Highlights
-
•
Global retinoblastoma incidence increased from 1990 to 2021, while the overall burden declined.
-
•
Retinoblastoma burden remains higher in low SDI regions, reflecting socioeconomic disparities.
-
•
International aid has reduced retinoblastoma burden in some low SDI regions.
-
•
Females under two bear a higher retinoblastoma burden than males.
Keywords: Retinoblastoma, Global burden of disease, Public health, Socio-demographic index
Abstract
Background
Retinoblastoma (Rb), the primary intraocular malignancy in children, poses significant risks, yet its overall burden remains inadequately assessed. This study aims to analyze global Rb trends using Global Burden of Disease, Injuries, and Risk Factors study (GBD) 2021 data.
Methods
GBD 2021 data was analyzed to assess Rb incidence, mortality, and disability-adjusted life years (DALYs) from 1990 to 2021. Average annual percentage changes (AAPCs) were calculated across genders, age groups (0-9 years), and geographic regions categorized by socio-demographic index (SDI) quintiles.
Results
From 1990 to 2021, the global Rb age-standardized incidence rate (ASIR) increased from 0.08 (per 100,000, range: 0.05 to 0.10) to 0.09 (per 100,000, range: 0.06 to 0.13). ASIR was not significantly correlated with SDI (R = -0.095, P = 0.18), while age-standardized DALYs rate (R = -0.693, P < 0.001) and age-standardized mortality rate (ASMR) (R = -0.71, P < 0.001) were significantly and negatively correlated with SDI. Increases in ASIR were concentrated in Asia, Europe, and northern and southern Africa. The highest standardized DALYs and ASMR were noted in certain countries in Asia, Europe, and South Africa. Among age groups, the highest disease burdens were observed in the “0-6 days” and “2-4 years” groups. There were no significant gender differences in Rb burden globally.
Conclusions
Despite global progress, regions with lower SDI face elevated Rb burden and mortality. Females exhibit higher burdens during infancy, necessitating further investigation. Effective Rb management in resource-limited areas requires international collaboration focused on health education, early diagnosis, and prenatal screening for high-risk families.
Introduction
Retinoblastoma (Rb) is an aggressive eye cancer of infancy and childhood, which, if left untreated, results in death within 1-2 years.[1] Early diagnosis and prompt treatment significantly improve both vision and survival outcomes.[2] In low-income countries, the median age at diagnosis is 30.5 months, compared with 14.1 months in high-income countries.[3] Consequently, the prognosis for Rb patients in high-income countries is significantly better, with survival rates approaching 100 %, compared to about 50 % in low-income countries due to delayed diagnosis and poorer prognosis.[4,5]
Most previous research with Rb has been clinical and mechanistic, often lacking a global perspective on the disease burden. It was not until the Global Burden of Disease, Injuries, and Risk Factors study (GBD) 2021 that Rb was reported for the first time, providing data on the incidence, deaths, disability-adjusted life years (DALYs), and other relevant metrics at global and regional levels from 1990 to 2021.[6,7] Therefore, this study aims to summarize the global burden of Rb over the past 30 years. The objective is to thoroughly evaluate the burden of Rb using the GBD dataset by performing a detailed analysis of overall trends, disparities across socio-demographic index (SDI) quintiles, and differences in patient burden by region and gender. This study aims to bridge existing research gaps and provide novel perspectives on the current global burden of Rb.
Methods
Overview
This study utilized data from the GBD 2021 study which provides comprehensive and up-to-date estimates of the epidemiology of 371 diseases and injuries, 288 causes of death, and 88 risk factors in 21 geographic regions, 204 countries, and 811 subnational countries for different sex and age groups from 1990 to 2021.[[6], [7], [8]] (21 geographic regions and 204 countries: Supplementary Table 1) Detailed information on the study design and methodology of the GBD study is available in the existing literature.[6,7,9] Data from the GBD study are publicly available online through the Global Health Data Exchange platform (GHDx, https://collab2021.healthdata.org/gbd-results/). For the first time, the GBD has subdivided the under-5 age group and provided data on the disease burden for children aged 0-9. We extracted data on incidence, DALYs, mortality, and their age-standardized rates (ASR, per 100,000) for Rb from GHDx and performed secondary analyses.[6] This study does not contain personal or medical information about an identifiable living individual, and involves no animal subjects.
Data sources
GBD 2021 utilized 100,983 data sources to estimate DALYs, years of life lost (YLLs), disability life years (YLDs), and healthy life expectancy (HALE) for 371 diseases and injuries. These sources encompass vital registration systems, verbal autopsies, censuses, household surveys, disease-specific registries, health service linkage data, and other resources.[6] For most diseases and injuries, prevalence and incidence were modeled using DisMod-MR 2.1 (Disease Modeling Meta-Regression; version 2.1). Additionally, GBD 2021 estimated mortality and YLLs for 288 causes of death across 204 countries and territories from 1990 to 2021. These data sources included vital registries and cause-of-death inferences for all 288 causes, surveys, censuses, surveillance, cancer registries, police records, open-source databases, and minimally invasive tissue sampling.[7] YLDs were calculated by multiplying the cause-age-sex-location-year-specific prevalence of each disease and injury sequelae by their respective disability weights. YLLs were computed by multiplying the cause-age-sex-location-year of death by the standardized life expectancy at the time of death. DALYs represent the total number of years lost due to illness, disability, or premature death and are the sum of YLDs and YLLs.
Socio-demographic index and geographic regions
SDI is a composite indicator used to assess the socio-economic development level across different regions, with values ranging from 0 to 100. A higher SDI value typically indicates a higher per capita income, greater average level of schooling, lower fertility rate, and more abundant healthcare resources within the area.[6] In GBD 2021, 201 countries were categorized into five SDI groups: high, medium-high, medium, medium-low, or low SDI regions. The SDI effectively reflects the social, economic and health situation in different regions and identifies social inequalities.
Statistical analyses
All analyses were performed using R software (version 4.3.1) and Joinpoint software (version 5.0). The incidence, mortality, and DALYs of Rb at national and regional levels from 1990 to 2021 were analyzed.6ASRs were calculated to understand the relative burden of Rb across different genders, age groups, years, and regions. Global trends in Rb were analyzed using Joinpoint regression analysis. This analysis involved several steps: firstly, corresponding standard errors (SE) were obtained through logarithmic transformation and binomial approximation. Secondly, using a geometric weighted calculation based on population, gender, and age group values, we calculated the average annual percentage changes (AAPCs) of ASR and their corresponding 95 % confidence intervals (CIs). Specifically, the Annual Percentage Changes (APCs) represent the percentage changes over the study period (1990–2021), while the annualized rates of change are calculated by taking the difference in the natural logarithm of the values at the start and end of the time interval, divided by the number of years in the interval. AAPCs and CIs greater than 0 indicate an increasing trend, while those <0 indicate a decreasing trend.[10] Throughout the study, we analyzed the association between the SDI and the age-standardized incidence, mortality, and DALYs rates of Rb using locally weighted regression. Spearman's rank correlation tests were employed to examine these correlations. A P-value of less than 0.05 indicates a statistically significant association between the variables.
