Abstract
Advanced genetic and nutritional strategies aimed at modulating fat deposition can significantly reduce production costs and enhance profitability in the poultry industry. Melanophilin (MLPH) is recognized as a key gene regulating pigmentation as shown by diluted hair and feather coloration in MLPH mutant animals, including avian models. However, the effects of MLPH during fat accretion have not been studied yet. Therefore, the objectives of the current study are to measure the temporal expression of the MLPH gene during the adipocyte differentiation in vitro and in vivo and to investigate the effect of MLPH loss on fat accretion and adipocyte sizes in vivo using MLPH knockout quail model. The current in vitro studies reveal that MLPH gene expression levels were considerably elevated during adipogenesis in avian cells [101-fold in DF-1, 28.5-fold in chicken embryonic fibroblasts (CEF) and 4-fold in quail embryonic fibroblasts (QEF), compared to the undifferentiated cells of each cell type, p < 0.05]. In addition, fractionated fat cells (FC) showed increased expression levels of MLPH (5.7-fold, p < 0.05) compared to stromal-vascular cells (SVC). Using the MLPH knockout quail, disruption of the MLPH gene resulted in significantly reduced body weight (BW) and subcutaneous fat (S. Fat) pad weights compared to the wild type (WT) (p < 0.05). Further analysis through sectioning and staining of the fat tissues revealed that the mutation in Rab binding domain (RBD) of quail MLPH resulted in decreased fat cell sizes (p < 0.01). Overall, our data clearly demonstrated that MLPH can be a potential adipogenic marker gene, and MLPH may be associated with fat accretion in the gene edited quail model, highlighting the important role of MLPH in adipogenesis.
Keywords: Melanophilin, Adipocyte Differentiation, Genome Editing, Quail, Adipose hypotrophy
Introduction
Accumulation of fat in poultry species can greatly affect their health and productivity. In production settings, obesity in poultry can adversely impact reproductive efficiency and meat quality, resulting in economic losses. On the other hand, inadequate fat reserves can disrupt energy balance, especially during high-demand periods like molting, egg production, and growth. Maintaining proper regulation of adipose tissue is crucial for optimal health and productivity, ensuring that poultry have sufficient energy reserves to support those physiological phenomena while avoiding the negative effects of both excessive and insufficient fat accumulation.
Melanophilin (MLPH), as a member of the exophilin family, mainly consists of three binding domains: the Rab binding domain (RBD), the myosin-Va binding domain (MBD), and the actin binding domain (ABD), arranged from the N-terminus to the C-terminus (Fukuda et al., 2002). Natural or induced mutations of MLPH resulted in a conserved phenotype of hypopigmentation in humans (Ménasché et al., 2003), mice (Matesic et al., 2001), rabbits (Lehner et al., 2013), mink (Cirera et al., 2013), chickens (Vaez et al., 2008), and quail (Lee et al., 2019). Recently, we reported that MLPH regulates dendritogenesis in melanocytes and melanosome transportation, affecting feather pigmentation in quail (Kim et al., 2024). This consistent phenotype highlights the pivotal role of MLPH in pigmentation. However, the role of MLPH in adipose deposition has not been studied yet. The current study aims to investigate temporal expression of the MLPH gene during adipogenic differentiation of avian cells. In addition, using the previously developed MLPH knockout quail (Lee et al., 2019), the effect of MLPH loss on adipogenesis in vivo was investigated for the first time.
