Abstract
Ralstonia solanacearum causes bacterial wilt, a devastating disease in solanaceous crops. The pathogenicity of R. solanacearum depends on its type III secretion system, which delivers a suite of type III effectors into plant cells. The disordered core effector RipAO is conserved across R. solanacearum species and affects plant immune responses when transiently expressed in Nicotiana benthamiana. Specifically, RipAO impairs pathogen-associated molecular pattern–triggered reactive oxygen species production, an essential plant defense mechanism. RipAO fused to yellow fluorescent protein initially localizes to filamentous structures, resembling the cytoskeleton, before forming large punctate aggregates around the nucleus. Consistent with these findings, tubulin alpha 6 (TUA6) and tubulin beta-1, building blocks of microtubules, were identified as putative targets of RipAO in immunoprecipitation and mass spectrometry analyses. In the presence of RipAO, TUA6-labeled microtubules fragmented into puncta, mimicking the effects of oryzalin, a microtubule polymerization inhibitor. These effects were corroborated in a N. benthamiana transgenic line constitutively expressing green fluorescent protein-labeled TUA6, where RipAO reduced microtubule density and stability at an accumulation level that did not induce aggregation. Moreover, oryzalin treatment further enhanced RipAO’s impairment of reactive oxygen species production, suggesting that RipAO disrupts microtubule networks via its association with tubulins, leading to immune suppression. Further research into RipAO’s interaction with the microtubule network will enhance our understanding of bacterial strategies to subvert plant immunity.
Keywords: Cytoskeleton, Heterologous expression, Reactive oxygen species, Type III secreted effector, Virulence
INTRODUCTION
Bacterial pathogens rely on virulence factors known as effectors to subvert host defenses and promote infection (Büttner, 2016, Gohre and Robatzek, 2008). These effectors are delivered directly into host cells via the type III secretion system and play diverse roles during infection, primarily to enhance bacterial proliferation (Deslandes and Rivas, 2012, Macho, 2016). A major function of effectors is the suppression of plant defense responses, specifically pattern-triggered immunity (PTI), which is activated by the recognition of pathogen-associated molecular patterns (Jones and Dangl, 2006, Macho and Zipfel, 2015). In addition to their immunosuppressive functions, some effectors manipulate host cellular processes, such as hormone signaling and vesicle trafficking, which are not directly related to immunity (Macho, 2016). By targeting specific host proteins and signaling pathways, effectors act as molecular probes, revealing critical components of the plant defense network (Jeon and Segonzac, 2023, Motion et al., 2015). This information can be used to identify susceptibility genes, which are valuable targets for crop protection and breeding programs aimed at developing resistant cultivars (Win et al., 2012).
Ralstonia solanacearum, a soil-borne pathogen, can infect over 200 plant species, including major solanaceous crops (Mansfield et al., 2012). R. solanacearum genetic diversity can be divided into 4 phylotypes based on geographic origin (Genin and Denny, 2012) or more recently into 3 species (Prior et al., 2016). During infection, R. solanacearum delivers over 70 type III effectors, termed Ralstonia-injected proteins, which are tightly regulated throughout the infection cycle (Peeters et al., 2013, Valls et al., 2006). Some of these effectors are reported to suppress plant immunity and enhance bacterial virulence (Fujiwara et al., 2016, Mukaihara et al., 2016, Nakano et al., 2017, Nakano and Mukaihara, 2018, Qi et al., 2024, Sang et al., 2018, Sun et al., 2019). However, the biochemical and biological functions of most R. solanacearum effectors remain poorly understood, primarily due to functional redundancy and interplay within the pathogen's extensive effector repertoire.
To overcome these challenges, heterologous expression systems, such as transient expression in Nicotiana benthamiana, are frequently used to assess effector activity. These systems are particularly useful for studying how effectors modulate key hallmarks of PTI, such as reactive oxygen species (ROS) production. Biochemical approaches are also used to identify host protein interactors through immunoprecipitation/mass spectrometry and yeast 2-hybrid assays (González-Fuente et al., 2020, Mukhtar et al., 2011). Additionally, live-cell imaging of effector-fluorescent protein fusions helps determine their subcellular localization, revealing the specific cellular compartments they target (Aung et al., 2017, Khan et al., 2018). These combined approaches enable a more detailed understanding of individual effector functions.
Microtubules (MT) are key components of the cytoskeleton and play a crucial role in mediating defense responses alongside actin filaments (Li and Day, 2019, Li and Staiger, 2018). Upon pathogen detection, MT undergo rapid rearrangement, acting as platforms for signal perception and transduction during immune responses (Wang et al., 2022). The disruption of MT by bacterial effectors has been shown to increase plant susceptibility to infection (Cheong et al., 2014, Guo et al., 2016, Lee et al., 2012). Therefore, MT have emerged as regulators of immune signaling and pathogen resistance, functioning beyond their structural role.
In a previous work, we used a transient expression system in the model Solanaceae host N. benthamiana to screen R. solanacearum effectors that modulate plant immunity (Jeon et al., 2020). Among these, RipAO, a conserved core effector, was identified to have immune-suppressive activity (Peeters et al., 2013, Jeon et al., 2020). Here, we focus on RipAO’s role in impairing ROS production and investigate its dynamic subcellular localization that varies with the accumulation of this disordered protein. RipAO contributes to bacterial virulence by associating with and disrupting the MT network, offering new insights into the role of MT in plant immunity.
MATERIALS AND METHODS
Sequence Analysis
In total, 135 RipAO protein sequences present in Ralstonia spp. were collected from the Ralsto T3E database (https://iant.toulouse.inra.fr/bacteria/annotation/site/prj/T3Ev3/) for phylogenetic analysis. Out of 65 unique sequences, 37 sequences between 450 and 600 amino acid-long were aligned to build the phylogenetic tree using jukes-cantor/neighbor-joining method by Geneious Prime software. The first hit outside of Ralstonia genus from BLASTp in nonredundant database, KJK04938.1, was included as an outgroup. To predict intrinsically disordered regions (IDRs) on RipAOGMI1000 protein sequence, disorder score of each residue was determined using 3 different predictors: DISORDERED3 (Jones and Cozzetto, 2015), IUPred (Erdős et al. 2021), and VSL2 (Peng et al., 2006) from the prediction portal CAID (https://caid.idpcentral.org/) (Conte et al. 2023). Secondary structures and 3D structural model of RipAOGMI1000 were generated using AlphaFold3 (Abramson et al., 2024).
Molecular Constructs
RipAO coding sequence from the R. solanacearum reference strain GMI1000 was synthesized with codon optimization for expression in tobacco (Cosmogenetech). Golden gate–compatible RipAO modules flanked by BsaI restriction sites were amplified from the synthetic gene fragment (Engler et al., 2008). Coding sequences of NbTUA6 and NbTUB1 were extracted from the publicly available NbDE dataset (Kourelis et al., 2019) and amplified from N. benthamiana cDNA using BsaI site–flanking primers. The resulting PCR products were ligated into the entry vector pICH41021. The modules were assembled with a fluorescent tag under the control of the cauliflower mosaic virus 35S promoter in the binary vectors pICH86988 and pICH86966 for C-terminal and N-terminal fusion, respectively. For Pseudomonas syringae–mediated effector delivery, RipAO modules were assembled into the broad host range vector pBBR1 in fusion with AvrRps4 promoter (128 bp), AvrRps4 N-terminus (1-136 aa), and either N-terminal or C-terminal fluorescent tag (Sohn et al., 2007).