Role of the funding source
The funders had no role in the study design, data collection, analysis, interpretation, or report writing. All authors had full access to the study data and accepted responsibility for the decision to submit the manuscript for publication.
Results
Global Trends in Incidence, DALYs rate and Mortality Associated with Rb from 1990 to 2021
Globally, the ASIR for Rb showed an overall increasing trend, rising from 0.08 (per 100,000, range: 0.05 to 0.1) in 1990 to 0.09 (per 100,000, range: 0.06 to 0.13) in 2021 (AAPC = 0.62, range: 0.42 to 0.82) (Table 1 and Fig. 1A). This upward trend persisted until 2019, followed by a significant decrease from 2019 to 2021 (APC = -8.99) (Fig. 1). The total number of incident Rb cases increased to 6275 (range: 3033 to 5953) in 2021, reflecting a 34.27 % rise (range: 4.23 % to 64.09 %) from 1990 (Supplementary Tables 2 and 3). Globally, DALYs reached 243,204 (range: 147,358 to 330,474) in 2021 from a baseline of 279,006 (range: 159,656 to 365,150) in 1990. The age-standardized DALYs rate declined from 4.54 (per 100,000, range: 2.60 to 5.94) in 1990 to 3.65 (per 100,000, range: 2.21 to 4.96) in 2021 (AAPC = -0.69, range: -0.86 to -0.52) (Table 1 and Supplementary Table 2, Fig. 1B). Similarly, ASMR decreased from 0.05 (per 100,000, range: 0.03 to 0.07) to 0.04 (per 100,000, range: 0.03 to 0.06), with mortality cases of 3180 (range: 1819 to 4173) and 2762 (range: 1666 to 3761) in 1990 and 2021 respectively (Table 1 and Supplementary Table 2, Fig. 1C)
Table 1.
Age-standardized rates of Incidence, DALYs and Deaths due to Retinoblastoma in global and different regions in 1990 and 2021.
| location | ASIR |
ASDR |
ASMR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2021 | AAPC | P.Value | 1990 | 2021 | AAPC | P.Value | 1990 | 2021 | AAPC | P.Value | |
| Global | 0.08 (0.05 to 0.1) | 0.09 (0.06 to 0.13) | 0.62 (0.42 to 0.82) | <0.001 | 4.54 (2.6 to 5.94) | 3.65 (2.21 to 4.96) | -0.69 (-0.86 to -0.52) | <0.001 | 0.05 (0.03 to 0.07) | 0.04 (0.03 to 0.06) | -0.7 (-0.87 to -0.53) | <0.001 |
| SDI groups | ||||||||||||
| High SDI | 0.13 (0.11 to 0.15) | 0.12 (0.09 to 0.15) | -0.26 (-0.78 to 0.26) | 0.324 | 0.63 (0.52 to 0.74) | 0.26 (0.2 to 0.32) | -2.72 (-3.3 to -2.14) | <0.001 | 0.01 (0.01 to 0.01) | 0 (0 to 0) | -3.44 (-4.24 to -2.63) | <0.001 |
| High-middle SDI | 0.07 (0.04 to 0.1) | 0.13 (0.07 to 0.19) | 2.08 (1.29 to 2.87) | <0.001 | 2.03 (1.14 to 3.1) | 0.78 (0.39 to 1.16) | -3.17 (-3.35 to -2.98) | <0.001 | 0.02 (0.01 to 0.04) | 0.01 (0 to 0.01) | -3.4 (-3.62 to -3.18) | <0.001 |
| Middle SDI | 0.05 (0.03 to 0.07) | 0.08 (0.05 to 0.11) | 1.81 (1.51 to 2.12) | <0.001 | 2.85 (1.69 to 3.77) | 1.51 (0.86 to 2.04) | -2.12 (-2.32 to -1.92) | <0.001 | 0.03 (0.02 to 0.04) | 0.02 (0.01 to 0.02) | -2.18 (-2.39 to -1.98) | <0.001 |
| Low-middle SDI | 0.07 (0.04 to 0.1) | 0.08 (0.05 to 0.12) | 0.47 (0.33 to 0.62) | <0.001 | 5.79 (3.13 to 8.09) | 4.2 (2.52 to 5.82) | -1.02 (-1.24 to -0.8) | <0.001 | 0.07 (0.04 to 0.09) | 0.05 (0.03 to 0.07) | -1.03 (-1.25 to -0.81) | <0.001 |
| Low SDI | 0.13 (0.07 to 0.18) | 0.1 (0.06 to 0.15) | -0.73 (-0.86 to -0.6) | <0.001 | 11.38 (6.32 to 15.64) | 7.8 (4.7 to 11.24) | -1.19 (-1.35 to -1.02) | <0.001 | 0.13 (0.07 to 0.18) | 0.09 (0.05 to 0.13) | -1.18 (-1.34 to -1.02) | <0.001 |
| Geographic regions (21) | ||||||||||||
| Andean Latin America | 0.11 (0.06 to 0.19) | 0.18 (0.1 to 0.3) | 1.73 (1.06 to 2.39) | <0.001 | 8.09 (4.77 to 13.88) | 3.73 (2.31 to 6.08) | -2.54 (-2.91 to -2.18) | <0.001 | 0.09 (0.05 to 0.16) | 0.04 (0.03 to 0.07) | -2.6 (-2.97 to -2.22) | <0.001 |
| Australasia | 0.05 (0.04 to 0.07) | 0.02 (0.01 to 0.03) | -3.52 (-6.26 to -0.69) | 0.015 | 0.15 (0.12 to 0.2) | 0.02 (0.01 to 0.04) | -6 (-9.21 to -2.68) | <0.001 | 0 (0 to 0) | 0 (0 to 0) | -6.95 (-9.34 to -4.5) | <0.001 |
| Caribbean | 0.03 (0.02 to 0.05) | 0.01 (0.01 to 0.02) | -3.25 (-5.26 to -1.19) | 0.002 | 1.2 (0.76 to 1.71) | 0.41 (0.2 to 0.75) | -3.27 (-4.93 to -1.57) | <0.001 | 0.01 (0.01 to 0.02) | 0 (0 to 0.01) | -3.26 (-4.92 to -1.58) | <0.001 |
| Central Asia | 0.03 (0.01 to 0.07) | 0.06 (0.03 to 0.1) | 1.55 (1.15 to 1.96) | <0.001 | 1.52 (0.7 to 2.86) | 1.28 (0.7 to 2.27) | -0.6 (-1.17 to -0.03) | 0.04 | 0.02 (0.01 to 0.03) | 0.01 (0.01 to 0.03) | -0.64 (-1.21 to -0.06) | 0.03 |
