses. PLoS One. 2018;13(11):e0207395. [DOI] [PMC free article] [PubMed]
- 62.Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003;42(4):289–317. [DOI] [PubMed] [Google Scholar]
- 63.Monroig Ó, Kabeya N. Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: a comprehensive review. Fish Sci. 2018;84(6):911–28. [Google Scholar]
- 64.Kainz M, Brett MT, Arts MT. Lipids in aquatic ecosystems. Verlag New York: Springer; 2009. [Google Scholar]
- 65.Wang K, Shen Y, Yang Y, Gan X, Liu G, Hu K, Li Y, Gao Z, Zhu L, Yan G, et al. Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nat Ecol Evol. 2019;3(5):823–33. [DOI] [PubMed] [Google Scholar]
- 66.Usui K, Hiraki T, Kawamoto J, Kurihara T, Nogi Y, Kato C, Abe F. Eicosapentaenoic acid plays a role in stabilizing dynamic membrane structure in the deep-sea piezophile Shewanella violacea: a study employing high-pressure time-resolved fluorescence anisotropy measurement. Biochim Biophys Acta. 2012;1818(3):574–83. [DOI] [PubMed] [Google Scholar]
- 67.Su H-M, Moser AB, Moser HW, Watkins PA. Peroxisomal straight-chain acyl-CoA oxidase and D-bifunctional protein are essential for the retroconversion step in docosahexaenoic acid synthesis. J Biol Chem. 2001;276(41):38115–20. [DOI] [PubMed] [Google Scholar]
- 68.Arístegui J, Gasol JM, Duarte CM, Herndld GJ. Microbial oceanography of the dark ocean’s pelagic realm. Limnol Oceanogr. 2009;54(5):1501–29. [Google Scholar]
- 69.Li J, Zhan Z, Li Y, Sun Y, Zhou T, Xu K. Chromosome-level genome assembly of a deep-sea Venus flytrap sea anemone sheds light upon adaptations to an extremely oligotrophic environment. Mol Ecol. 2024;33(18):e17504. [DOI] [PubMed] [Google Scholar]
- 70.Ginn F, Beisel U, Barua M. Flourishing with awkward creatures: togetherness, vulnerability, killing. Environ Humanities. 2014;4(1):113–23. [Google Scholar]
- 71.Staljanssens D, Azari EK, Christiaens O, Beaufays J, Lins L, Van Camp J, Smagghe G. The CCK (-like) receptor in the animal kingdom: functions, evolution and structures. Peptides. 2011;32(3):607–19. [DOI] [PubMed] [Google Scholar]
- 72.von Elert E, Agrawal MK, Gebauer C, Jaensch H, Bauer U, Zitt A. Protease activity in gut of Daphnia magna: evidence for trypsin and chymotrypsin enzymes. Comp Biochem Physiol B Biochem Mol Biol. 2004;137(3):287–96. [DOI] [PubMed] [Google Scholar]
- 73.Hu MYA, Hagen W, Jeng MS, Saborowski R. Metabolic energy demand and food utilization of the hydrothermal vent crab Xenograpsus testudinatus (Crustacea: Brachyura). Aquat Biol. 2012;15(1):11–25. [Google Scholar]
- 74.Datta MS, Gore J. Evolution: ‘snowed’ in with the enemy. Curr Biol. 2014;24(1):R33–5. [DOI] [PubMed] [Google Scholar]
- 75.Muhlia-Almazán A, Sánchez-Paz A, García-Carreño FL. Invertebrate trypsins: a review. J Comp Physiol B. 2008;178:655–72. [DOI] [PubMed] [Google Scholar]
- 76.Boetius A, Felbeck H. Digestive enzymes in marine invertebrates from hydrothermal vents and other reducing environments. Mar Biol. 1995;122:105–13. [Google Scholar]
- 77.Sogin EM, Kleiner M, Borowski C, Gruber-Vodicka HR, Dubilier N. Life in the dark: phylogenetic and physiological diversity of chemosynthetic symbioses. Annu Rev Microbiol. 2021;5:695–718. [DOI] [PubMed] [Google Scholar]
- 78.Guan H, Feng D, Birgel D, Kiel S, Peckmann J, Li S, Tao J. Lipid biomarker patterns reflect nutritional strategies of seep-dwelling bivalves from the South China Sea. Front Mar Sci. 2022;9:831286. [Google Scholar]
- 79.Carginale V, Trinchella F, Capasso C, Scudiero R, Parisi E. Gene amplification and cold adaptation of pepsin in Antarctic fish. A possible strategy for food digestion at low temperature. Gene. 2004;336(2):195–205. [DOI] [PubMed] [Google Scholar]
- 80.De Luca V, Maria G, De Mauro G, Catara G, Carginale V, Ruggiero G, Capasso A, Parisi E, Brier S, Engen JR, et al. Aspartic proteinases in Antarctic fish. Mar Genomics. 2009;2(1):1–10. [DOI] [PubMed] [Google Scholar]
- 81.Archambault P, Bowden DA, Rowden AA, Thurber AR, Baco AR, Levin LA, Smith CR. Cold seep epifaunal communities on the Hikurangi Margin, New Zealand: composition, succession, and vulnerability to human activities. PLoS One. 2013;8(10):e76869. [DOI] [PMC free article] [PubMed]
- 82.Teacă A, Begun T, Mureșan M. Annelid invaders in the Black Sea region: the distribution of Streblospio gynobranchiata and first occurrence of Laonome xeprovala. Global Ecol Conserv. 2021;32:e01920. [Google Scholar]
- 83.Lu L, Wu RSS. Recolonization and succession of marine macrobenthos in organic-enriched sediment deposited from fish farms. Environ Pollut. 1998;101(2):241–51. [DOI] [PubMed] [Google Scholar]
- 84.LaFont R. The endocrinology of invertebrates. Ecotoxicology. 2000;9(1/2):41–57. [Google Scholar]
- 85.Blake JA, Maciolek NJ, Meißner K. 7.4 Sedentaria: Sabellida/Spionida. In: Westheide W, editor. Volume 2 Pleistoannelida, Sedentaria II. Berlin, Boston: De Gruyter; 2020: 1–103.