Bacterial Strains
Binary constructs were mobilized into Agrobacterium tumefaciens AGL1 strain by electroporation and the strains were grown at 28ºC on LB medium supplemented with 100 μg/ml carbenicillin and 50 μg/ml kanamycin. Broad host range vector constructs were mobilized into P. syringae pv. tomato DC3000 effectorless mutant D36E (Wei et al., 2015) by triparental mating and the strains were grown at 28ºC on King’s B medium supplemented with 50 μg/ml rifampicin, 100 μg/ml spectinomycin, and 20 μg/ml gentamycin. Cells were collected from overnight liquid culture by centrifugation and resuspended in infiltration medium (10 mM MgCl2 and 10 mM MES-KOH, pH = 5.6 for A. tumefaciens, 10 mM MgCl2 for P. syringae) for subsequent experiments.
Plant Materials
N. benthamiana plants were grown in a growth chamber at 25ºC under long-day conditions (16-hour light/8-hour dark). Transgenic N. benthamiana line stably expressing GFP-fused Arabidopsis TUA6 (GFP-AtTUA6; Gillespie et al., 2002) was kindly provided by Dr. Jessica Lee Erickson (Leibniz Institute for Plant Biochemistry).
Agrobacterium-Mediated Transient Expression
A. tumefaciens AGL1 cells grown overnight were centrifuged and resuspended in infiltration medium to reach OD600 = 0.1 to 0.6 depending on subsequent experiments as previously reported (Kim et al., 2024). The suspensions were infiltrated into fully expanded leaves of 5-week-old N. benthamiana plants using a needless syringe.
Measurement of ROS Production
N. benthamiana leaves were infiltrated with A. tumefaciens carrying RipAO-YFP or GFP (OD600 = 0.4) and leaf disks were collected at 24 h post infiltration (hpi) using a 5-mm biopsy punch. The leaf disks were recovered on 150 μl of distilled water overnight. At 36 hpi, the water was replaced with 100 μl of assay solution containing 100 μM luminol (Sigma-Aldrich), 2 μg of horseradish peroxidase (Sigma-Aldrich), and 50 nM of flg22 (Peptron) as an elicitor. Luminescence was measured in relative light unit (RLU) for either 60 minutes or 6 hours using SYNERGY HTX multimode microplate reader (BioTek).
Bacterial Growth Assay
N. benthamiana leaves were infiltrated with Pst D36E suspensions adjusted to an OD600 of 0.002. Two leaf disks from each infiltrated area, a total of 10 leaf disks for 5 replicates, were collected using an 8-mm biopsy punch at 3 hours and 3 days after inoculation. The leaf disks were ground in 10 mM MgCl2, and serial dilutions were plated on solid King’s B medium supplemented with selective antibiotics and 50 μg/ml nystatin (Sigma-Aldrich). After incubation at 28ºC for 3 days, colony-forming units were enumerated.
Confocal Microscopy
For the observation of RipAO localization, N. benthamiana leaves were infiltrated with a 1:1 mixture of A. tumefaciens strains carrying RipAO-YFP (OD600 = 0.4 for 24 hpi, OD600 = 0.1 for 36 and 48 hpi) and mCherry (OD600 = 0.4). For colocalization of RipAO and NbTUA6, A. tumefaciens carrying YFP-NbTUA6 was infiltrated at an OD600 = 0.6 first. A. tumefaciens carrying RipAO-cyan fluorescent protein (CFP) was infiltrated after 2 hours, 12 hours (OD600 = 0.1), and 24 hours (OD600 = 0.4) in the NbTUA6-infiltrated area. All the infiltrated spots were observed 2 days after NbTUA6 infiltration. Confocal microscopy was conducted using a SP8X confocal laser scanning microscope (Leica) with a 40× water-immersion objective. CFP, GFP/YFP, and mCherry fluorophores were excited with a 405-nm diode laser, a 488-nm argon laser, and a 561-nm argon laser, respectively. Emission was collected in the following channels: 460 to 480 nm (CFP), 490 to 520 nm (GFP), 520 to 540 nm(YFP), and 590 to 610 nm (mCherry). Confocal z-stacks of epidermal cells were captured at 1-µm intervals, spanning a total of 10 to 30 steps. LasX software controlled the microscope and performed image processing to convert z-stacks into single images by maximum projection as shown in the figures.
Drug Treatments
Oryzalin (Sigma-Aldrich) was prepared at 100 mM in dimethyl sulfoxide (DMSO) and subsequently diluted to 100 μM in distilled water. This solution was infiltrated into agroinfiltrated spots using a needleless syringe 3 hours before confocal microscopy observation. For ROS measurement, the water in which leaf disks were floated was replaced with a 100 μM oryzalin solution 3 hours before measurement. ROS production was then elicited using assay solution containing 50 nM flg22 and 100 μM oryzalin. Taxol (Paclitaxel, Sigma-Aldrich) was dissolved in DMSO at 20 mM, diluted in distilled water to 20 μM, and infiltrated to A. tumefaciens inoculation spots 4 hours after agroinfiltration. For each treatment, DMSO controls were diluted and applied in parallel under the same conditions.
Protein Detection and Immunoprecipitation
Leaf tissues infiltrated with A. tumefaciens (OD600 = 0.4) were frozen at indicated time points and ground into a fine powder in liquid nitrogen. Total proteins were extracted in an equal volume of GTEN buffer (10% glycerol, 50 mM Tris-HCl pH 7.5, 2 mM EDTA pH 8, and 150 mM NaCl) supplemented with 5 mM DTT, 0.5% IGEPAL (Sigma-Aldrich), 1% PVPP, and 1 tablet of cOmplete protease inhibitor cocktail per 40 ml (Roche). The extracts were clarified by centrifugation at 15,000 × g for 10 minutes at 4ºC and filtered through MiraCloth (Millipore). The clarified extracts were mixed with 3 × SDS sample buffer and denatured at 96ºC for 10 minutes. For immunoprecipitation, 1.5 ml of diluted extracts were incubated with 30 μl of GFP-Trap agarose beads (ChromoTek) for 2 hours at 4ºC. The beads were washed 3 times with GTEN buffer supplemented with 5 mM DTT, 0.5% IGEPAL, and cOmplete protease inhibitor cocktail. The bound proteins were released in 40 μl of 3 × SDS sample buffer at 96ºC for 10 minutes. Proteins were separated by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with anti-GFP antibodies (Santa Cruz Biotechnology) followed by secondary antimouse-horseradish peroxidase antibodies (Sigma-Aldrich). Immunoblots were developed using SuperSignal West substrate (Thermo Scientific) and detected with an Azure 400 CCD imager (Azure Biosystem). For detection of P. syringae–delivered RipAO fused with the mCherry tag, total proteins were extracted from N. benthamiana leaves infiltrated with Pst D36E strains (OD600 = 0.1) using the same procedure described above. The extracts were incubated with 30 μl of RFP-Trap agarose beads (ChromoTek). The bound proteins were eluted and detected by immunoblotting with anti-mCherry antibodies (Agrisera).