| Central Europe | 0.03 (0.02 to 0.06) | 0.03 (0.02 to 0.05) | 0.36 (-1.79 to 2.56) | 0.744 | 0.8 (0.4 to 1.6) | 0.2 (0.13 to 0.32) | -3.93 (-5.31 to -2.53) | <0.001 | 0.01 (0 to 0.02) | 0 (0 to 0) | -4.16 (-5.54 to -2.76) | <0.001 |
| Central Latin America | 0.06 (0.04 to 0.08) | 0.08 (0.05 to 0.12) | 0.65 (0.26 to 1.04) | 0.001 | 3.58 (3.06 to 4.23) | 1.62 (1.1 to 2.3) | -2.72 (-3.38 to -2.06) | <0.001 | 0.04 (0.04 to 0.05) | 0.02 (0.01 to 0.03) | -2.77 (-3.43 to -2.11) | <0.001 |
| Central Sub-Saharan Africa | 0.05 (0.02 to 0.09) | 0.03 (0.01 to 0.07) | -1.33 (-1.45 to -1.21) | <0.001 | 4.53 (2.06 to 7.75) | 2.74 (1.08 to 5.26) | -1.65 (-1.75 to -1.56) | <0.001 | 0.05 (0.02 to 0.09) | 0.03 (0.01 to 0.06) | -1.64 (-1.74 to -1.54) | <0.001 |
| East Asia | 0.05 (0.03 to 0.08) | 0.14 (0.06 to 0.22) | 3.16 (2.53 to 3.79) | <0.001 | 2.45 (1.27 to 3.76) | 0.88 (0.37 to 1.32) | -3.38 (-4.15 to -2.6) | <0.001 | 0.03 (0.01 to 0.04) | 0.01 (0 to 0.01) | -3.65 (-4.43 to -2.86) | <0.001 |
| Eastern Europe | 0.06 (0.04 to 0.09) | 0.05 (0.04 to 0.07) | -0.91 (-1.62 to -0.2) | 0.012 | 1.23 (0.9 to 1.66) | 0.4 (0.3 to 0.54) | -3.48 (-5.05 to -1.9) | <0.001 | 0.01 (0.01 to 0.02) | 0 (0 to 0.01) | -3.61 (-5.2 to -1.99) | <0.001 |
| Eastern Sub-Saharan Africa | 0.28 (0.16 to 0.39) | 0.2 (0.12 to 0.31) | -1.05 (-1.17 to -0.93) | <0.001 | 23.71 (13.9 to 33.35) | 14.67 (9.7 to 22.54) | -1.51 (-1.61 to -1.4) | <0.001 | 0.27 (0.16 to 0.38) | 0.17 (0.11 to 0.26) | -1.51 (-1.61 to -1.4) | <0.001 |
| High-income Asia Pacific | 0.13 (0.1 to 0.16) | 0.16 (0.11 to 0.21) | 0.8 (-0.61 to 2.23) | 0.266 | 0.74 (0.55 to 1.01) | 0.29 (0.21 to 0.4) | -2.88 (-3.88 to -1.87) | <0.001 | 0.01 (0.01 to 0.01) | 0 (0 to 0) | -3.79 (-4.73 to -2.84) | <0.001 |
| High-income North America | 0.21 (0.17 to 0.25) | 0.11 (0.08 to 0.16) | -2.06 (-3.12 to -0.98) | <0.001 | 0.68 (0.6 to 0.77) | 0.24 (0.18 to 0.32) | -3.44 (-4.57 to -2.31) | <0.001 | 0.01 (0.01 to 0.01) | 0 (0 to 0) | -3.84 (-5 to -2.67) | <0.001 |
| North Africa and Middle East | 0.02 (0.01 to 0.04) | 0.04 (0.02 to 0.06) | 1.62 (1.29 to 1.96) | <0.001 | 1.35 (0.82 to 2.12) | 0.63 (0.39 to 1) | -2.43 (-2.71 to -2.14) | <0.001 | 0.02 (0.01 to 0.02) | 0.01 (0 to 0.01) | -2.5 (-2.93 to -2.06) | <0.001 |
| Oceania | 0.01 (0 to 0.04) | 0.02 (0.01 to 0.05) | 0.72 (0.05 to 1.39) | 0.035 | 1.19 (0.37 to 3.34) | 1.33 (0.37 to 4.39) | 0.32 (-0.36 to 1.01) | 0.352 | 0.01 (0 to 0.04) | 0.02 (0 to 0.05) | 0.32 (-0.36 to 1.01) | 0.354 |
| South Asia | 0.06 (0.03 to 0.09) | 0.08 (0.04 to 0.12) | 0.86 (0.6 to 1.13) | <0.001 | 5.23 (2.47 to 7.82) | 3.68 (2.06 to 5.48) | -1.09 (-1.3 to -0.87) | <0.001 | 0.06 (0.03 to 0.09) | 0.04 (0.02 to 0.06) | -1.1 (-1.32 to -0.89) | <0.001 |
| Southeast Asia | 0.04 (0.02 to 0.07) | 0.05 (0.03 to 0.09) | 1.25 (0.89 to 1.62) | <0.001 | 2.32 (1.01 to 3.86) | 1.47 (0.65 to 2.24) | -1.5 (-1.71 to -1.28) | <0.001 | 0.03 (0.01 to 0.04) | 0.02 (0.01 to 0.03) | -1.53 (-1.74 to -1.32) | <0.001 |
| Southern Latin America | 0.08 (0.04 to 0.14) | 0.06 (0.03 to 0.09) | -1.18 (-2.36 to 0.01) | 0.053 | 1.33 (0.88 to 2.11) | 0.32 (0.21 to 0.47) | -4.78 (-5.84 to -3.71) | <0.001 | 0.01 (0.01 to 0.02) | 0 (0 to 0) | -5.02 (-6.1 to -3.93) | <0.001 |
| Southern Sub-Saharan Africa | 0.03 (0.02 to 0.06) | 0.06 (0.02 to 0.11) | 1.96 (1.32 to 2.6) | <0.001 | 2.36 (1.24 to 3.57) | 3.31 (1.35 to 6) | 1 (0.42 to 1.58) | 0.001 | 0.03 (0.01 to 0.04) | 0.04 (0.02 to 0.07) | 0.98 (0.4 to 1.57) | 0.001 |
| Tropical Latin America | 0.05 (0.03 to 0.07) | 0.04 (0.03 to 0.06) | -0.52 (-0.98 to -0.06) | 0.026 | 3.14 (2.45 to 3.99) | 1 (0.67 to 1.38) | -3.64 (-4.09 to -3.19) | <0.001 | 0.04 (0.03 to 0.05) | 0.01 (0.01 to 0.02) | -3.67 (-4.12 to -3.22) | <0.001 |
| Western Europe | 0.13 (0.1 to 0.15) | 0.17 (0.12 to 0.21) | 0.91 (-0.66 to 2.51) | 0.258 | 0.5 (0.44 to 0.57) | 0.29 (0.22 to 0.38) | -1.57 (-3.07 to -0.05) | 0.043 | 0 (0 to 0.01) | 0 (0 to 0) | -2.57 (-4.18 to -0.93) | 0.002 |
| Western Sub-Saharan Africa | 0.11 (0.05 to 0.16) | 0.1 (0.04 to 0.16) | -0.51 (-0.7 to -0.32) | <0.001 | 9.74 (4.64 to 14.09) | 6.69 (2.81 to 10.58) | -1.23 (-1.46 to -1) | <0.001 | 0.11 (0.05 to 0.16) | 0.08 (0.03 to 0.12) | -1.23 (-1.46 to -0.99) | <0.001 |
ASIR, Age-standardized Incidence rates; ASDR, Age-standardized DALYs rate; ASMR, Age-standardized Mortality rates; AAPC, average annual percentage changes.