Materials and methods
Cell Culture and Inducing Adipogenesis
DF-1 cells (ATCC, #CRL-12203, a chicken fibroblast cell line) were cultured in Dulbecco's modified Eagle's medium (DMEM, #11965, Gibco) containing 10 % fetal bovine serum (#F4135, Sigma-Aldrich) and 1 % antibiotics (Antibiotic-Antimycotic, #15240112, Gibco). For culturing embryonic cells, chicken embryonic cells (CEF) and quail embryonic cells (QEF) were isolated at embryonic day (E) 6 or 5 and cultured as described in our previous study (Kim et al., 2020). To induce adipogenic differentiation, cells were grown in DMEM containing 10 % chicken serum (#16110, Gibco) and 1 % antibiotics for 3 days for DF-1 cells, or for 4 days for QEF and CEF. To isolate fat cell fractions, subcutaneous adipose tissue was collected from chicken. Tissue was fractionated into stromal-vascular cells (SVC) and fat cells (FC) as described previously (Song et al., 2013).
Oil red O staining
To visualize lipid droplets, cells were stained with Oil Red O (ORO, #O0625, Sigma-Aldrich) as described in our previous study (Kim et al., 2021b). Briefly, after fixation with 10 % neutral buffered formalin for 1 h, cells were washed with distilled water and stained with 60 % ORO solution for 1 h, and then images of stained cells were captured using an EVOS cell imaging system (Thermo Fisher Scientific).
RNA extraction and quantitative RT-PCR
Total RNA was isolated from DF-1 cells, CEF, and QEF using Trizol reagent (#15596026, Life Technologies Inc.) according to the manufacturer's instructions. cDNA was synthesized from the RNA and used to perform quantitative RT-PCR (qPCR) as described in our previous study (Kim et al., 2020). All the primer sequences used in this study were described in a previous study (Kim et al., 2020) except MLPH (NCBI reference number: XM_015868094.2, F: 5′-GACCTGAAGTGCAAGATAGACCA and R: 5′-CTAGAAGAGCTGAATTCCCCTTC). The relative quantification of gene expression was determined by using the 2−ΔΔCt method (Livak and Schmittgen, 2001) by normalizing to those of the endogenous RPS13 gene.
Animal usages and generation of MLPH knockout quail line
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University (Protocol No. 2015A00000013-R1). MLPH knockout quail were produced using procedures described in our previous study (Lee et al., 2019). All quail eggs were incubated in the same conditions, time, temperature, and humidity, and then all hatched quail were raised in the same circumstances, such as room temperature, the size of brooder cages, the same kind of feed, and free access to food and water. To compare fat weights and sizes between wild type and MLPH knockout quail (WT and HO, respectively), adipose tissues were collected from 12-week-old male quail.
Histological processing and measurement of number and size of adipose tissues
After collection of adipose tissues, they were fixed in 10 % neutral buffered formalin for 2 days and then sectioned into 5 μm slices after embedding in paraffin. The sections were stained with hematoxylin and eosin, and subsequently sizes were analyzed using NIH image J software (ImageJ, Ver. 1.52, http://imagej.nih.gov/ij) as following our previous study (Kim et al., 2021a).
Statistical analysis
Multiple means were compared by one-way ANOVA followed by Tukey's multiple comparison test. To compare characteristics between WT and HO quail, the data were analyzed by t-tests. p-value, p < 0.05, was considered as a statistically significant difference. All data were expressed as means ± SEM, and statistical analyses were performed by using the GraphPad Prism software (ver. 6.02).
