Abstract
Objectives
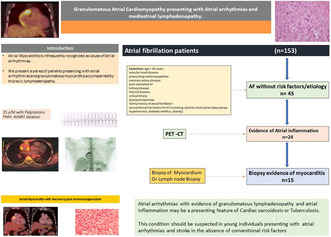
We present a case series of patients with granulomatous myocarditis presenting as atrial arrhythmias accompanied by lymphadenopathy.
Background
Atrial myocarditis (AM) may be the cause of atrial fibrillation (AF) in patients without risk factors.
Methods
Patients with atrial fibrillation without risk factors underwent 18F‐Fluorodeoxyglucose positron emission tomography (18F‐FDG‐PET). We performed biopsy of lymph nodes or myocardium in patients with atrial uptake of 18F‐FDG‐PET.
Results
AM was observed in 15 patients. The median age of the patients was 42 years and left ventricular ejection fraction (LVEF) at presentation was 45%. All patients had AF, atrial flutter was noted in 4 patients (26.7%) and 2 patients (13.3%) had atrioventricular nodal reentrant tachycardia (AVNRT). 18F‐FDG‐PET uptake was noted in the atria in all patients and in the ventricles in 3 patients (20%). Cardiac sarcoidosis was the diagnosis in 12 patients (80%) while 3 patients (20%) had tuberculosis. The median CHA2DS2 VASc score was 1. Four patients (26.7%) presented with ischemic stroke. All patients were treated with disease‐specific therapy in addition to antiarrhythmic medications. Over a median follow up of 26 months, a significant improvement in clinical status commensurate with a decline in atrial uptake was noted. A non‐significant improvement in LVEF to 56% with disease‐specific therapy was observed. (p = 0.09).
Conclusion
Atrial fibrillation with granulomatous lymphadenopathy may be a presenting feature of AM. The risk of stroke is high in these individuals. AM should be suspected in young individuals presenting with atrial fibrillation and stroke without conventional risk factors.
Keywords: Atrial fibrillation, Atrial myocarditis
Atrial myocarditis characterized by granulomatous inflammation typical of sarcoidosis or tuberculosis, can be a cause of atrial fibrillation in young patients who don't exhibit classical risk factors. It can present with extracardiac features such as lymphadenopathy and stroke. This emphasizes the importance of recognizing and managing the underlying inflammatory condition.
1. INTRODUCTION
The pathophysiology of atrial fibrillation (AF) involves complex changes such as electrical remodelling and structural remodelling, with fibrosis being a central pathological feature. 1 Replacement fibrosis where connective tissue ousts the atrial myocardium is the net result of myriad insults to the myocardium. Atrial inflammation has been recognized to be an important feature of certain AF etiologies, namely post‐operative AF, obesity, infection, and autoimmune diseases. 2 , 3 The role of myocarditis involving predominantly the atrial musculature has been recognized but its role in the pathogenesis of AF has not been firmly established. 4 , 5 The potential causes of inflammatory atrial myocarditis (AM) include infections, sarcoidosis, giant cell myocarditis, rheumatic heart disease, connective tissue disorders and drugs. The manifestations of AM could range from atrial arrhythmias, stroke, valve dysfunction to sinus nodal dysfunction. 6 , 7
Detection of inflammation and its management has been established in the management of ventricular arrhythmias due to granulomatous myocarditis. 8 The important diagnostic modalities in this aspect are nuclear imaging and biopsies, either of the myocardium or draining lymph nodes. In this study we describe the presentation, clinical features, diagnostic strategies, and management of patients presenting with atrial arrhythmias with atrial inflammation on imaging and histopathological studies, in the absence of conventional risk factors.