Fig. 1.
Retinoblastoma related burden from 1990 to 2021 across SDI quintiles.
Age-standardized incidence(A), DALYs (B) and mortality (C) rates of retinoblastoma. DALYs, disability-adjusted life years; SDI, socio-demographic index; APC, annual percentage change.
Among all SDI quintiles, high-middle SDI regions exhibited the sharpest increase in the overall ASIR (AAPC = 2.08, range: 1.29 to 2.87), rising by 100.58 % (range: 21.82 % to 222.23 %) from 0.07(per 100,000, range: 0.11 to 0.15) in 1990 to 0.13(per 100,000, range: 0.07 to 0.19) in 2021(Table 1 and Supplementary Table 3, Fig. 1A). Conversely, low-middle SDI regions showed a slower increase in ASIR (AAPC = 0.47, range: 0.33 to 0.62), from 0.07 (per 100,000, range: 0.04 to 0.10) in 1990 to 0.08 (per 100,000, range: 0.05 to 0.12) in 2021, remaining consistently lowest between 2003 and 2021 (Table 1, Fig. 1A).
The age-standardized DALYs rate and ASMR rankings across the five SDI groups were consistent over the years. All regions demonstrated a slight downward trend from 1990 to 2021 (Fig. 1B-C). Low SDI regions consistently had the highest values for both age-standardized DALYs rate and ASMR, but also demonstrated the largest downward trend changes. The age-standardized DALYs rate for low SDI regions decreased from 11.38 per 100,000 (range: 6.32 to 15.64) in 1990 to 7.8 per 100,000 (range: 4.70 to 11.24) in 2021 (AAPC = -1.19, range: -1.35 to -1.02) (Table 1, Fig. 1B). Similarly, the ASMR for low SDI regions decreased from 0.13 per 100,000 (range: 0.07 to 0.18) to 0.09 per 100,000 (range: 0.05 to 0.13) (AAPC = -0.18, range: -1.34 to -1.02) (Table 1, Fig. 1C).
Among the 21 geographical regions, five regions consistently had ASIR of Rb greater than 0.1 per 100,000 from 1990 to 2021: Eastern Sub-Saharan Africa, Andean Latin America, Western Europe, High-income Asia Pacific, and High-income North America (Supplementary Figure 1A). Among these, Eastern Sub-Saharan Africa ranked the highest in both 1990 and 2021, despite a slight drop of ASIR (AAPC = -1.05, range: -1.17 to -0.93) from 0.28 (per 100,000, range: 0.16 to 0.39) in 1990 to 0.2 (per 100,000, range: 0.12 to 0.31) in 2021 (Table 1). Notably, in 2013, the ASIR in High-income Asia Pacific peaked at 0.34 (per 100,000, range: 0.27 to 0.41) before declining rapidly to 0.16 (per 100,000, range: 0.11 to 0.21) in 2021 (Table 1). Additionally, from 1990 to 2021, the top five regions for age-standardized DALYs rate and ASMR were consistently Eastern Sub-Saharan Africa, Western Sub-Saharan Africa, Andean Latin America, South Asia, and Southern Sub-Saharan Africa. All regions experienced a declining trend (Supplementary Figure 1B-C). Eastern Sub-Saharan Africa demonstrated the largest change in both age-standardized DALYs rate (AAPC = -1.51, range: -1.61 to -1.40) and ASMR (AAPC = -1.51, range: -1.61 to -1.20) (Table 1).
Correlations between SDI and the burden of Rb
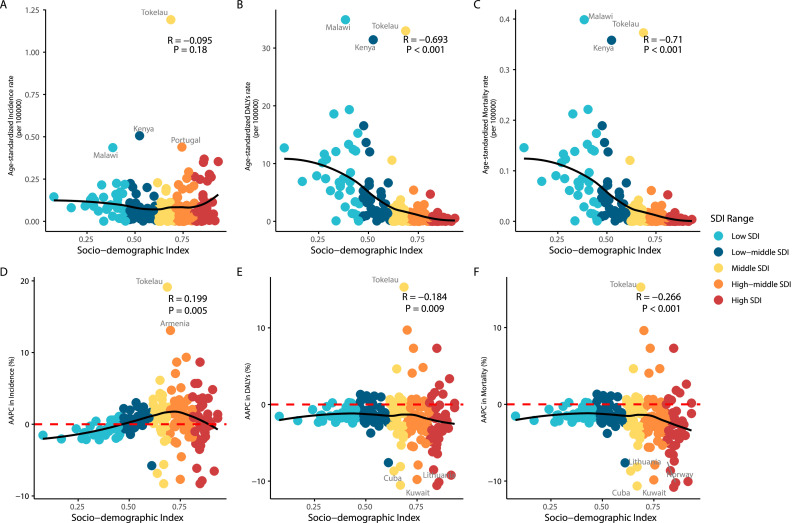
In 2021, the ASIR of Rb showed no significant correlation with SDI level (R = -0.095, P = 0.18) (Fig. 2A). However, the age-standardized rate and ASMR were significantly negatively correlated with the SDI level (R = -0.693, P < 0.001 and R = -0.71, P < 0.001 respectively) (Fig. 2B-C). The AAPC in ASIR was positively correlated with the SDI level (R = 0.199, P = 0.005), while the AAPC in age-standardized DALYs rate and ASMR were negatively correlated with the SDI level (R = -0.184, P = 0.009; R = -0.0266, P < 0.001, respectively) (Fig. 2D-F).
Fig. 2.
Retinoblastoma related burden and their AAPCs with different SDI levels.
Age-standardized incidence (A, D), DALYs (B, E) and mortality (C, F) rates of retinoblastoma. DALYs, disability-adjusted life years; SDI, socio-demographic index; AAPC, average annual percentage change. AAPC>0 represents an increase in the rate, and AAPC<0 represents a decrease in the rate.