Results and discussion
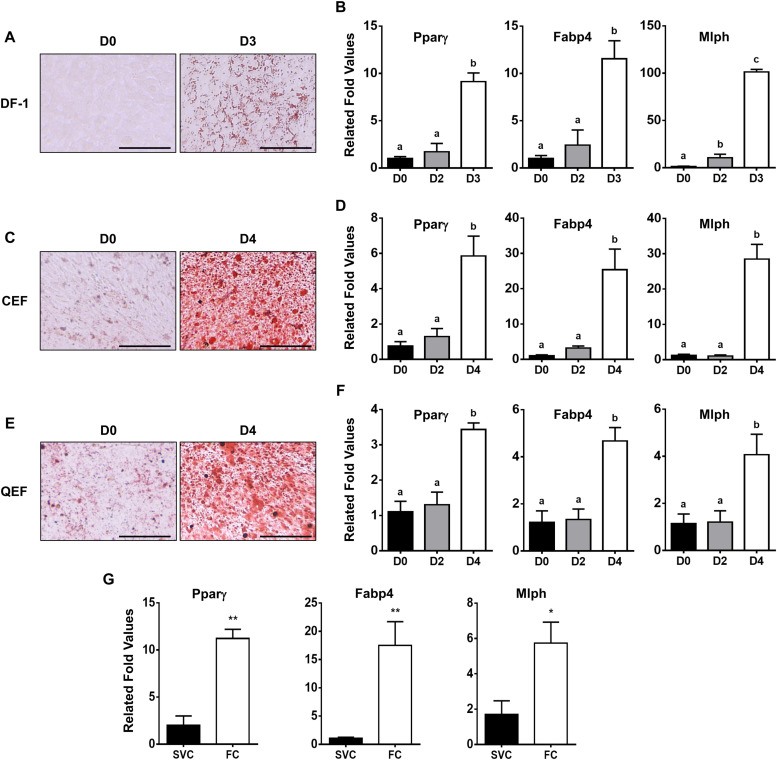
Our previous studies (Kim et al., 2021b; Lee et al., 2021) demonstrated that avian cells, such as DF-1, CEF, and QEF, have the potential to differentiate into adipocytes, which results in increased expression of genes involved in adipogenic differentiation. In the current study, adipogenic differentiation was induced in DF-1, CEF, and QEF (Fig. 1A, 1C and 1E), and pro-adipogenic marker genes, PPARγ and FABP4, were significantly increased in DF-1 at D3, and CEF and QEF at D4, following the induction of adipogenic differentiation, compared to their levels at D0. More specifically, the increase in fold changes for PPARγ and FABP4 were 9.1-fold and 11.6-fold in DF-1, and 5.9-fold and 25.4-fold in CEF, 3.4-fold and 4.7-fold in QEF, respectively (p < 0.05, Fig. 1B, 1D and 1F). In addition, the expression levels of the MLPH gene were considerably up-regulated in the avian cells during adipogenic differentiation, with fold changes of 101 in DF-1, 28.5 in CEF, and 4 in QEF, respectively (p < 0.05, Fig. 1B, 1D and 1F). Moreover, higher expression levels of PPARγ and FABP4 genes (11.2-fold and 17.5-fold, respectively, p < 0.05, Fig. 1G) were observed in the FC compared to the SVC in chicken adipose tissues, indicating successful separation of the two cell fractionations. MLPH expression was higher in the FC than the SVC (5.7-fold, p < 0.05, Fig. 1G), suggesting up-regulated expression of MLPH in mature adipocytes. These data, in the current study, support that MLPH could be a novel gene involved in adipogenesis in avian cells.
Fig. 1.
Increased expression of the MLPH gene during adipogenic differentiation in poultry in vitro. To induce adipogenic differentiation, cells were incubated with a medium, DMEM containing 10 % chicken serum, for 3 days (D3) for (A) DF-1 or 4 days (D4) for (C) CEF and (E) QEF. Oil Red O staining was visualized under a microscope. Scale bar: 100 μm. Relative expression levels of genes, PPARγ and FABP4 as major pro-adipogenic marker genes, and MLPH by qPCR (B, D and F). (G) Subcutaneous adipose tissues were separated into SVC and FC, and expression levels of the genes were analyzed. RPS13 was used as a house keeping gene. All data are shown as mean ± SEM (n = 4). For statistical analysis, one-way ANOVA was used for DF-1, CEF, and QEF, and t-test was used for SVC and FC by the GraphPad PRISM 6.02 program, p < 0.05. Abbreviations: CEF, chicken embryonic fibroblasts; QEF, quail embryonic fibroblasts; SVC, stromal-vascular cell; FC, fat cell; qPCR, quantitative real-time PCR.