2. METHODS
2.1. Patient population
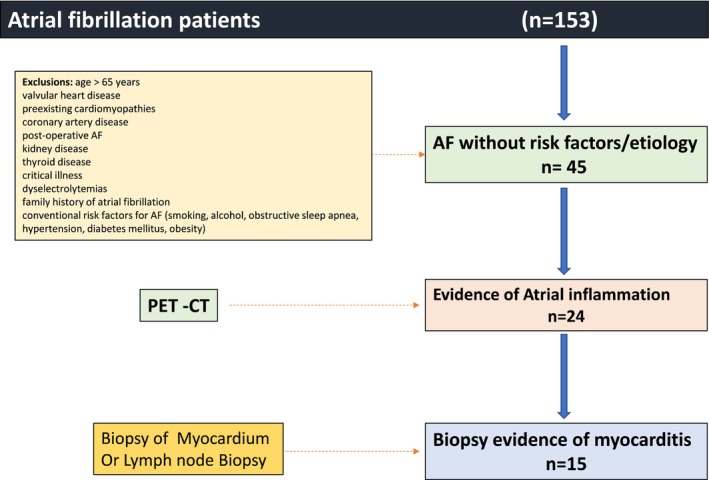
We evaluated 153 patients with age <65 years referred to our center for AF between January 2015 and July 2018. We excluded patients with age >65 years, valvular heart disease, preexisting cardiomyopathies, coronary artery disease, post‐operative AF, kidney disease, thyroid disease, critical illness, significant dyselectrolytemias, family history of AF, and patients with conventional risk factors for AF (smoking, alcohol, obstructive sleep apnea, hypertension, diabetes mellitus, obesity). 9 We also excluded patients with ventricular arrhythmias. In this study, we describe the clinical features of these patients presenting with sustained atrial arrhythmias (AF and atrial flutter) with evidence of inflammation on imaging and biopsy.
We evaluated the patient charts and reports for information on past illness, procedures, and therapies. Informed consent was obtained from all patients.
2.2. Diagnostic evaluation
Detailed history and clinical examination were performed in all patients. Laboratory tests for complete blood counts (CBC), erythrocyte sedimentation rate (ESR), hs CRP, renal function, liver function, thyroid function, and electrolytes were performed in all patients. Coronary artery disease was ruled out by stress testing and coronary angiography when indicated. A 12‐lead electrocardiogram at baseline and during the tachyarrhythmia was evaluated in all patients. The diagnostic evaluation protocol that was used has been summarized in the Figure 1. The following imaging modalities were also used in all patients:
Echocardiography—A detailed echocardiogram was conducted for biventricular function, regional wall motion, and valve dysfunction. We also measured the left atrial (LA), right atrial (RA) volumes, and the left ventricular ejection fraction (LVEF). The American Society of Echocardiography (ASE) recommendations for chamber quantification were used for these measurements. 10 We used the 2012 WHF echocardiographic criteria for rheumatic heart disease to rule out rheumatic heart disease. We also ruled out acute rheumatic fever with the modified Jones criteria. 11
Delayed gadolinium enhancement Cardiac Magnetic Resonance (CMR)—A CMR was performed for ventricular function and delayed enhancement in the ventricular myocardium in 6 of these patients.
Positron Emission Tomography (18 FDG‐PET‐CT)—Was performed in all the patients according to standard protocols with a 16‐slice scanner (Siemens). The 18F‐FDG‐PET/CT images were obtained with patients on a high fat, low carbohydrate diet for 24 hwith an overnight fast of 12–16 h prior to the imaging. The images were interpreted visually for evidence of uptake in the atrial or ventricular myocardium. We recorded the site of uptake in the atria and classified it according to the following regions: (1) Right atrial appendage (RAA) (2) Right atrial free wall (RA) (3) Interatrial septum (IAS) (4) Left atrial appendage (LAA) (5) Left atrial anterior wall (LAAW) and left atrial posterior wall (LAPW)
Computerized tomography and magnetic resonance imaging of the brain was performed in patients presenting with stroke.