Between 1990 and 2021, countries in the middle SDI and high-middle SDI regions predominantly showed an increasing trend in ASIR (Fig. 2D). Most countries and regions experienced ASIR changes of less than 10 %, with the exceptions of Tokelau and Armenia, which had AAPC values of 19.13 (range: 14.98 to 23.42) and 13.08 (range: 12.32 to 13.84), respectively (Supplementary Table 4). During this period, 79.7 % of countries saw a decrease in age-standardized DALYs rate, especially Cuba (AAPC = -10.53, range: -15.11 to -5.70), Kuwait (AAPC = -10.57, range: -12.67 to -8.41), and Lithuania (AAPC = -10.14, range: -11.51 to -8.74) (Supplementary Table 4). Similarly, 79.7 % of countries experienced a decline in ASMR, particularly Cuba (AAPC = -10.64, range: -15.20 to -5.83), Kuwait (AAPC = -10.80, range: -12.10 to -8.64), Lithuania (AAPC = -10.22, range: -11.63 to -8.80), and Norway (AAPC = -10.05, range: -12.10 to -7.96) (Supplementary Table 4). Notably, Tokelau was the only region showing a significant increase in both age-standardized DALYs rate and ASMR, with AAPC exceeding 10 (Fig. 2E-F).
Burden of Rb in 1990 and 2021, as well as AAPCs from 1990 to 2021 in 204 countries and territories
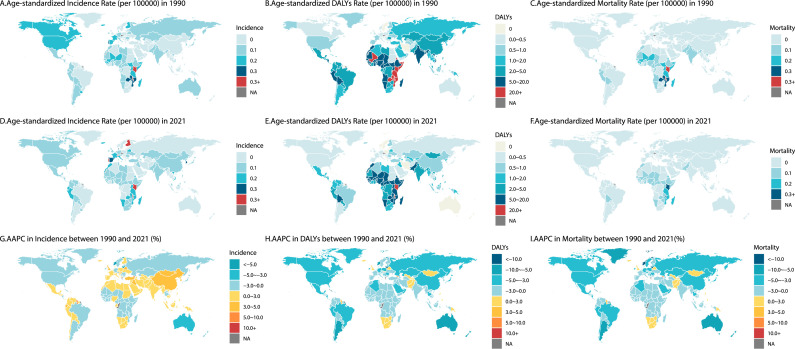
From 1990 to 2021, the age-standardized incidence rate (ASIR) of retinoblastoma (Rb) increased in 122 countries and regions (Fig. 3G). However, only 39 countries and regions showed an increase in the age-standardized DALYs rate, and 38 countries and regions exhibited an increase in the ASMR (Fig. 3H and I). Countries with rising ASIR were predominantly concentrated in Asia, Europe, northern and southern Africa, and certain Latin American nations. Additionally, some countries in Asia, Europe, and southern Africa also reported increases in the age-standardized DALYs rate.
Fig. 3.
Retinoblastoma related burden and their AAPCs from 1990 to 2021 in 204 countries and territories.
The incidence (A, D), DALYs (B, E) and mortality (C, F) rates for retinoblastoma in 1990 and 2021, respectively, as well as AAPCs in incidence (G), DALYs (H) and mortality (I) rates from 1990 to 2021 in 204 countries and territories. DALYs, disability-adjusted life years; AAPC, average annual percentage change. AAPC>0 represents an increase in the rate, and AAPC<0 represents a decrease in the rate.
According to Fig. 3 and Supplementary Table 4, in 1990, Kenya (0.60 per 100,000, range: 0.31 to 0.93), Malawi (0.55 per 100,000, range: 0.27 to 1.06), and Singapore (0.37 per 100,000, range: 0.26 to 0.48) had the highest ASIRs globally. However, by 2021, Tokelau (1.19 per 100,000, range: 0.25 to 3.86) emerged as the country with the highest ASIR. Malawi maintained the highest age-standardized DALYs rate both in 1990 and 2021 (47.89 per 100,000, range: 23.82 to 91.91, 34.90 per 100,000, range: 14.34 to 77.09 respectively). Between 1990 and 2021, Kenya and Malawi consistently had the highest ASMR, though both saw slight declines. Kenya's ASMR decreased from 0.54 (range: 0.28 to 0.81) to 0.36 (range: 0.20 to 0.57), and Malawi's ASMR dropped from 0.55 (range: 0.27 to 1.05) to 0.40 (range: 0.16 to 0.88).
The most pronounced upward trends in ASIR were seen in Tokelau (AAPC = 19.13, range: 14.98 to 23.42), Armenia (AAPC = 13.08, range: 12.32 to 13.84), and Cook Islands (AAPC = 9.34, range: 8.18 to 10.51). Tokelau also exhibited the fastest-growing age-standardized DALYs rate (AAPC = 15.32) and ASMR (AAPC = 15.27), followed by Armenia (AAPC = 9.71 & 9.61) and Georgia (AAPC = 7.33 & 7.30). Conversely, Cuba (AAPC = -8.30 & -8.29), Kuwait (AAPC = -8.29 & -7.89), and Lithuania (AAPC = -7.89 & -6.54) showed the most significant declines in these metrics.
Disease burden of Rb in seven age groups in 1990 and 2021
A bimodal distribution was observed with peak incidences occurring in the “0-6 days” and “2-4 years” age groups. In 2021, the ASIR for Rb was highest in the “0-6 days” age group at 1.02 (per 100,000, range: 0.63 to 1.41). This group also recorded the highest age-standardized DALYs rate and ASMR of 39.07 (per 100,000, range: 21.25 to 54.53) and 0.43 (per 100,000, range: 0.23 to 0.60), respectively (Supplementary Table 5, Fig. 4A-C). The ASIR increased sequentially across the five age groups: “7-27days”, “1-5months”, “6-11 months”, “12-23 months”, and “2-4 years”, with a second peak at “2-4 years” (ASIR = 0.91, per 100,000, range: 0.54 to 1.27). Similarly, the age-standardized DALYs rate and ASMR exhibited a secondary peak in this age group (Age-standardized DALYs rate = 38.79, per 100,000, range: 23.30 to 54.43; ASMR = 0.44, per 100,000, range: 0.26 to 0.62) (Supplementary Table 5, Fig. 4A-C).
Fig. 4.
The burden of retinoblastoma in different age groups.
(A-C) Age-standardized incidence (A), DALYs (B), mortality (C) rates of retinoblastoma in different age groups in 2021. (D) Proportion of retinoblastoma burden by age group in countries and regions in 1990 and 2021. DALYs, disability-adjusted life years.