Mass Spectrometry Analysis
Total proteins were extracted from RipAO-YFP or YFP-expressing N. benthamiana leaves frozen at 48 hpi and subjected to GFP-Trap affinity purification. The bound proteins were eluted in 30 μl of 3 × SDS sample buffer and separated by SDS-PAGE. The entire lanes, visualized by Coomassie blue staining, were excised and sent to National Instrumentation Center for Environmental Management, Seoul National University for in-gel digestion, subsequent mass spectrometry analysis, and data processing. Nano-LC-MS/MS was performed with the Q Exactive LC-MS/MS system (Thermo Scientific). The resulting spectra were searched against the NbDE dataset (Kourelis et al., 2019). Peptide matches commonly identified in 2 out of 3 biological replicates of RipAO-YFP or YFP control samples were listed. Matches unique to the RipAO-YFP list were selected as putative interactor of RipAO.
Bimolecular Fluorescence Complementation Assay
RipAO fused with the N-terminal part of the YFP (YFPn) at the C-terminus and NbTUA6 or NbTUB1 fused with the C-terminal part of the YFP (YFPc) at the N-terminus were cloned into A. tumefaciens AGL1. Equal volumes of A. tumefaciens carrying RipAO-YFPn (OD600 = 0.4) and free YFPc or YFPc-tubulin (OD600 = 0.4) were mixed and infiltrated to N. benthamiana leaves. DMSO or taxol was treated at 4 hpi and yellow fluorescence was detected at 48 hpi.
Microtubule Disruption Assays and Quantitative Analysis
A. tumefaciens carrying mCherry-RipAO or mCherry (OD600 = 0.6) and Pst D36E delivering mCherry-fused RipAO or mCherry (OD600 = 0.1) were infiltrated into 5-week-old GFP-AtTUA6 transgenic N. benthamiana leaves. Confocal images were acquired 2 days after agroinfiltration or 12 and 24 hours after Pst D36E infiltration. Images obtained by maximum projection of z-stacks were analyzed using Fiji (Schindelin et al., 2012) for MT destabilization. To quantify MT intensity, MTs longer than 10 µm were marked using the line tool in the GFP channel and the mean gray values were measured. The mean gray value of a neighboring cytosolic region, parallel to each marked MT, was measured to account for the background. Relative MT fluorescence was calculated by dividing MT brightness by the cytosolic background. To quantify MT density, a random 30-µm-long line was drawn within a cell, and the number of MTs intersecting this line was counted.
Statistical Analysis
All the statistical analyses were conducted with the GraphPad Prism 10 software using merged data from at least 3 independent experiments. Significant differences were determined by 1-way ANOVA at the 95% level followed by Tukey’s multiple comparisons test.
RESULTS
RipAO Impairs flg22-Triggered ROS Production and Enhances Bacterial Growth in N. benthamiana
RipAO is conserved in 136 out of the 158 sequenced strains of the RSSC (Supplementary Fig. S1A), suggesting that it may play important roles in the interaction with host plants and virulence of the pathogen (Peeters et al., 2013). Sequence analysis of RipAO protein from the reference strain GMI1000 showed that more than 70% of residues were predicted to be intrinsically disordered (Supplementary Fig. S1B and S1C). This disordered nature is corroborated by the absence of known functional domains or motifs.
To investigate a possible impact of RipAO on plant immune responses, we measured flg22-triggered ROS production in N. benthamiana leaves transiently expressing RipAO (Fig. 1A). RipAO significantly reduced the initial ROS burst amplitude compared with the GFP control. While ROS levels in GFP-expressing control tissues returned to baseline within 1 hour and exhibited a secondary peak at 2 hours post elicitation, RipAO-expressing tissues showed a prolonged first peak with a gradual decline and no detectable secondary peak. Consistent with these ROS kinetics, the total ROS production over 6-hour measurements was significantly lower in RipAO-expressing tissues.
Fig. 1.
RipAO impairs flg22-triggered ROS production and enhances bacterial growth in N. benthamiana. (A) flg22-triggered ROS production in N. benthamiana leaf tissues expressing RipAO. RipAO-YFP or GFP were transiently expressed via agroinfiltration. Leaf disks were challenged with water or flg22 at 36 hours post agroinfiltration and ROS production was monitored for 6 hours. Solid line and shaded band present the mean values ± standard error of relative light unit (RLU) from a representative experiment (n = 12). Individual values of the total ROS production from 3 independent experiments are shown in boxes and whiskers (n = 32) on the right. (B) Growth of P. syringae pv. tomato DC3000 effectorless mutant D36E delivering RipAO in N. benthamiana. D36E carrying the empty vector (EV), RipP2, or RipAO were infiltrated into N. benthamiana leaves and the bacterial populations were enumerated at 0 and 3 days post inoculation (dpi) as colony-forming unit (CFU). Individual values from 3 independent experiments are shown in boxes and whiskers (n = 15). Different letters indicate significant differences determined by 1-way ANOVA followed by Tukey’s multiple comparisons test (P < .0001).
Additionally, we evaluated the impact of RipAO on bacterial proliferation by employing the effectorless polymutant P. syringae pv. tomato DC3000 D36E (hereafter referred to as Pst D36E) to deliver RipAO into plant cells via the type III secretion system (Sohn et al., 2007, Wei et al., 2015). The delivery of RipAO enhanced Pst D36E growth in N. benthamiana at 3 days post infiltration, although to a lesser extent compared with the well-characterized PTI suppressor RipP2 (Fig. 1B) (Le Roux et al., 2015). Collectively, these findings indicate that RipAO can dampen plant immune responses and facilitate bacterial growth.
RipAO Exhibits Dynamic Changes in Subcellular Localization During Transient Expression in N. benthamiana
We previously reported that 48 hours after agroinfiltration, RipAO-YFP localizes to and around nuclei, as well as to cytoplasmic puncta in N. benthamiana epidermal cells (Jeon et al., 2020). To better characterize the subcellular localization of RipAO, we examined RipAO-YFP fluorescence at earlier time points after agroinfiltration. As the accumulation of RipAO-YFP protein increased in agroinfiltrated tissues (Supplementary Fig. S2), RipAO-YFP fluorescence showed distinct localization patterns (Fig. 2A). At 24 hpi, RipAO-YFP labeled a filamentous network. By 36 hpi, RipAO-YFP-labeled puncta distributed on cytoplasmic tracks, alongside bundles of fragmented filaments. Lastly, by 48 hpi, large puncta were primarily observed aggregating on the nuclear surface, with fewer puncta remaining along cytoplasmic tracks.
Fig. 2.