Evaluation of etiology—A detailed physical examination for evidence of rheumatic fever, sarcoidosis, and tuberculosis. A previous history of tuberculosis was also sought. A tuberculin skin test was performed in all patients with 5 tuberculin units of purified protein derivative. An induration of greater than 10 mm at 48 h was interpreted as a positive test. A surgical biopsy of the right atrial appendage was performed in 1 patient and a biopsy of enlarged lymph nodes was performed in all the patients. The biopsy specimens were evaluated by histopathology with Gram's staining and haematoxylin and eosin staining. Staining and cultures for mycobacterium and fungi were performed in all the biopsy specimens. A polymerase chain reaction for tuberculosis was performed in all the patients on tissue biopsy specimens. The diagnosis of cardiac sarcoidosis was according to the Expert Consensus Recommendation Criteria by Birnie et al. 12 The diagnosis of cardiac sarcoidosis was based on Histopathological or clinical criteria and confirmed as definite with a histological diagnosis from myocardial tissue or probable based on clinical criteria and extracardiac biopsy diagnosis.
FIGURE 1.
Diagnostic strategy in patients suspected of having Atrial myocarditis.
2.3. Management
2.3.1. Antiarrhythmic therapy
Initially all patients were treated with rate control medications such as ß‐blockers, digoxin, and calcium channel antagonists. The rhythm control medications used were sotalol, flecainide, and amiodarone. Oral anticoagulation was recommended in all patients with evidence of myocarditis.
2.3.2. Electrophysiology study (EPS) and radiofrequency ablation
An EPS with ablation of atrial flutter (AFL) was performed in 4 patients who continued to have arrhythmias despite AADs. The ablation was performed with three‐dimensional mapping system (CARTO, Biosense Webster), a Stockert radiofrequency generator (Stockert GmBh, Freiburg, Germany), and an 8‐F irrigated tip ablation catheter. The success of atrial flutter ablation was defined as bidirectional block and atrioventricular nodal reentrant tachycardia (AVNRT) ablation was defined as non‐inducibility.
2.3.3. Management of underlying disease
Therapy of myocarditis in the form of either sarcoidosis or tuberculosis was added to the management of patients after appropriate diagnosis. Patients were treated with oral corticosteroids (prednisolone 0.5 mg/kg/day to a maximum dose of 60 mg/day) for 8 weeks initially. Patients were evaluated at every follow‐up visit by clinical evaluation, ECG, echocardiography, and 18FDG‐PET scans. After 8 weeks, the corticosteroids were tapered, and oral methotrexate started concurrently at a dose of 7.5 mg/week. Methotrexate was continued for 2 years. The duration and titration of therapy were guided by disease response. The response was assessed by 18F‐FDG‐PETs and clinical evaluation. After the initial phase, patients were followed up at 3–6 monthly intervals. In patients with evidence of tuberculosis, anti‐tuberculosis therapy was instituted according to standard recommendations.
2.4. Statistical methods
Continuous variables that did not show a normal distribution were expressed as a median with interquartile range. Categorical variables were represented as frequencies and percentages. The paired t‐test was used to show the effect of treatment on LA, RA dimensions, LVEF, and inflammatory parameters.
3. RESULTS
Out of the 153 patients referred with AF, there were 45 patients without an underlying etiology or risk factors for AF. We evaluated these 45 patients for evidence of atrial inflammation. 18F FDG‐PET demonstrated atrial uptake in 24 patients. Biopsy evidence of myocarditis was found in 15 out of these 24 patients. Atrial tachyarrhythmias with evidence of atrial inflammation and mediastinal lymphadenopathy were observed in 15 patients (30%).
3.1. Baseline characteristics
The median age of the study population at presentation was 42 years with a male predominance (73.3%). The left ventricular ejection fraction at presentation was 45% and the left atrial volume was 35 mL/m2. None of the patients had the clinical features of acute rheumatic fever, tuberculosis, or systemic sarcoidosis. Baseline clinical characteristics are presented in Table 1. One of the patients had a pacemaker for sinus nodal dysfunction. Median follow‐up was 26 months. None of the patients had ventricular arrhythmias at presentation. At presentation, the NYHA class was 3. Four patients (26.7%) presented with an ischemic stroke and one patient had a left atrial thrombus (6.7%). Spontaneous echo contrast was noted in 3 of the 15 patients (20%). The mean CHA2DS2 VASc score of the patients at presentation was 1.