Globally, Rb was most prevalent in the “2-4 years” age group (1990: 60.7 %; 2021: 58.2 %) with an increase in the percentage of cases in the “12-23 months” age group compared to 1990. In 2021, high-middle SDI regions exhibited a higher proportion of children with Rb onset before the age of two years (35.6 %), compared to low SDI regions, which had a significantly lower proportion (22.7 %). Among 21 geographical regions, Oceania and Western Sub-Saharan Africa had a lower percentage of Rb onset before the age of two. Conversely, these regions had the highest incidence percentages in the “2-4 years” age group in 2021 (91.4 % and 78.1 %, respectively). Regions with relatively earlier disease onset included East Asia, the Caribbean, North Africa and the Middle East (Fig. 4D).
Disease burden of Rb in different sexes from 1990 to 2021
Between 1990 and 2021, the ASIR of Rb generally increased, while the age-standardized DALYs rate and ASMR decreased. Overall, there were no significant differences in trends between genders. (Fig. 5G-I). However, analysis by age group and region revealed notable gender disparities in disease burden for the “0-6 days” age group (Fig. 5A-C). Specifically, in 2021, the ASIR was higher in females (1.34 per 100,000, range: 0.65 to 2.01) compared to males (0.73 per 100,000, range: 0.47 to 0.99) (Supplementary Table 5). The gender gap in age-standardized DALYs and ASMR further widened, with female age-standardized DALYs rate and ASMR of 56.13 per 100 000 (range: 23.15 to 84.10) and 0.62 per 100 000 (range: 0.25 to 0.92), which were more than twice as high as those in males (age-standardized DALYs rate: 23.15; ASMR: 0.25). In the “2-4 years” age group, ASIR was high in both sexes (Female: 0.89, Male: 0.92), with no significant gender disparity in age-standardized DALYs rate and ASMR (Supplementary Table 5). Globally, there was no significant gender difference in the burden of Rb (ASIR: Female = 0.09, Male = 0.09; age-standardized DALYs rate: Female = 3.67, Male = 3.64; ASMR: Female = 0.04, Male = 0.04) (Supplementary Table 5). However, regional variations in gender burden exist. In Eastern Europe, Oceania, and Eastern Sub-Saharan Africa, ASIR was higher amongst males, whereas Asia, Pacific, and East Asia had higher ASIR among females. Gender differences in age-standardized DALYs and ASMR were also observed in Oceania, Eastern Sub-Saharan Africa, and Western Sub-Saharan Africa (Supplementary Table 5).
Fig. 5.
The burden of retinoblastoma by gender.
(A-C) Age-standardized incidence (A), DALYs (B), mortality (C) rates of retinoblastoma in different age groups in 2021 by gender. (D-F) Age-standardized incidence (D), DALYs (E) and mortality (F) rates of retinoblastoma by gender in different countries and territories in 2021. (G-I) Age-standardized rates of incidence (G), DALYs (H) and mortality (I) of retinoblastoma by gender from 1990 to 2021. DALYs, disability-adjusted life years.
Discussion
Rb is the most prevalent malignant intraocular tumor among children and remains a significant global public health challenge, particularly in economically underdeveloped regions. The GBD 2021 study spans an analysis period from 1990 to 2021, encompassing 21 geographic regions and 204 countries. Utilizing this comprehensive dataset, we elucidate the dynamic changes of global Rb burden across SDI quintiles, nine age groups and genders. Our findings aim to underpin the development of more efficacious prevention and treatment strategies based on robust scientific evidence.
From 1990 to 2021, there was an upward trend in the ASIR of Rb. This increase is likely due to improvements in global medical care, which have enhanced disease detection capabilities. In high-middle and middle SDI regions, the ASIR continued to rise until 2019, likely driven by better economic conditions and healthcare systems such as comprehensive child wellness checkups and eye screenings.[11,12] In contrast, the ASIR declined in low SDI regions. This decrease may reflect a lower frequency of reported and treated cases, potentially due to limited ophthalmic screening and general health awareness.[3] Additionally, Rb is classified into hereditary and non-hereditary types. [13] In higher-income regions, where healthcare access is generally better, individuals who survive the disease may carry pathogenic RB1 genes, resulting in a higher proportion of familial cases.[3] On another note, the global decline in ASIR after 2019 may be partially attributable to the COVID-19 pandemic, which disrupted healthcare services worldwide and likely hindered disease diagnosis and reporting.[14]
Globally, the age-standardized DALYs rate and ASMR of Rb have declined from 1990 to 2021, yet significant disparities in disease burden persist across SDI groups. Interestingly, low SDI countries have witnessed the most substantial reductions in these rates, despite their disease burden remaining significantly higher than other regions. This suggests an enhanced recognition of early Rb symptoms and a boost in healthcare investments in low-income countries. International aid initiatives, such as those led by One Retinoblastoma World (1RBW)[15], World Eye Cancer Hope (WE C Hope)[16], and Retinoblastoma Network (Rb-NET)[16], which connects centres in higher and lower resource countries, have played crucial roles in raising awareness, resources, and support. These collaborative efforts have contributed to the reduction of age-standardized DALYs rate and ASMR in low SDI regions. In contrast, high SDI countries have experienced a lower and steadily decreasing burden of Rb, attributable to early screening protocols for ocular diseases in infants and children. For example, in the United States, American ophthalmic oncologists and pathologists have implemented screening guidelines targeted at high-risk families, underlining the critical role of early detection and intervention.[17]
From 1990 to 2021, the ASIR of Rb has exhibited an upward trend in Asia, North and South Africa, Europe, and several Latin American countries. According to the Global Retinoblastoma Study Group and study by Tomar et al., over 50 % of the global Rb cases are reported in Asia, followed by Africa.[3,18] Given that they are no significant racial differences in Rb presentations in previous studies[19,20], regional disparities are more likely to be attributed to environmental risk factors, parental occupational exposures (particularly for sporadic Rb cases)[21], and improved disease detection capabilities due to advancements in medical care.[3] Despite the observed increase in ASIR in certain regions, the overall DALYs rate and ASMR have decreased globally, signaling that improvements in care and treatment are having a positive effect in reducing the global disease burden. The African region, particularly countries like Kenya and Malawi, continues to carry the highest Rb-related mortality rates. However, international collaborations are beginning to make a positive impact. For example, the Retinoblastoma Network (Rb-NET) is working to improve treatment outcomes in six sub-Saharan African countries, whilst WE C Hope has developed the Kenya National Retinoblastoma Strategy (KNRbS) aiming to improve survival rates for children with Rb in the region.[22] Our findings indicate a reduction in the disease burden in Kenya and Malawi, suggesting that international cooperation has been impactful. These efforts could serve as models for addressing Rb in other underserved regions.