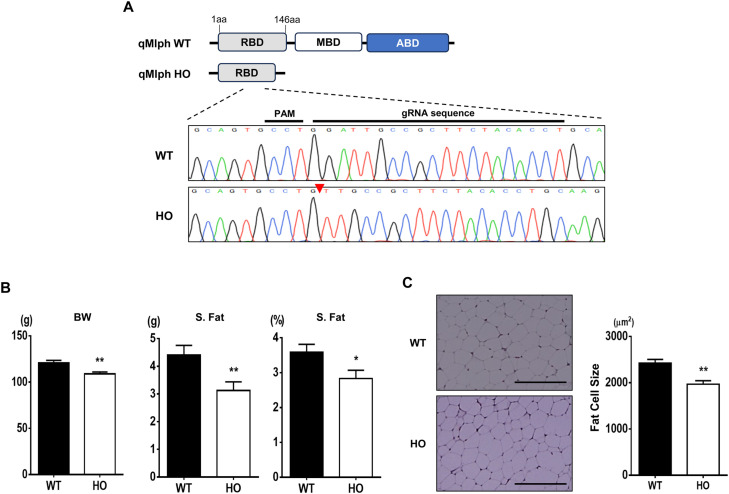
The MLPH knockout quail possessed a two base pair deletion in the RBD that resulted in a premature stop codon (Fig. 2A). Only male quail (WT and HO) were analyzed as female quail have different adipose tissue dynamics due to egg laying. The HO showed significantly reduced body weight (BW) and weights of S. Fat compared to the WT (WT vs. HO, 120.7 g vs. 108.8 g in BW, 4.4 g vs. 3.1 g in S. Fat, p < 0.01, Fig. 2B). When the S. fat weights were calculated as percentages of the total body weights, the HO had significantly reduced percentages of the fat pads (WT vs. HO, 3.6 % vs. 2.8 %, p < 0.05, Fig. 2B). Further analysis through sectioning and staining of adipose tissues revealed that the HO showed significantly decreased fat cell sizes compared to the WT (WT vs. HO, 2424 μm2 vs. 1968 μm2, p < 0.01, Fig. 2C). Overall, our data clearly demonstrate that MLPH is a candidate gene for regulating adipocyte differentiation, and disruption of MLPH exerts anti-adipogenic effects in the quail model, highlighting the important roles of MLPH in adipogenesis.
Fig. 2.
Phenotypic comparisons of adipose tissues between male MLPH WT and HO quail at 12-weeks-old. (A) Targeting loci of quail MLPH and sequencing analysis. To knockout MLPH in quail, the RBD was targeted, and results from sequencing analysis indicated 2 base pair deletion of the MLPH gene resulting in a premature stop codon. RBD, Rab-binding domain; MBD, myosin-Va binding domain; ABD, actin-binding domain. (B) Comparisons of body weight (BW) and subcutaneous fat tissues (S. Fat) in male quail at 12-weeks-old. *p < 0.05. **p < 0.01. n = 12 for WT and 11 for HO. (C) Histological differences in leg and neck fat tissues between WT and HO quail at 12-weeks-old. Hematoxylin and Eosin staining of leg and neck fat tissue. To measure fat cell size, five areas of each of the stained slides were randomly imaged, measured, and averaged. **p < 0.01. n = 5 for both WT and HO. t-test was used for the statistical analysis between WT and HO using the Graphpad PRISM 6.02 program. Scale bar: 200 μm.