TABLE 1.
Baseline characteristics.
| Clinical characteristic | |
|---|---|
| Age (years) a | 42 (34–52) |
| Gender (M) | 11 (73.3%) |
| Ejection fraction (LVEF %) a | 45% (37–60) |
| LA volume (mL/m2) a | 35 (30–38) |
| Systolic Blood pressure (mm Hg) a | 122 (106–134) |
| Diastolic Blood pressure (mm Hg) a | 73 (67–83) |
| Body mass index (BMI) a | 25 (23–28) |
| Hemoglobin (grams/dL) a | 13.3 (11–13.9) |
| White blood cell count (WBC/μL) a | 9211 (6180–10,867) |
| Erythrocyte sedimentation rate (ESR mm/h) a | 17.5 (13–19) |
| High sensitivity C‐reactive protein (hs CRP mg/dL) a | 9.2 (6.6–12) |
| Estimated glomerular filtration rate (GFR) a | 82 (78–87) |
| CHA2DS2 VASc score a | 1 (1–1.5) |
| Arrhythmia | |
| Atrial fibrillation | 15 (100%) |
| Atrial flutter | 4 (26.7%) |
| SVT | 2 (13.3%) |
| Ischemic stroke | 4 (26.7%) |
| Antiarrhythmic Medications | |
| Amiodarone | 3 (20%) |
| Flecainide | 2 (13.3%) |
| Sotalol | 3 (20%) |
| Rate control medications | |
| ß‐Blockers | 13 (86.7%) |
| Calcium channel blockers | 2 (13.3%) |
| Digoxin | 3 (20%) |
| Diagnosis | |
| Cardiac Sarcoidosis | 12 (80%) |
| Cardiac sarcoidosis/tuberculosis | 3 (20%) |
| Follow‐Up (months) | 30.5 |
Age, EF, LA volume, systolic blood pressure, diastolic blood pressure, BMI, hemoglobin, WBC count, ESR, hs CRP, GFR, and CHA2DS2VASc score have been expressed as Median with interquartile ranges.
3.2. Atrial arrhythmias
AF was the presenting arrhythmia in all these patients and AFL was observed in 4 patients (26.7%). Two patients (13.3%) also had typical AVNRT. One of the patients presented with AF, AFL, and an AVNRT and 5 patients had another dysrhythmia in addition to AF. The AF was paroxysmal in 8 patients (53.3%) and persistent in 7 patients (46.7%). The patients had significant symptoms such as dyspnoea (85.7%), chest discomfort (57.1%), palpitations (78.6%), and light‐headedness (50%). None of the patients had syncope. Four of these patients presented with stroke (26.7%) during which AF was detected. All the strokes were ischemic and none of the patients had hemorrhagic stroke. Cardioversion was performed in 9 of these patients (60%). At follow‐up, 4 (26.7%) of these patients had recurrent AF episodes.
3.3. Imaging
18F FDG‐PET scans showed evidence of atrial inflammation in all patients. The atrial uptake was seen in the right atrial appendage in 7 patients (46.7%), right atrial wall in 13 patients (86.7%), interatrial septum in 2 patients (13.3%), left atrial appendage in 4 patients (26.7%), anterior left atrial wall in 5 patients (33.3%), and posterior atrial wall in 4 patients (26.7%). (Table 2) Biatrial involvement was observed in 5 patients (33.3%). In addition, increased uptake in the left ventricular myocardium was noted in 3 patients (20%). This was noted in the basal septal region in 2 patients and at the LV apex in 2 patients and LV lateral wall in one patient.
TABLE 2.