Rb most significantly impacts newborns aged 0-6 days and children aged 2-4 years, with delays in diagnosis observed in low SDI countries. The median age at diagnosis was 14.1 months in high-income countries compared to 30.5 months in low-income countries.[3] The earlier diagnosis in high-income countries can be attributed to better access to healthcare, including prenatal genetic testing and post-birth screenings for symptoms like leukocoria (white pupils).[23,24] Some Rb tumors develop in utero, and 7-10 % of Rb cases and 44-71 % of familial Rb cases are diagnosed during the neonatal period in developed countries.[25] In contrast, neonates with Rb are rarely diagnosed at birth in developing countries, often leading to delayed treatment and poorer outcomes.[26] Prompt diagnosis and treatment of Rb in infants lead to significantly improved vision and overall health outcomes.[27] The disease burden of Rb rises progressively with age, reaching a second peak at 2-4 years of age. This might be associated with non-sporadic cases of Rb, which accounts for 60-70 % of all presentations and has a later onset.[28] Given that Rb is a rapidly progressive malignant tumor, early diagnosis and treatment are crucial to improving prognostic outcomes.[29] Genetic counseling and screening are especially integral for high-risk families affected by familial Rb.[17] In less developed regions, urgent priorities include enhancing educational efforts and promoting prenatal screening to diagnose and treat Rb at earlier stages.
Overall, there was no significant gender difference in Rb globally from 1990 to 2021. Interestingly, however, females showed higher ASIR, age-standardized DALYs rate and ASMR in the first two years of life compared to males. Our results highlight a greater prevalence of female patients diagnosed with Rb before the age of two, which is consistent with a recent study in the US.[20] Notably, in the “0-6 days” age group, females exhibited a higher ASIR, indicating a greater risk for female infants during this critical period. Genetically, the incidence of Rb should be approximately equal between males and females since the RB1 pathogenic gene is located on chromosome 13 and can be inherited from either parent.[30] However, factors such as sex hormones and suprachoroidal receptor levels may contribute to the higher prevalence of disease burden in females during the first two years of life.[31,32] Although some studies have suggested a potential male predominance in Rb pathogenesis[33], recent research generally indicates no significant gender difference.[13,19,34] Additionally, the observed gender differences in the age-standardized DALYs rate and ASMR can largely be attributable to the barriers women face in accessing medical resources, driven by gender inequality, particularly in regions with pronounced gender discrimination.[34,35] Similar gender disparities in disease burden have been observed in other eye conditions, such as cataracts[36], diabetic retinopathy[37] and uncorrected refractive error.[38]
There are several limitations to note in this study. Firstly, like other studies based on the GBD database, the methodological limitations of GBD 2021 may introduce data source bias, particularly intensified in low-income areas where barriers to data collection and underreporting are more prevalent. Secondly, GBD 2021 data for Rb are limited to individuals nine years of age and younger. In practice, older children and adults can be diagnosed with Rb, especially if the tumor is slow-growing or if early symptoms go unnoticed.[39] These factors may result in an underestimation or skewed picture of the true global disease burden of Rb. Thirdly, this study focused solely on the overall global burden of Rb and did not explore data for hereditary and sporadic Rb in detail, despite the significant differences in their age of onset, clinical presentation, and risk of secondary tumors.[40] Lastly, variations in healthcare access and quality across regions could impact the accuracy of our findings as regions with limited healthcare infrastructure may have higher rates of undiagnosed or misdiagnosed cases. Therefore, more prospective cohort studies and mechanistic investigations are required to provide a clearer picture of the global burden of Rb.
Conclusion
From 1990 to 2021, the global Rb burden has significantly decreased. However, disparities persist, with low SDI regions continuing to bear a disproportionate share of the disease burden. Despite these challenges, progress driven by international organizations and policy interventions is evident. In particular, our analysis highlights a higher incidence of Rb among female newborns, particularly in the first two years of life, which warrants further investigation. Leveraging data from the GBD 2021 database, this study provides a comprehensive overview of the global impact of Rb over three decades. These insights aim to guide future epidemiological studies and bolster the development of targeted policies and healthcare strategies on both national and international fronts.
Funding
The work was supported by Key Research and Development Program of Zhejiang Province [grant number 2024C03204]; the National Natural Science Foundation Regional Innovation and Development Joint Fund [grant number U20A20386]; National Natural Science Foundation of China [grant number 82330032]; the grant from startup found of Zhejiang A & F University under Grant [grant number 203402007101]; and Leading Innovative and Entrepreneur Team of Zhejiang Province, MobiDrop (Zhejiang) Co., Ltd. [grant number 2022R02005]. The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
CRediT authorship contribution statement
Linyan Wang: Writing – original draft, Visualization, Software, Data curation. Jianing Chen: Writing – original draft, Validation, Software, Data curation. Yunhan Shen: Writing – review & editing, Visualization, Software. Grace Loy Ming Hooi: Writing – review & editing. Shuohan Wu: Writing – review & editing. Feng Xu: Writing – review & editing. Hao Pei: Writing – review & editing, Funding acquisition. Jianpeng Sheng: Writing – review & editing. Tiansheng Zhu: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Juan Ye: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Hao Pei is the shareholder of MobiDrop (Zhejiang) Co., Ltd. No conflict of interest for other authors.
Contributor Information
Tiansheng Zhu, Email: tszhu@fudan.edu.cn.
Juan Ye, Email: yejuan@zju.edu.cn.