RipAO exhibits dynamic changes in subcellular localization during transient expression in N. benthamiana. (A) Subcellular localization of RipAO at different time points after agroinfiltration. RipAO-YFP and mCherry were coexpressed in N. benthamiana leaves and the subcellular localization in lower epidermal cells was analyzed by confocal microscopy at 24, 36, and 48 hours post infiltration (hpi). (B) Effect of microtubule inhibitors on RipAO-labeled structures. Leaf disks expressing RipAO-YFP were floated on a solution of oryzalin for 3 hours before observation. Taxol was infiltrated to the leaf area expressing RipAO-YFP 4 hours after agroinfiltration. Scale bars = 20 µm.
As the early filamentous network was reminiscent of the MT organization, RipAO-YFP localization was observed in tissues pretreated with the MT polymerization inhibitor oryzalin or with the MT-stabilizing agent taxol (Fig. 2B) (Caillaud, 2022). At 24 hpi when RipAO-YFP localized to the filamentous network, oryzalin treatment caused a dramatic shift in localization to punctate aggregates, while taxol had a minimal effect. By 36 hpi, oryzalin treatment accelerated the aggregation of RipAO-marked puncta and filament fragments compared with the DMSO control. At 48 hpi when RipAO mainly localized to the large perinuclear aggregates, oryzalin did not further alter the localization. On the contrary, taxol treatment at 36 and 48 hpi aligned small RipAO puncta along cytoplasmic tracks, forming a network of dotted lines. These observations indicate that RipAO could be associated with the MT network and that this localization pattern dynamically changes as the protein accumulates.
RipAO Is Associated With Tubulins In Planta
In line with the observed association of RipAO with the MT network, peptides corresponding to 2 tubulin subunits, tubulin alpha 6 (NbTUA6) and tubulin beta-1 (NbTUB1), were identified from LC-MS/MS analysis of RipAO-YFP-enriched protein extracts following immunopurification on GFP-trap beads (Supplementary Table S1). To further explore the potential association between RipAO and tubulin, bimolecular fluorescence complementation assay was conducted (Fig. 3). Coexpression of RipAO with either NbTUA6 or NbTUB1 resulted in YFP fluorescence reconstitution as short, rod-shaped fragments that were restored into dashed filamentous structures by taxol treatment. From this, we inferred that RipAO can interact with tubulins in existing MT. Additionally, we assessed the direct interaction between RipAO and tubulins using a yeast 2-hybrid assay; however, no positive interactions were detected (Supplementary Fig. S3). These results suggest that while RipAO resides in close proximity with polymerized tubulins, this interaction may be mediated by additional factors.
Fig. 3.
RipAO associates with tubulins in planta. Bimolecular fluorescence complementation (BiFC) analysis of RipAO association with tubulin. RipAO fused with the N-terminal part of the YFP (RipAO-YFPn) was coexpressed with free C-terminal part of the YFP (YFPc), YFPc-NbTUA6, or YFPc-NbTUB1. Taxol or DMSO were infiltrated to the same leaf area 4 hours after agroinfiltration. The YFP signals in lower epidermal cells were detected by confocal microscopy at 48 hours post agroinfiltration. BF, bright field. Scale bars = 20 µm.
RipAO Destabilizes NbTUA6-Labeled Microtubule Networks, Leading to Aggregation of Microtubule Fragments
The localization of RipAO and its association with tubulins prompted us to investigate the dynamics of the MT network in the presence of RipAO. To this end, we agroinfiltrated RipAO fused with CFP at various time points into leaf tissues expressing YFP-NbTUA6 (Fig. 4). Consistent with early subcellular localization of RipAO-YFP, RipAO-CFP colocalized with the MT network labeled by YFP-NbTUA6. As RipAO accumulated and aggregated, it led to the aggregation of NbTUA6-labeled MT fragments into fusiform structures. By 48 hpi, NbTUA6 aggregates were observed around the nucleus, with partial colocalization with RipAO. As YFP-NbTUA6 protein stability is not significantly affected by the coexpression with RipAO (Supplementary Fig. S4), these observations indicate that the gradual accumulation of RipAO disrupts the MT network, leading to the formation of aggregated structures.
Fig. 4.
RipAO destabilizes NbTUA6-labeled microtubule networks, leading to aggregation of microtubule fragments. Dynamics of RipAO and NbTUA6 colocalization. RipAO-CFP and YFP-NbTUA6 were coexpressed in N. benthamiana leaves and the subcellular localization in lower epidermal cells was analyzed by confocal microscopy at 24, 36, and 48 hours post infiltration (hpi). Fluorescence intensity profiles along the dashed line are shown in the right panels. Scale bars = 20 µm.
RipAO Can Destabilize Microtubule Filaments at the Non–Aggregate-Triggering Level
To better characterize the impact of RipAO on MT network organization, transgenic N. benthamiana line constitutively expressing GFP-AtTUA6 was utilized for quantification of MT destabilization (Gillespie et al., 2002, Ortmann et al., 2023). Instead of using RipAO fused with a C-terminal fluorescent tag, we employed mCherry-RipAO to assess its effects on MT dynamics. Notably, mCherry-RipAO–labeled MT at a later time point compared with RipAO-mCherry. While RipAO-mCherry formed large aggregates at 48 hpi, mCherry-RipAO signals began to diminish (Supplementary Fig. S5A). We confirmed by immunoblotting that mCherry-RipAO did not accumulate to the same extent as C-terminally tagged RipAO (Supplementary Fig. S5B). This differential stability might be due to the fluorescent protein fusion interaction with the disordered regions of RipAO. Nonetheless, as mCherry-RipAO accumulation is not sufficient to induce aggregation, we used this N-terminally tagged protein fusion to evaluate the MT network at nonaggregating levels of RipAO. Free mCherry or mCherry-RipAO were expressed in the GFP-AtTUA6 leaves and confocal images were acquired at 48 hpi (Fig. 5A). In the presence of mCherry-RipAO, both MT density and fluorescence were significantly reduced compared with the mCherry control (Fig. 5B). This reduction suggests that RipAO interferes with the stability or the organization of the MT network before causing fragmentation and aggregation.
Fig. 5.
RipAO can destabilize microtubule filaments at the non–aggregate-triggering level. Destabilization of TUA6-labeled microtubule (MT) filaments by RipAO expression. (A) Confocal images of lower epidermal cells of GFP-AtTUA6 transgenic N. benthamiana–expressing mCherry or mCherry-RipAO 48 hours after agroinfiltration. Scale bars = 20 µm. (B) Quantification of MT destabilization by RipAO expression. Density and relative fluorescence of MT filaments were measured from the confocal images acquired in (A). Dots and bars present individual values and the median from 3 independent experiments, respectively (n = 15 for MT number/μm, n = 60 for relative MT fluorescence). Different letters indicate significant differences determined by 1-way ANOVA followed by Tukey’s multiple comparisons test (P < .05).