Clinical features of patients with atrial arrhythmias with inflammatory cardiomyopathy.
| Patient number | Age (years) | Sex | LVEF (%) | Follow‐up (months) | Arrhythmia | Site of lymphadenopathy | Lymph nodes with FDG‐PET uptake | Atrial FDG‐PET uptake | Biopsied node | PPD | M.tb PCR | AFB culture | Diagnosis | Disease‐specific therapy | Presentation | CHA2DS2‐Vasc score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 61 | 24 | AF | M, C | M, C | RA | C | P | N | P | CS/TB | PDN + ATT | Symptomatic AF | 2 |
| 2 | 53 | M | 68 | 26 | AF, AFL | M | M | RA, RAA, IAS, LAA, LAPW, LA | M | N | N | N | CS | PDN | Symptomatic AFL/AF | 2 |
| 3 | 48 | F | 60 | 24 | AF | M | M | RA | M | P | N | N | CS | PDN | Symptomatic AF | 1 |
| 4 | 25 | M | 60 | 42 | AF, AFL, AVNRT | M, A | M | RA, RAA | RAA biopsy | N | N | N | CS | PDN | Initially with AVNRT followed by symptomatic AF/AFL | 2 |
| 5 | 62 | F | 32 | 31 | AF | M, C | M, C | RA, RAA, IAS, LAA, LA | C | N | N | N | CS | PDN | Heart failure and AF | 2 |
| 6 | 37 | M | 65 | 28 | AF | M | M | RA | M | N | N | N | CS | PDN | Symptomatic AF | 0 |
| 7 | 42 | F | 35 | 22 | AF | M, S | M, S | LAA, LAPW, LA | S | P | P | NA | CS/TB | PDN + ATT | Heart failure and AF | 2 |
| 8 | 58 | M | 45 | 42 | AF | M | M | RA | M | P | NA | P | CS/TB | PDN + ATT | Symptomatic AF | 0 |
| 9 | 21 | M | 54 | 62 | AF | M | M | RA, RAA | M | N | N | N | CS | PDN | Symptomatic AF | 0 |
| 10 | 42 | F | 35 | 51 | AF, AFL | M | M | RA | M | N | N | N | CS | PDN | Heart failure and AFL | 2 |
| 11 | 52 | M | 36 | 8 | AF | M | M | LAA, LAPW, LA | M | N | N | N | CS | PDN | Symptomatic AF | 2 |
| 12 | 32 | M | 39 | 19 | AF, AVNRT | M | M | RA, RAA | M | N | N | N | CS | PDN | AF with AVNRT | 0 |
| 13 | 28 | M | 43 | 24 | AF | M, C | M | RA | M | N | N | N | CS | PDN/MT | Symptomatic AF | 0 |
| 14 | 48 | M | 49 | 30 | AF, AFL | M | M | RA, RAA | M | N | N | N | CS | PDN | Symptomatic AFL | 0 |
| 15 | 40 | M | 39 | 25 | AF | M | M | RA, RAA, LA, LAPW | M | P | N | N | CS | PDN/MT | Symptomatic AF | 1 |
Abbreviations: 18FDG, 18fluorodeoxyglucose; A, axillary lymphadenopathy; AF, atrial fibrillation; AFB, acid fast bacilli; AFL, atrial flutter; ATT, anti‐tuberculosis therapy; AVNRT, atrioventricular nodal reentrant tachycardia; C, cervical lymphadenopathy; CS, cardiac sarcoidosis; IAS, interatrial septum; LA, left atrium; LAA, left atrial appendage; LAPW, posterior wall of left atrium; LVEF, left ventricular ejection fraction; M, mediastinal lymphadenopathy; M.tb PCR, polymerase chain reaction to detect Mycobacterium tuberculosis; Mtx, methotrexate; N, negative; NA, not available; P, positive; PDN, prednisolone; RA, right atrium; RAA, Right atrial appendage; S, supraclavicular lymphadenopathy; TB, tuberculosis.