- 86.Zimmer RK, Butman CA. Chemical signaling processes in the marine environment. Biol Bull. 2000;198(2):168–87. [DOI] [PubMed] [Google Scholar]
- 87.Chandler GT, Shipp MR, Donelan TL. Bioaccumulation, growth and larval settlement effects of sediment-associated polynuclear aromatic hydrocarbons on the estuarine polychaete, Streblospio benedicti (Webster). J Exp Mar Biol Ecol. 1997;213:95–110. [Google Scholar]
- 88.Huggett MJ. Settlement of generalist marine invertebrate herbivores in response to bacterial biofilms and other cues. UNSW, Sydney: University of New South Wales; 2006. [Google Scholar]
- 89.Lindasy SM. Ecology and biology of chemoreception in polychaetes. Zoosymposia. 2009;2:339–67. [Google Scholar]
- 90.Conzelmann M, Williams EA, Tunaru S, Randel N, Shahidi R, Asadulina A, Berger J, Offermanns S, Jékely G. Conserved MIP receptor–ligand pair regulates Platynereis larval settlement. Proc Natl Acad Sci U S A. 2013;110(20):8224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sokolinskaya EL, Kolesov DV, Lukyanov KA, Bogdanov AM. Molecular principles of insect chemoreception. Acta Naturae. 2020;12(3):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Derby CD, Kozma MT, Senatore A, Schmidt M. Molecular mechanisms of reception and perireception in crustacean chemoreception: a comparative review. Chem Senses. 2016;41(5):381–98. [DOI] [PubMed] [Google Scholar]
- 93.Saina M, Busengdal H, Sinigaglia C, Petrone L, Oliveri P, Rentzsch F, Benton R. A cnidarian homologue of an insect gustatory receptor functions in developmental body patterning. Nat Commun. 2015;6(1):6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morinaga S, Nagata K, Ihara S, Yumita T, Niimura Y, Sato K, Touhara K. Structural model for ligand binding and channel opening of an insect gustatory receptor. J Biol Chem. 2022;298(11):102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schulz DJ, Kozma MT, Ngo-Vu H, Wong YY, Shukla NS, Pawar SD, Senatore A, Schmidt M, Derby CD. Comparison of transcriptomes from two chemosensory organs in four decapod crustaceans reveals hundreds of candidate chemoreceptor proteins. PLoS One. 2020;15(3):e0230266. [DOI] [PMC free article] [PubMed]
- 96.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X. Ionotropic receptor 25a C isoform is responsible for temperature and chemical sensing in Drosophila melanogaster. Brandeis University; 2016.
- 98.Task D, Lin CC, Vulpe A, Afify A, Ballou S, Brbic M, Schlegel P, Raji J, Jefferis GSXE, Li H, et al. Chemoreceptor co-expression in Drosophila melanogaster olfactory neurons. eLife. 2022;11:e72599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weston JNJ, Jensen EL, Hasoon MSR, Kitson JJN, Stewart HA, Jamieson AJ. Barriers to gene flow in the deepest ocean ecosystems: evidence from global population genomics of a cosmopolitan amphipod. Sci Adv. 2022;8(43):eabo6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dixon DR, Pruski AM, Dixon LR. The effects of hydrostatic pressure change on DNA integrity in the hydrothermal-vent mussel Bathymodiolus azoricus: implications for future deep-sea mutagenicity studies. Mutat Res. 2004;552(1–2):235–46. [DOI] [PubMed] [Google Scholar]
- 101.Basantani MK, Gupta D, Mehrotra R, Mehrotra S, Vaish S, Singh A. An update on bioinformatics resources for plant genomics research. Curr Plant Biol. 2017;11–12:33–40. [Google Scholar]
- 102.Moran D, Softley R, Warrant EJ. The energetic cost of vision and the evolution of eyeless Mexican cavefish. Sci Adv. 2015;1(8): e1500363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Andrews S. FastQC: a quality control tool for high throughput sequence data. In.: Cambridge, United Kingdom; 2010.