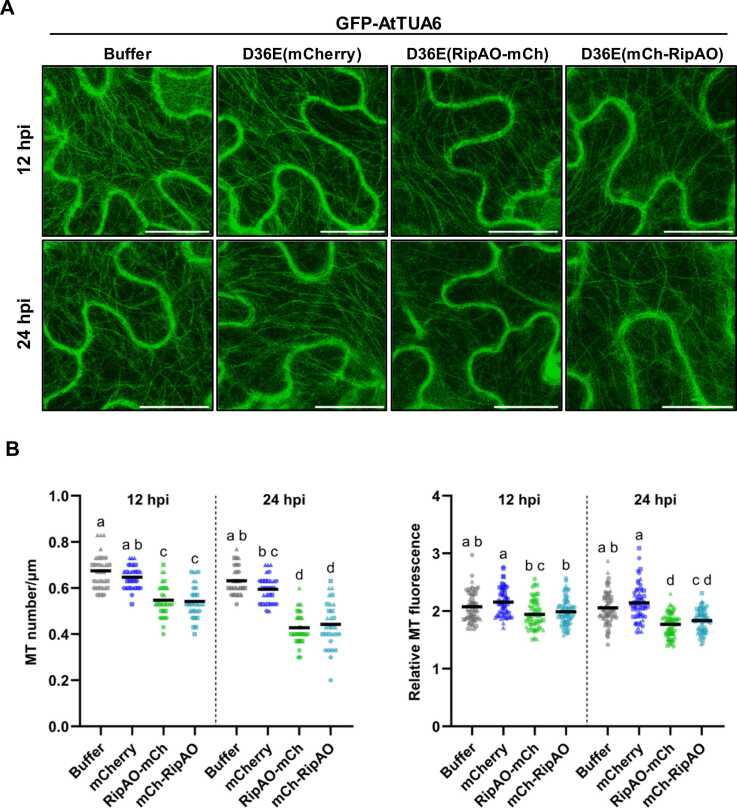
To confirm these findings under physiological conditions, we conducted a similar experiment using the Pst D36E strains carrying the expression plasmid for mCherry or mCherry-fused RipAO (Fig. 6A). The delivery of RipAO by Pst D36E type III secretion system was validated by immunoprecipitation on RFP-trap beads of protein extracted from Pst D36E-infiltrated tissues (Supplementary Fig. S6). Consistent with the transient expression assay, both MT density and fluorescence were significantly reduced in the presence of RipAO, regardless of the mCherry tag position (Fig. 6B). Altogether, these results suggest that RipAO may exert a similar disruptive effect on the MT network when secreted by R. solanacearum.
Fig. 6.
Type III secretion system–delivered RipAO destabilizes microtubule filaments. Destabilization of TUA6-labeled microtubule (MT) filaments by P. syringae pv. tomato DC3000 effectorless mutant D36E delivering RipAO. (A) Confocal images of lower epidermal cells of GFP-AtTUA6 transgenic N. benthamiana infiltrated with D36E carrying mCherry, RipAO-mCherry (RipAO-mCh), or mCherry-RipAO (mCh-RipAO) at 12 and 24 hours post infiltration (hpi). Scale bars = 20 µm. (B) Quantification of MT destabilization by RipAO delivery. Density and relative fluorescence of MT filaments were measured from the confocal images acquired in (A). Dots and bars present individual values and the median from 3 independent experiments, respectively (n = 30 for MT number/μm, n = 60 for relative MT fluorescence). Different letters indicate significant differences determined by 1-way ANOVA followed by Tukey’s multiple comparisons test (P < .05).
Oryzalin Treatment Exacerbates RipAO Suppression of flg22-Triggered ROS Production
Lastly, we attempted to link RipAO-induced perturbation of the MT network and its suppressive effect on plant immunity. To this end, N. benthamiana tissues expressing either free GFP or RipAO-YFP were pretreated with the MT polymerization inhibitor oryzalin (Fig. 7). While oryzalin did not significantly affect flg22-triggered ROS production in GFP-expressing tissues, it notably enhanced the reduction in ROS amplitude in RipAO-expressing tissues. This suggests that disruption of the MT network exacerbates the inhibitory effect of RipAO on PTI.
Fig. 7.
Oryzalin treatment exacerbates RipAO suppression of flg22-triggered ROS production. flg22-triggered ROS production in N. benthamiana leaf tissues expressing RipAO and pretreated with DMSO or oryzalin for 3 hours. The leaf disks were challenged with flg22 solution containing DMSO or oryzalin at 36 hours post agroinfiltration and ROS production was monitored for 60 minutes. Solid line and shaded band present the mean values ± standard error of relative light unit (RLU) from a representative experiment (n = 12). Individual values of the total ROS production from 4 independent experiments are shown in boxes and whiskers (n = 48). Different letters indicate significant differences determined by 1-way ANOVA followed by Tukey’s multiple comparisons test (P < .05).
DISCUSSION
Using a heterologous expression system, we show that the R. solanacearum effector RipAO disrupts MT networks via an association with tubulins to inhibit immune signaling. Although heterologous expression systems cannot fully replicate the natural ordered secretion of multiple effectors during infection, transient expression in N. benthamiana and Arabidopsis transgenic lines expressing the effectors are widely used to explore R. solanacearum virulence mechanisms (Kim et al., 2023, Landry et al., 2020, Lonjon et al., 2016). For example, RipN, the endoplasmic reticulum and nuclear-localized effector, suppresses PTI by manipulating NADH/NAD+ homeostasis and promotes growth of Pst DC3000 in Arabidopsis (Sun et al., 2019). RipAL targets chloroplast lipids to suppress PTI and salicylic acid signaling through the activation of the antagonistic jasmonic acid pathway in N. benthamiana (Nakano and Mukaihara, 2018). RipAL also enhances virulence of Pst DC3000 in Arabidopsis, which is in accordance with the reduced growth of ripAL deletion mutant in pepper. These findings support that isolating the function of single effector is an essential and efficient approach for functional characterization of effectors.
Genomic studies on the RSSC have identified RipAO as a core effector present in at least 95% of sequenced strains (Landry et al., 2020, Peeters et al., 2013, Sabbagh et al., 2019). This high conservation suggests that RipAO is likely essential for the pathogen's ability to infect a wide range of hosts. However, due to the intrinsically disordered structure of RipAO, comparative structural analyses between variants have been challenging. While these IDRs complicate functional predictions, they often confer structural flexibility, enabling low-affinity interactions with multiple host targets (Marín et al., 2013). The disordered structure of RipAO aligns with its dynamic localization in plant cells, which mirrors the behavior of other effectors that target multiple subcellular compartments (Khan et al., 2018). Of note, IDRs determine MT binding of many MT-associated proteins, which enable a fine-tuned regulation of MT dynamics (Gonzalez et al., 2023). Moreover, mounting experimental evidence has revealed that IDRs that encode multivalency promote biological phase separation and condensate formation (Emenecker et al., 2021, Wang and Gu, 2021). An increase in the protein concentration enables interactions between IDRs to drive condensates formation at a specific threshold, and at higher concentration, condensates may form filaments and aggregates with solid-like properties (Solis-Miranda et al., 2023). While we have not directly demonstrated phase separation of RipAO, its localization pattern—from dispersed on the MT network to large aggregates at higher level of accumulation—suggests that similar process might be at play.