A CMR was performed in 6 patients and was normal in 5 patients. One patient had evidence of delayed enhancement in the midmyocardial aspect of the anterolateral LV. No atrial abnormalities were detected on MRI. Figures 2 and 3 depict two representative cases.
FIGURE 2.
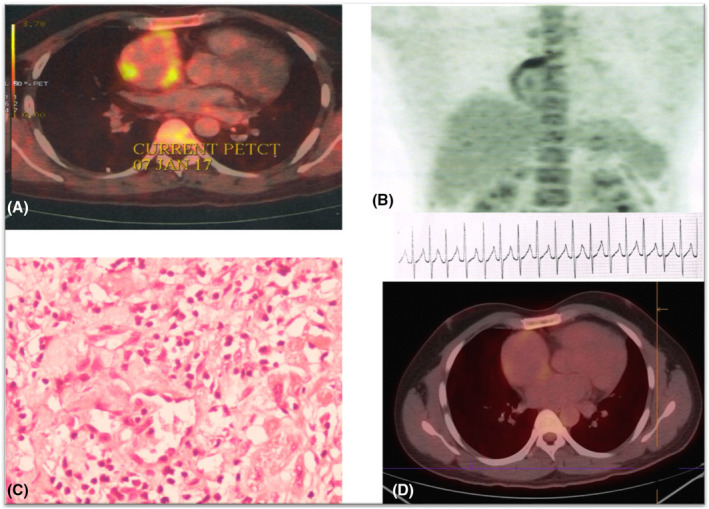
(A, B) 18‐Fluorodeoxyglucose positron emission tomography (18FDG‐PET) scan showing atrial uptake. (C) Atrial biopsy specimen showing inflammatory infiltrate and granuloma. (D) Shows resolution of inflammation after institution of disease‐specific therapy with corticosteroids.
FIGURE 3.
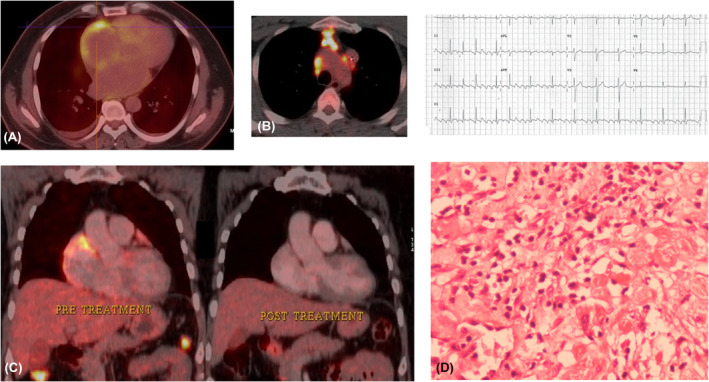
(A) 18‐Fluorodeoxyglucose positron emission tomography (18FDG‐PET) scan showing atrial uptake and (B) Mediastinal lymph node uptake. (C) 18FDG‐PET scans showing resolution of inflammation after disease‐specific therapy. (D) Histopathology showing granulomatous infiltrate.
3.4. Extracardiac involvement
Lymphadenopathy was the only extracardiac involvement and this was observed in the mediastinum in 12 patients (80%), axillary region in 1 patient (6.7%), cervical region in 2 (13.3%), and supraclavicular region in 1 patient (6.7%). 18F‐FDG‐PET showed uptake in mediastinal nodes in 15 patients (100%) and in other regions in 3 patients (20%) (Table 2). The HRCT of the chest showed no abnormalities in the pulmonary parenchyma. There was no clinical evidence of rheumatological disease.
3.5. Histopathology
Eleven of the patients underwent needle aspiration of the mediastinal lymph nodes, while the remaining patients had lymph node biopsy from cervical, supraclavicular, or axillary regions. One patient had biopsy of the RA appendage (surgical). There was evidence of inflammation in all patients either on myocardial biopsy or biopsy of lymph nodes. 14 (93.3%) patients showed evidence of granulomatous inflammation. Granulomatous inflammation without caseation was noted in 11 patients (73.3%) while caseation or necrosis was observed in 3 (20%) patients. One patient showed evidence of lymphocytic infiltrate suggestive of lymphocytic myocarditis.