References
- 1.Zhao J., Feng Z., Gallie B.L. Natural history of untreated retinoblastoma. Cancers. (Basel) 2021;13(15) doi: 10.3390/cancers13153646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byroju V.V., Nadukkandy A.S., Cordani M., Kumar L.D. Retinoblastoma: present scenario and future challenges. Cell Commun. Signal. 2023;21(1):226. doi: 10.1186/s12964-023-01223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Retinoblastoma Study G. Fabian I.D., Abdallah E., et al. Global retinoblastoma presentation and analysis by national income level. JAMa Oncol. 2020;6(5):685–695. doi: 10.1001/jamaoncol.2019.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Retinoblastoma Study G The Global Retinoblastoma Outcome Study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob. Health. 2022;10(8):e1128–e1e40. doi: 10.1016/S2214-109X(22)00250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong E.S., Choy R.W., Zhang Y., et al. Global retinoblastoma survival and globe preservation: a systematic review and meta-analysis of associations with socioeconomic and health-care factors. Lancet Glob. Health. 2022;10(3):e380–e3e9. doi: 10.1016/S2214-109X(21)00555-6. [DOI] [PubMed] [Google Scholar]
- 6.Diseases GBD, Injuries C Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024 doi: 10.1016/S0140-6736(24)00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborators GBDCoD Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024 doi: 10.1016/S0140-6736(24)00367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborators GBDRF Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2162–2203. doi: 10.1016/S0140-6736(24)00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborators GBDU-M Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet. 2021;398(10303):870–905. doi: 10.1016/S0140-6736(21)01207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Dimaras H., Kimani K., Dimba E.A., et al. Retinoblastoma. Lancet. 2012;379(9824):1436–1446. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 12.Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol. Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 13.Fabian I.D., Sagoo M.S. Understanding retinoblastoma: epidemiology and genetics. Community Eye Health. 2018;31(101):7. [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian I.D., Stacey A.W., Bowman R., et al. Retinoblastoma management during the COVID-19 pandemic: a report by the Global Retinoblastoma Study Group including 194 centers from 94 countries. Pediatr. Blood Cancer. 2021;68(1):e28584. doi: 10.1002/pbc.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girdler H., Flegg K., Prochaska J., Dimaras H. Characterization of international partnerships in global retinoblastoma care and research: a network analysis. PLOS. Glob. Public Health. 2021;1(12) doi: 10.1371/journal.pgph.0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman R. Retinoblastoma: a curable, rare and deadly blinding disease. Community Eye Health. 2018;31(101):1–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Skalet A.H., Gombos D.S., Gallie B.L., et al. Screening children at risk for retinoblastoma: consensus report from the American association of ophthalmic oncologists and pathologists. Ophthalmology. 2018;125(3):453–458. doi: 10.1016/j.ophtha.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Tomar A.S., Finger P.T., Gallie B., et al. Global retinoblastoma treatment outcomes: association with national income level. Ophthalmology. 2021;128(5):740–753. doi: 10.1016/j.ophtha.2020.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Shields C.L., Dockery P.W., Yaghy A., et al. Intra-arterial chemotherapy for retinoblastoma in 341 consecutive eyes (1,292 infusions): comparative analysis of outcomes based on patient age, race, and sex. J. AAPOS. 2021;25(3):150. doi: 10.1016/j.jaapos.2020.12.006. e1- e9. [DOI] [PubMed] [Google Scholar]
- 20.Black A.K., Kahn A.E., Lamy C., Warman R., Barengo N.C. The association between race and age of diagnosis of retinoblastoma in United States children. J. AAPOS. 2024;28(1) doi: 10.1016/j.jaapos.2023.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Koochakzadeh L., Yekta A., Hashemi H., Pakzad R., Heydarian S., Khabazkhoob M. Epidemiological aspect of retinoblastoma in the world: a review of recent advance studies. Int. J. Ophthalmol. 2023;16(6):962–968. doi: 10.18240/ijo.2023.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J.A., Kimani K., White A., et al. Achieving optimal cancer outcomes in East Africa through multidisciplinary partnership: a case study of the Kenyan National Retinoblastoma Strategy group. Global. Health. 2016;12(1):23. doi: 10.1186/s12992-016-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh L., Chinnaswamy G., Meel R., et al. Epidemiology, diagnosis and genetics of retinoblastoma: ICMR consensus guidelines. Indian J. Pediatr. 2024 doi: 10.1007/s12098-024-05085-2. [DOI] [PubMed] [Google Scholar]
- 24.Neriyanuri S., Raman R., Rishi P., Govindasamy K., Ramprasad V.L., Sharma T. Prenatal genetic diagnosis of retinoblastoma–clinical correlates on follow-up. Indian J. Ophthalmol. 2015;63(9):741–742. doi: 10.4103/0301-4738.170979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivela T.T., Hadjistilianou T. Neonatal retinoblastoma. Asia Pac. J. Oncol. Nurs. 2017;4(3):197–204. doi: 10.4103/apjon.apjon_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chantada G.L., Dunkel I.J., Qaddoumi I., et al. Familial retinoblastoma in developing countries. Pediatr. Blood Cancer. 2009;53(3):338–342. doi: 10.1002/pbc.21970. [DOI] [PubMed] [Google Scholar]
- 27.Soliman S.E., Dimaras H., Khetan V., et al. Prenatal versus postnatal screening for familial retinoblastoma. Ophthalmology. 2016;123(12):2610–2617. doi: 10.1016/j.ophtha.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Galvez C.C., Ordaz-Favila J.C., Villar-Calvo V.M., Cancino-Marentes M.E., Bosch-Canto V. Retinoblastoma: review and new insights. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.963780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramson D.H., Schefler A.C., Beaverson K.L., Rollins I.S., Ruddat M.S., Kelly C.J. Rapid growth of retinoblastoma in a premature twin. Arch. Ophthalmol. 2002;120(9):1232–1233. doi: 10.1001/archopht.120.9.1232. [DOI] [PubMed] [Google Scholar]
- 30.Mallipatna A., Marino M., Singh A.D. Genetics of retinoblastoma. Asia Pac. J. Ophthalmol. (Phila) 2016;5(4):260–264. doi: 10.1097/APO.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 31.Gamulescu M.A. [Gender medicine in ophthalmology: the "small difference" between women and men] Ophthalmologe. 2020;117(8):831–842. doi: 10.1007/s00347-020-01174-7. [DOI] [PubMed] [Google Scholar]
- 32.Field M.G., Kuznetsoff J.N., Zhang M.G., et al. RB1 loss triggers dependence on ESRRG in retinoblastoma. Sci. Adv. 2022;8(33):eabm8466. doi: 10.1126/sciadv.abm8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong J.R., Tucker M.A., Kleinerman R.A., Devesa S.S. Retinoblastoma incidence patterns in the US Surveillance, epidemiology, and end results program. JAMa Ophthalmol. 2014;132(4):478–483. doi: 10.1001/jamaophthalmol.2013.8001. [DOI] [PubMed] [Google Scholar]
- 34.Fabian I.D., Khetan V., Stacey A.W., et al. Sex, gender, and retinoblastoma: analysis of 4351 patients from 153 countries. Eye (Lond) 2022;36(8):1571–1577. doi: 10.1038/s41433-021-01675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khera R., Jain S., Lodha R., Ramakrishnan S. Gender bias in child care and child health: global patterns. Arch. Dis. Child. 2014;99(4):369–374. doi: 10.1136/archdischild-2013-303889. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert C.E., Lepvrier-Chomette N. Gender inequalities in surgery for bilateral cataract among children in low-income countries: a systematic review. Ophthalmology. 2016;123(6):1245–1251. doi: 10.1016/j.ophtha.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 37.Khandekar R., Mohammed AJ. Gender inequality in vision loss and eye diseases: evidence from the Sultanate of Oman. Indian J. Ophthalmol. 2009;57(6):443–449. doi: 10.4103/0301-4738.57153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou L., Liu X., Tang X., Wang L., Ye J. Gender inequality in global burden of uncorrected refractive error. Am. J. Ophthalmol. 2019;198:1–7. doi: 10.1016/j.ajo.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Meel R., Kashyap S., Bakhshi S., Singh Bajaj M., Wadhwani M. Retinoblastoma in children older than 6 years of age. Ocul. Oncol. Pathol. 2020;6(6):395–404. doi: 10.1159/000509040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Othman I.S. Retinoblastoma major review with updates on Middle East management protocols. Saudi. J. Ophthalmol. 2012;26(2):163–175. doi: 10.1016/j.sjopt.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]