The cytoskeleton plays an essential role in plant immune responses by mediating the internalization and positioning of immune receptors on the plasma membrane (Bücherl et al., 2017, Li and Day, 2019, Nagawa et al., 2012). It also acts as a scaffold for the transport of vesicles and antimicrobial peptides to infection sites (Brandizzi and Wasteneys, 2013, Li and Day, 2019, Li and Staiger, 2018). While actin dynamics in plant immunity are well-documented, MT are less understood in this context (Henty-Ridilla et al., 2013, Li et al., 2015). MT reorganization has been observed during fungal and oomycete infections, often showing localized depolymerization at pathogen contact points (Hardham, 2013, Quentin et al., 2016, Takemoto et al., 2003). However, in early bacterial infections, there are fewer noticeable changes in MT structure (Guo et al., 2016, Lee et al., 2012), and treatments with pathogen-associated molecular patterns do not significantly alter MT (Binet et al., 2001, Chang and Nick, 2012). This suggests MT may not respond directly to early immune signals. In our study, we observed no significant change in MT density or fluorescence after infiltration with Pst D36E delivering mCherry, in GFP-AtTUA6 transgenic plants. However, MT appear more aligned with fewer cross-linked filaments after Pst D36E inoculation compared with buffer-infiltrated controls. This suggests MT might play a role in PTI after bacteria recognition. However, the precise connection between MT remodeling and immune signaling remains unclear and requires further study.
Despite limited understanding of MT's role in plant immunity, evidence shows that proper MT dynamics are critical for defense against pathogens. Chemical disruption of MT networks with oryzalin has been reported to enhance susceptibility to virulent bacteria (Guo et al., 2016, Hiles et al., 2023, Lee et al., 2012). These studies have shown different mode of action for bacterial effectors that target and disrupt the MT networks, either by directly modifying tubulins or by association with other cytoskeleton components. The Pst effector HopZ1a directly interacts with and acetylates tubulin, destabilizing the MT network, which leads to suppression of the plant secretory pathway and cell wall–based defenses (Lee et al., 2012). The Xanthomonas euvesicatoria effector, XopL, also directly associates with MT and causes strong cell death when transiently expressed in N. benthamiana (Erickson et al., 2018, Ortmann et al., 2023). Comparative sequence analyses identified a proline-rich region/α-helical region of XopL is important for the MT localization that is correlated with the cell death phenotype. In contrast with HopZ1a or XopL, RipAO cannot directly bind tubulin and no catalytic activity could be predicted from its disordered structure. However, RipAO sequence is enriched in proline and arginine residues in patches that could contribute to its association with MT.
AvrBsT, a homolog of HopZ1a from Xanthomonas euvesicatoria, acetylates Arabidopsis ACIP1 (for ACETYLATED INTERACTING PROTEIN) that positively regulates plant immune responses (Cheong et al., 2014). ACIP1 colocalizes with MT in the form of filaments and some small puncta (Cheong et al., 2014). AvrBsT triggers the formation of large GFP-ACIP1 aggregates, in an acetyltransferase-dependent manner (Cheong et al., 2014). This AvrBsT-induced aggregation of ACIP1 is reminiscent of RipAO localization at 36 hpi, although RipAO forms larger aggregates with thick MT fragments, which suggests involvement of other MT-associated proteins in RipAO-MT interaction. Lastly, RipU, a core effector from R. solanacearum K60, directly interacts with both actin and tubulin and disrupts actin and MT cytoskeleton (Denne et al., 2021, Hiles et al., 2023). RipU suppresses flg22-triggered ROS production in N. benthamiana, and R. solanacearum mutants lacking RipU show reduced wilting symptoms and root colonization in tomato plants (Hiles et al., 2023). In parallel to our RipAO study, this supports the idea that R. solanacearum employs effectors to remodel the MT networks and promote virulence. Although the precise mechanisms by which RipU and RipAO disrupt the MT network remain unknown, these findings underscore the critical role of MT in plant defense and reveal how pathogens evolve diverse strategies to manipulate the cytoskeleton during plant infection.
Author Contributions
H.J. and C.S. conceived the experiments. H.J. and W.K. performed and analyzed the experiments. H.J. and C.S. wrote the paper. All the authors approved the paper before submission.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was carried out with the support of the National Research Foundation of Korea (NRF) projects No. 2022R1I1A1A01066399 and No. 2018R1A5A1023599.
Footnotes
Supplemental material associated with this article can be found online at: doi:10.1016/j.mocell.2024.100167.
ORCID
Hyelim Jeon: 0000-0002-9784-2321.
Wanhui Kim: 0000-0002-9622-1355.
Cécile Segonzac: 0000-0002-5537-7556.
Appendix A. Supplemental material
Supplementary material
.
References
- Abramson J., Adler J., Dunger J., Evans R., Green T., Pritzel A., Ronneberger O., Willmore L., Ballard A.J., Bambrick J., et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. doi: 10.1038/s41586-024-07487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K., Xin X., Mecey C., He S.Y. Subcellular localization of Pseudomonas syringae pv. tomato effector proteins in plants. Methods Mol. Biol. 2017;1531:141–153. doi: 10.1007/978-1-4939-6649-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet M.N., Humbert C., Lecourieux D., Vantard M., Pugin A. Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 2001;125:564–572. doi: 10.1104/pp.125.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F., Wasteneys G.O. Cytoskeleton-dependent endomembrane organization in plant cells: an emerging role for microtubules. Plant J. 2013;75:339–349. doi: 10.1111/tpj.12227. [DOI] [PubMed] [Google Scholar]
- Bücherl C.A., Jarsch I.K., Schudoma C., Segonzac C., Mbengue M., Robatzek S., MacLean D., Ott T., Zipfel C. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife. 2017;6 doi: 10.7554/eLife.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D. Behind the lines—actions of bacterial type III effector proteins in plant cells. FEMS Microbiol. Rev. 2016;40:894–937. doi: 10.1093/femsre/fuw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud M.C. Tools for studying the cytoskeleton during plant cell division. Trends Plant Sci. 2022;27:1049–1062. doi: 10.1016/j.tplants.2022.05.006. [DOI] [PubMed] [Google Scholar]
- Chang X., Nick P. Defence signalling triggered by flg22 and harpin is integrated into a different stilbene output in Vitis cells. PLOS One. 2012;7 doi: 10.1371/journal.pone.0040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong M.S., Kirik A., Kim J.G., Frame K., Kirik V., Mudgett M.B. AvrBsT acetylates Arabidopsis ACIP1, a protein that associates with microtubules and is required for immunity. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A.D., Mehdiabadi M., Bouhraoua A., Miguel M.A., Tosatto S.C.E., Piovesan D. Critical assessment of protein intrinsic disorder prediction (CAID) - results of round 2. Proteins. 2023;91:1925–1934. doi: 10.1002/prot.26582. [DOI] [PubMed] [Google Scholar]