3.6. Underlying disease
The tuberculin skin test was positive in 5 patients (33.3%) out of these 3 patients showed evidence of caseation and necrosis in biopsy specimens. M. tuberculosis deoxyribonucleic acid PCR was positive in 1 of the 15 patients (6.7%) and Mycobacterium tuberculosis was cultured in two patients on lymph node biopsy sample (13.3%). None of these patients exhibited evidence of pulmonary tuberculosis either at presentation or anytime in the past. So, tuberculosis was the underlying etiology of granulomatous inflammation in 3 patients (20%).
Sarcoidosis was diagnosed based on the Expert Consensus Diagnostic criteria in the remaining 12 patients (80%).
3.7. Electrophysiological study (EPS)
Out of these 15 patients, an EPS with radiofrequency (RF) ablation of AFL was performed in 3 patients (20%) and one patient had a successful slow pathway ablation for typical AVNRT. Of the 3 patients with AFL, 2 patients received a cavotricuspid isthmus line for right atrial flutter, which was successful. One patient had a left atrial flutter ablation which was unsuccessful.
3.8. Management
All patients received disease‐specific therapy in the form of immunosuppression or anti‐tuberculosis therapy. Immunosuppression therapy included prednisolone or methotrexate. Patients received rate control with ß‐blockers, calcium channel antagonists, and digoxin. Antiarrhythmic drugs used for rhythm control were flecainide, amiodarone, and sotalol. The clinical characteristics and therapies are summarized in Table 2. RFA of AFL and supraventricular tachycardia was performed at the onset in 3 patients along with disease‐specific therapy. One patient who had an unsuccessful ablation and was treated with disease‐specific therapy and had no recurrence of arrhythmia.
Overall, the initiation of disease‐specific therapy reduced the incidence of atrial arrhythmias at 26 months of follow‐up. Table 3 shows the changes in clinical parameters at baseline and at follow‐up. This corresponded to resolution of atrial inflammation on 18F‐FDG‐PET‐CTs. There was a significant improvement in functional class of patients from NYHA 3 to NYHA 1 at follow‐up (p = 0.005). Importantly, there was an improvement in LV ejection fraction from 48.1% to 56% but this did not achieve statistical significance (p = 0.0853). The RA and LA volumes were not significantly different from baseline. There was a significant decline in inflammatory parameters, with the hs CRP declining from a baseline value of 11–2.9 at follow‐up (p = 0.02). Recurrence of atrial arrhythmias was noted in 4 patients at follow‐up and these two of the patients needed another cardioversion and escalation of immunosuppression due to persistent 18F‐FDG‐PET uptake.
TABLE 3.
Clinical parameters at baseline and at follow‐up after disease‐specific therapy.
| Baseline | Follow‐up of 26 months | p‐value | |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 45 | 56 | 0.08 |
| NYHA functional class | III | I | 0.005 |
| LA volume (mL/m2) | 35 | 34 | 0.89 |
| High sensitivity C‐reactive protein (hs CRP mg/dL) | 11 | 2.9 | 0.02 |
One patient had a decline in LVEF at follow‐up and had Ventricular uptake on 18FDG‐PET‐CT.
3.9. Oral anticoagulation
Fourteen out of the 15 patients were treated with oral anticoagulation with vitamin K antagonists to maintain an INR of 2–3. One patient was not anticoagulated based on patient preference.
4. DISCUSSION
The main findings of this study are: (1) granulomatous myocarditis may present as recurrent atrial arrhythmia including AF. (2) These patients have a higher risk of stroke (26.7%) and predisposition to thromboembolism in the absence of traditional risk factors. (3) Isolated atrial involvement was observed in over 80% of this patient population. The etiology of granulomatous myocarditis could either be cardiac sarcoidosis or tuberculosis. (4) They respond well to immunosuppression (Sarcoidosis) and anti‐tuberculosis therapy (TB).