Taken together, the current study clearly demonstrates that MLPH is upregulated during adipogenesis in vitro, and that disruption of the gene causes reduced adipose weights and fat cell sizes in quail. Our previous study (Kim et al., 2024) demonstrated that disruption of MLPH function negatively affected cellular trafficking and localization of melanosomes. Although it has not been completely elucidated what components are involved in regulating cellular trafficking of lipid droplets in the adipocytes, increasing evidence suggests that Rab family members are associated on lipid droplets and regulate fat accretion in adipocytes (Pulido et al., 2011; Li and Yu, 2016). Further studies are needed to determine if the MLPH/Rabs complex is involved in cellular trafficking of lipid droplets. In this regard, disruption of MLPH might impair trafficking and redistribution of lipid droplets, which could be a hypothetical mechanism of decreased fat accretion in MLPH knockout quail. To the best of our knowledge, this is the first study to reveal the anti-adipogenic effects of MLPH knockout in quail. Moreover, this study identifies MLPH as a candidate biomarker for adipogenic differentiation.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to Cameron McCurdy for the assistance on a part of histological examinations. This research was funded by the United States Department of Agriculture National Institute of Food and Agriculture Grant (Project No. 2022-67015-36482).
References
- Cirera S., Markakis M.N., Christensen K., Anistoroaei R. New insights into the melanophilin (MLPH) gene controlling coat color phenotypes in American mink. Gene. 2013;527:48–54. doi: 10.1016/j.gene.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kuroda T.S., Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Lee J.., Kim S., Lillehoj H.S., Lee K. Hypertrophy of adipose tissues in quail embryos by in ovo injection of all-trans retinoic acid. Front. Physiol. 2021;12:1–8. doi: 10.3389/fphys.2021.681562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H., Lee J., Ko J.-K., Lee K. Melanophilin regulates dendritogenesis in melanocytes for feather pigmentation. Commun. Biol. 2024;7:592. doi: 10.1038/s42003-024-06284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Lee J.., Suh Y., Cressman M., Lee K. Adipogenic differentiation of embryonic fibroblasts of chicken, turkey, duck, and quail in vitro by medium containing chicken serum alone. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Lee J.., Suh Y., Cressman M., Lee S.S., Lee K. Adipogenic and myogenic potentials of chicken embryonic fibroblasts in vitro: combination of fatty acids and insulin induces adipogenesis. Lipids. 2020;55:163–171. doi: 10.1002/lipd.12220. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim D.H., Suh Y., Lee K. Potential usage of DF-1 cell line as a new cell model for avian adipogenesis. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Ma J., Lee K. Direct delivery of adenoviral CRISPR/Cas9 vector into the blastoderm for generation of targeted gene knockout in quail. Proc. Natl. Acad. Sci. U. S. A. 2019;116:13288–13292. doi: 10.1073/pnas.1903230116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner S., Gähle M., Dierks C., Stelter R., Gerber J., Brehm R., Distl O. Two-exon skipping within MLPH is associated with coat color dilution in rabbits. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yu S.S.B. Rab proteins as regulators of lipid droplet formation and lipolysis. Cell Biol. Int. 2016;40:1026–1032. doi: 10.1002/cbin.10650. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matesic L.E., Yip R.., Reuss A.E., Swing D.A., O'Sullivan T.N., Fletcher C.F., Copeland N.G., Jenkins N.A. Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10238–10243. doi: 10.1073/pnas.181336698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménasché G., Ho C.H., Sanal O., Feldmann J., Tezcan I., Ersoy F., Houdusse A., Fischer A., de Saint Basile G. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1) J. Clin. Invest. 2003;112:450–456. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido M.R., Diaz-Ruiz A.., Jiménez-Gómez Y., Garcia-Navarro S., Gracia-Navarro F., Tinahones F., López-Miranda J., Frühbeck G., Vázquez-Martínez R., Malagón M.M. Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS One. 2011;6:e22931. doi: 10.1371/journal.pone.0022931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ahn J., Suh Y., Davis M.E., Lee K. Identification of novel tissue-specific genes by analysis of microarray databases: a human and mouse model. PLoS One. 2013;8:e64483. doi: 10.1371/journal.pone.0064483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaez M., Follett S.A., Bed'hom B., Gourichon D., Tixier-Boichard M., Burke T. A single point-mutation within the melanophilin gene causes the lavender plumage colour dilution phenotype in the chicken. BMC Genet. 2008;9:1–9. doi: 10.1186/1471-2156-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]