- Denne N.L., Hiles R.R., Kyrysyuk O., Iyer-Pascuzzi A.S., Mitra R.M. Ralstonia solanacearum effectors localize to diverse organelles in Solanum hosts. Phytopathology. 2021;111:2213–2226. doi: 10.1094/PHYTO-10-20-0483-R. [DOI] [PubMed] [Google Scholar]
- Deslandes L., Rivas S. Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 2012;17:644–655. doi: 10.1016/j.tplants.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Emenecker R.J., Holehouse A.S., Strader L.C. Biological phase separation and biomolecular condensates in plants. Annu. Rev. Plant Biol. 2021;72:17–46. doi: 10.1146/annurev-arplant-081720-015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C., Kandzia R., Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PloS One. 2008;3 doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdős G., Pajkos M., Dosztányi Z. IUPred3: prediction of protein disorder enhanced with unambiguous experimental annotation and visualization of evolutionary conservation. Nucleic Acids Res. 2021;49:W297–W303. doi: 10.1093/nar/gkab408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.L., Adlung N., Lampe C., Bonas U., Schattat M.H. The Xanthomonas effector XopL uncovers the role of microtubules in stromule extension and dynamics in Nicotiana benthamiana. Plant J. 2018;93:856–870. doi: 10.1111/tpj.13813. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Kawazoe T., Ohnishi K., Kitagawa T., Popa C., Valls M., Genin S., Nakamura K., Kuramitsu Y., Tanaka N., et al. RipAY, a plant pathogen effector protein, exhibits robust gamma-glutamyl cyclotransferase activity when stimulated by eukaryotic thioredoxins. J. Biol. Chem. 2016;291:6813–6830. doi: 10.1074/jbc.M115.678953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin S., Denny T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012;50:67–89. doi: 10.1146/annurev-phyto-081211-173000. [DOI] [PubMed] [Google Scholar]
- Gillespie T., Boevink P., Haupt S., Roberts A.G., Toth R., Valentine T., Chapman S., Oparka K.J. Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of tobacco mosaic virus. Plant Cell. 2002;14:1207–1222. doi: 10.1105/tpc.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohre V., Robatzek S. Breaking the barriers: microbial effector molecules subvert plant immunity. Annu. Rev. Phytopathol. 2008;46:189–215. doi: 10.1146/annurev.phyto.46.120407.110050. [DOI] [PubMed] [Google Scholar]
- González-Fuente M., Carrère S., Monachello D., Marsella B.G., Cazalé A.C., Zischek C., Mitra R.M., Rezé N., Cottret L., Mukhtar M.S., et al. EffectorK, a comprehensive resource to mine for Ralstonia, Xanthomonas, and other published effector interactors in the Arabidopsis proteome. Mol. Plant Pathol. 2020;21:1257–1270. doi: 10.1111/mpp.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.P., Frandsen K.E.H., Kesten C. The role of intrinsic disorder in binding of plant microtubule-associated proteins to the cytoskeleton. Cytoskeleton. 2023;80:404–436. doi: 10.1002/cm.21773. [DOI] [PubMed] [Google Scholar]
- Guo M., Kim P., Li G., Elowsky C.G., Alfano J.R. A bacterial effector co-opts calmodulin to target the plant microtubule network. Cell Host Microbe. 2016;19:67–78. doi: 10.1016/j.chom.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Hardham A.R. Microtubules and biotic interactions. Plant J. 2013;75:278–289. doi: 10.1111/tpj.12171. [DOI] [PubMed] [Google Scholar]
- Henty-Ridilla J.L., Shimono M., Li J., Chang J.H., Day B., Staiger C.J. The plant actin cytoskeleton responds to signals from microbe-associated molecular patterns. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles R., Rogers A., Jaiswal N., Zhang W., Butchacas J., Merfa M.V., Klass T., Kaser E., Jacobs J.M., Staiger C.J., et al. A Ralstonia solanacearum type III effector alters the actin and microtubule cytoskeleton to promote bacterial virulence in plants. BioRxiv. 2023 doi: 10.1371/journal.ppat.1012814. 2023.11.01.565113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H., Kim W., Kim B., Lee S., Jayaraman J., Jung G., Choi S., Sohn K.H., Segonzac C. Ralstonia solanacearum type III effectors with predicted nuclear localization signal localize to various cell compartments and modulate immune responses in Nicotiana spp. Plant Pathol. J. 2020;36:43–53. doi: 10.5423/PPJ.OA.08.2019.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H., Segonzac C. Manipulation of the host endomembrane system by bacterial effectors. Mol. Plant. Microbe Interact. 2023;36:208–217. doi: 10.1094/MPMI-09-22-0190-FI. [DOI] [PubMed] [Google Scholar]
- Jones D.T., Cozzetto D. DISOPRED3: precise disordered region predictions with annotated protein-binding activity. Bioinformatics. 2015;31:857–863. doi: 10.1093/bioinformatics/btu744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Khan M., Seto D., Subramaniam R., Desveaux D. Oh, the places they'll go! A survey of phytopathogen effectors and their host targets. Plant J. 2018;93:651–663. doi: 10.1111/tpj.13780. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim J., Kim M., Park J.T., Sohn K.H. Comparative analysis on natural variants of fire blight resistance protein FB_MR5 indicates distinct effector recognition mechanisms. Mol. Cells. 2024;47 doi: 10.1016/j.mocell.2024.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Jeon H., Lee H., Sohn K.H., Segonzac C. The Ralstonia pseudosolanacearum type III effector RipL delays flowering and promotes susceptibility to Pseudomonas syringae in Arabidopsis thaliana. Mol. Cells. 2023;46:710–724. doi: 10.14348/molcells.2023.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourelis J., Kaschani F., Grosse-Holz F.M., Homma F., Kaiser M., van der Hoorn R.A.L. A homology-guided, genome-based proteome for improved proteomics in the alloploid Nicotiana benthamiana. BMC Genom. 2019;20:722. doi: 10.1186/s12864-019-6058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry D., González-Fuente M., Deslandes L., Peeters N. The large, diverse, and robust arsenal of Ralstonia solanacearum type III effectors and their in planta functions. Mol. Plant Pathol. 2020;21:1377–1388. doi: 10.1111/mpp.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux C., Huet G., Jauneau A., Camborde L., Tremousaygue D., Kraut A., Zhou B., Levaillant M., Adachi H., Yoshioka H., et al. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell. 2015;161:1074–1088. doi: 10.1016/j.cell.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Lee A.H.Y., Hurley B., Felsensteiner C., Yea C., Ckurshumova W., Bartetzko V., Wang P.W., Quach V., Lewis J.D., Liu Y.C., et al. A bacterial acetyltransferase destroys plant microtubule networks and blocks secretion. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Staiger C.J. Understanding cytoskeletal dynamics during the plant immune response. Annu. Rev. Phytopathol. 2018;56:513–533. doi: 10.1146/annurev-phyto-080516-035632. [DOI] [PubMed] [Google Scholar]