- 104.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27(6):764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vurture GW, Sedlazeck FJ, Nattestad M, Underwood CJ, Fang H, Gurtowski J, Schatz MC. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics. 2017;33(14):2202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roach MJ, Schmidt SA, Borneman AR. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinformatics. 2018;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manni M, Berkeley MR, Seppey M, Simão FA, Zdobnov EM. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021;38(10):4647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen N. Using Repeat Masker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics. 2004;5(1):4.10. 11-14.10. 14. [DOI] [PubMed] [Google Scholar]
- 111.Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110(1–4):462–7. [DOI] [PubMed] [Google Scholar]
- 112.Hubley R, Finn RD, Clements J, Eddy SR, Jones TA, Bao W, Smit AF, Wheeler TJ. The Dfam database of repetitive DNA families. Nucleic Acids Res. 2016;44(D1):D81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stanke M, Keller O, Gunduz I, Hayes A, Waack S, Morgenstern B. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34(suppl_2):W435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brůna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom Bioinform. 2021;3(1):lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cantarel BL, Korf I, Robb SM, Parra G, Ross E, Moore B, Holt C, Alvarado AS, Yandell M. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008;18(1):188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, White O, Buell CR, Wortman JR. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katoh K, Misawa K, Ki Kuma, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–77. [DOI] [PubMed] [Google Scholar]
- 123.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–91. [DOI] [PubMed] [Google Scholar]
- 125.De Bie T, Cristianini N, Demuth JP, Hahn MW. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22(10):1269–71. [DOI] [PubMed] [Google Scholar]
- 126.McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32(Web Server)::W20-W25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Birney E, Clamp M, Durbin R. GeneWise and genomewise. Genome Res. 2004;14(5):988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015;43(W1):W39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Team RC. R: a language and environment for statistical computing. Vienna: Foundation for Statistical Computing; 2013. [Google Scholar]
- 131.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. [DOI] [PubMed] [Google Scholar]
- 132.Chen S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta. 2023;2(2):e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kolde R, Kolde MR. Package ‘pheatmap.’ R package. 2015;1(7):790. [Google Scholar]
- 135.Yan Y. The genome of Lindaspio polybranchiata (Polychaeta: Spionidae)-Figshare. 2024. 10.6084/m9.figshare.26264636.v1.
- 136.Yan Y. The genome of Lindaspio polybranchiata (Polychaeta: Spionidae)-CNGB. 2024. https://db.cngb.org/search/project/CNP0006250.
- 137.Yan Y. The genome of Lindaspio polybranchiata (Polychaeta: Spionidae)-NCBI. 2024. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1188207.
- 138.Yan Y. The genome of Lindaspio polybranchiata (Polychaeta: Spionidae)-Github. 2024. https://github.com/ywddwed/Lindaspio_genome.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. The gene numbers annotated by multiple databases. Fig. S2. The KEGG enrichment of unique gene families in L. polybranchiata. Fig. S3. Phylogenetic tree and gene family analysis of L. polybranchiata and other annelids. Fig. S4. KEGG enrichment of genes under positive selection in L. polybranchiata. Fig. S5. Alignment of paired domain of Pax6. Fig. S6. Alignment of TM5–7 domains of GRLs from L. polybranchiata, S. benedicti, and C. teleta. Fig. S7. Phylogenetic tree of candidate IRs across Canalipalpata. Fig. S8. Location of sampling stations. Fig. S9. K-mer distribution of the Lindaspio polybranchiata genome sequences. Fig. S10. Anatomical regions of L. polybranchiata used for transcriptomic analysis.
Additional file 2: Table S1. The gene family analysis results with CAFE. Table S2. The GO enrichment of expanded gene families in L. polybranchiata. Table S3. Gene counts related to hydrogen sulfide tolerance and detoxification. Table S4. The KEGG enrichment of expanded gene families in spionids. Table S5. The KEGG enrichment of positively selected genes in spionids. Table S6. Taxonomic information and genome sizes for the annelids used in this study. Table S7. The reference sequences of chemoreceptor.
Data Availability Statement
The assemblies and protein sequences of published genome used in this study were from the NCBI repository: Streblospio benedicti (GCA_019095985.1) [21], Paraescarpia echinospica (GCA_020002185.1) [28], Lamellibrachia luymesi (GCA_009193005.1) [29], Lamellibrachia satsuma (GCA_022478865.1) [33], Branchipolynoe longqiensis (GCA_030323885.1) [34], Harmothoe impar (GCA_947462335.1) [35], Capitella teleta (GCA_000328365.1) [31], Helobdella robusta (GCA_000326865.1) [31], Owenia fusiformis (GCA_903813345.2) [27], Dimorphilus gyrociliatus (GCA_904063045.1) [27], and from the National Genomics Data Center: Eisenia andrei (GWHACBE00000000) [32]. Genome assembly and annotation of the Lindaspio polybranchiata are available at Figshare [135]. All sequencing data for the genome and transcriptome are also available at the CNGB Sequence Archive (CNSA) of the China National GeneBank DataBase (CNGBdb) under project CNP0006250 [136] and National Center for Biotechnology Information (NCBI) under project PRJNA1188207 [137]. The computer commands were shared on GitHub [138].