- Li J., Henty-Ridilla J.L., Staiger B.H., Day B., Staiger C.J. Capping protein integrates multiple MAMP signalling pathways to modulate actin dynamics during plant innate immunity. Nat. Commun. 2015;6:7206. doi: 10.1038/ncomms8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Day B. Battlefield cytoskeleton: turning the tide on plant immunity. Mol. Plant Microbe Interact. 2019;32:25–34. doi: 10.1094/MPMI-07-18-0195-FI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonjon F., Turner M., Henry C., Rengel D., Lohou D., van de Kerkhove Q., Cazale A.C., Peeters N., Genin S., Vailleau F. Comparative secretome analysis of Ralstonia solanacearum type 3 secretion-associated mutants reveals a fine control of effector delivery, essential for bacterial pathogenicity. Mol. Cell Proteom. 2016;15:598–613. doi: 10.1074/mcp.M115.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A.P., Zipfel C. Targeting of plant pattern recognition receptor-triggered immunity by bacterial type-III secretion system effectors. Curr. Opin. Microbiol. 2015;23:14–22. doi: 10.1016/j.mib.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Macho A.P. Subversion of plant cellular functions by bacterial type-III effectors: beyond suppression of immunity. New Phytol. 2016;210:51–57. doi: 10.1111/nph.13605. [DOI] [PubMed] [Google Scholar]
- Mansfield J., Genin S., Magori S., Citovsky V., Sriariyanum M., Ronald P., Dow M., Verdier V., Beer S.V., Machado M.A., et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín M., Uversky V.N., Ott T. Intrinsic disorder in pathogen effectors: protein flexibility as an evolutionary hallmark in a molecular arms race. Plant Cell. 2013;25:3153–3157. doi: 10.1105/tpc.113.116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motion G.B., Amaro T.M., Kulagina N., Huitema E. Nuclear processes associated with plant immunity and pathogen susceptibility. Brief. Funct. Genom. 2015;14:243–252. doi: 10.1093/bfgp/elv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaihara T., Hatanaka T., Nakano M., Oda K. Ralstonia solanacearum type III effector RipAY is a glutathione-degrading enzyme that is activated by plant cytosolic thioredoxins and suppresses plant immunity. mBio. 2016;7:e00359–00316. doi: 10.1128/mBio.00359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar M.S., Carvunis A.R., Dreze M., Epple P., Steinbrenner J., Moore J., Tasan M., Galli M., Hao T., Nishimura M.T., et al. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science. 2011;333:596–601. doi: 10.1126/science.1203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S., Xu T., Lin D., Dhonukshe P., Zhang X., Friml J., Scheres B., Fu Y., Yang Z. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Mukaihara T. Ralstonia solanacearum type III effector RipAL targets chloroplasts and induces jasmonic acid production to suppress salicylic acid-mediated defense responses in plants. Plant Cell Physiol. 2018;59:2576–2589. doi: 10.1093/pcp/pcy177. [DOI] [PubMed] [Google Scholar]
- Nakano M., Oda K., Mukaihara T. Ralstonia solanacearum novel E3 ubiquitin ligase (NEL) effectors RipAW and RipAR suppress pattern-triggered immunity in plants. Microbiology. 2017;163:992–1002. doi: 10.1099/mic.0.000495. [DOI] [PubMed] [Google Scholar]
- Ortmann S., Marx J., Lampe C., Handrick V., Ehnert T.M., Zinecker S., Reimers M., Bonas U., Erickson J.L. A conserved microtubule-binding region in Xanthomonas XopL is indispensable for induced plant cell death reactions. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1011263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters N., Carrere S., Anisimova M., Plener L., Cazale A.C., Genin S. Repertoire, unified nomenclature and evolution of the Type III effector gene set in the Ralstonia solanacearum species complex. BMC Genom. 2013;14:859. doi: 10.1186/1471-2164-14-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K., Radivojac P., Vucetic S., Dunker A.K., Obradovic Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinform. 2006;7:208. doi: 10.1186/1471-2105-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior P., Ailloud F., Dalsing B.L., Remenant B., Sanchez B., Allen C. Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species. BMC Genom. 2016;17:90. doi: 10.1186/s12864-016-2413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi P., Zhang D., Zhang Y., Zhu W., Du X., Ma X., Xiao C., Lin Y., Xie J., Cheng J., et al. Ubiquitination and degradation of plant helper NLR by the Ralstonia solanacearum effector RipV2 overcome tomato bacterial wilt resistance. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114596. [DOI] [PubMed] [Google Scholar]
- Quentin M., Baurès I., Hoefle C., Caillaud M.C., Allasia V., Panabières F., Abad P., Hückelhoven R., Keller H., Favery B. The Arabidopsis microtubule-associated protein MAP65-3 supports infection by filamentous biotrophic pathogens by down-regulating salicylic acid-dependent defenses. J. Exp. Bot. 2016;67:1731–1743. doi: 10.1093/jxb/erv564. [DOI] [PubMed] [Google Scholar]
- Sabbagh C.R.R., Carrere S., Lonjon F., Vailleau F., Macho A.P., Genin S., Peeters N. Pangenomic type III effector database of the plant pathogenic Ralstonia spp. PeerJ. 2019;7 doi: 10.7717/peerj.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y., Wang Y., Ni H., Cazale A.C., She Y.M., Peeters N., Macho A.P. The Ralstonia solanacearum type III effector RipAY targets plant redox regulators to suppress immune responses. Mol. Plant Pathol. 2018;19:129–142. doi: 10.1111/mpp.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K.H., Lei R., Nemri A., Jones J.D.G. The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell. 2007;19:4077–4090. doi: 10.1105/tpc.107.054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Miranda J., Chodasiewicz M., Skirycz A., Fernie A.R., Moschou P.N., Bozhkov P.V., Gutierrez-Beltran E. Stress-related biomolecular condensates in plants. Plant Cell. 2023;35:3187–3204. doi: 10.1093/plcell/koad127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li P., Shen D., Wei Q., He J., Lu Y. The Ralstonia solanacearum effector RipN suppresses plant PAMP-triggered immunity, localizes to the endoplasmic reticulum and nucleus, and alters the NADH/NAD(+) ratio in Arabidopsis. Mol. Plant Pathol. 2019;20:533–546. doi: 10.1111/mpp.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D., Jones D.A., Hardham A.R. GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J. 2003;33:775–792. doi: 10.1046/j.1365-313x.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- Valls M., Genin S., Boucher C. Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum. PLoS Pathog. 2006;2 doi: 10.1371/journal.ppat.0020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lian N., Zhang Y., Man Y., Chen L., Yang H., Lin J., Jing Y. The cytoskeleton in plant immunity: dynamics, regulation, and function. Int. J. Mol. Sci. 2022;23:15553. doi: 10.3390/ijms232415553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Gu Y. The emerging role of biomolecular condensates in plant immunity. Plant Cell. 2021;34:1568–1572. doi: 10.1093/plcell/koab240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H.L., Chakravarthy S., Mathieu J., Helmann, Tyler C., Stodghill P., Swingle B., Martin G.B., Collmer A. Pseudomonas syringae pv. tomato DC3000 type III secretion effector polymutants reveal an interplay between HopAD1 and AvrPtoB. Cell Host Microbe. 2015;17:752–762. doi: 10.1016/j.chom.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win J., Chaparro-Garcia A., Belhaj K., Saunders D.G.O., Yoshida K., Dong S., Kamoun S. Effector biology of plant-associated organisms: concepts and perspectives. Cold Spring Harbor Symp. Quant. Biol. 2012;77:235–247. doi: 10.1101/sqb.2012.77.015933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material