The entity of AM has been described in the past but has not received much attention. An important study by Frustaci et al. showed evidence of atria limited myocarditis in 66% of “lone” AF patients studied by endomyocardial biopsy of atria. 13 A study has also demonstrated antibodies against myosin in the sera of AF patients. 14 A series of 13 patients with AM has also shown that atrial giant cell myocarditis (GCM) has a favorable prognosis when compared to the classic variety of GCM. 4 Our data indicates that “isolated AM” could be a substrate for atrial arrhythmias, which could also be life threatening. 15
The sensitivity of 18F‐FDG‐PET in detecting cardiac involvement in sarcoidosis has been around 87.5%. 16 Cardiac MRI also aids in the diagnosis but its value in detecting atrial pathology is not well established. The sensitivity and predictive value of 18F‐FDG‐PET scan for detecting AF was 54% and 96.1% in a recent study. 17
An autopsy study of young sudden death victims with ventricular pre‐excitation showed evidence of AM in 50%. 15 Since inflammation is a potent pro‐thrombotic state, the risk of thromboembolic complications could be heightened in this population. In the present study, 26.7% of patients with AM presented with an ischemic stroke with LA thrombus detected in one patient. Inflammation may alter the electrical milieu, resulting in multiple atrial arrhythmias that could be refractory to conventional management. Our study had 33.3% of the patients presenting with more than one atrial arrhythmia. Involvement of the sinus node may lead to sinoatrial node disease and bradycardia. Other possible presentations could be idiopathic atrial enlargement, atrial tumor mimic, macro reentrant arrhythmias, and AF. 6 , 18
Corticosteroids have been conventionally used to manage myocarditis. A customized approach to granulomatous myocarditis has been shown to improve outcomes in patients presenting with ventricular arrhythmias. Patients with evidence of myocardial inflammation have been shown to benefit from immunosuppression and based on 18F‐FDG‐PET uptake. 19 All our patient's received immunosuppression with corticosteroids and methotrexate. After establishing an etiological diagnosis of granulomatous or lymphocytic myocarditis we managed our patients with immunosuppression in addition to standard rate and rhythm control for AF. At follow‐up there was recurrence of AF in 26.7% of patients. This was managed by intensification of immunosuppression along with standard antiarrhythmic medications and cardioversion.
5. LIMITATIONS
This is a single center study with a limited number of patients. The prospective cohort of patients is from a region where tuberculosis and sarcoidosis are prevalent. The etiology of myocarditis may vary in different parts of the world. Nevertheless, the study provides a framework for the evaluation of AF patients when no cause is readily evident. Larger studies from multiple regions may be required to shed light on the etiology of AM in different geographies.
Serological testing for viruses and anti‐myosin cardiac antibodies was not performed.
Brain imaging was performed only in patients presenting with stroke, so silent emboli may have been missed.
Though we could follow up with our patients for a significant period, longer follow‐up may be needed to observe the consequences of this condition.
6. CONCLUSIONS
AM could be prominent cause of AF in young patients without the classical risk factors. This could be characterized by granulomatous inflammation typical of sarcoidosis or tuberculosis. The prominent extracardiac features of this syndrome include thoracic lymphadenopathy and an exaggerated stroke risk. This entity has an excellent response to disease‐specific therapy.
FUNDING INFORMATION
There are no funding sources for this study.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interests for this article.
ETHIC STATEMENT
Informed consent was obtained from all patients before the procedures performed in the study. No individual or a group of individuals can be identified in the study or in the images provided.
Kumar S, Yalagudri S, Saggu D, Mansoor M, Tourani VK, Narasimhan C. Atrial arrhythmias with mediastinal lymphadenopathy presentation of isolated atrial myocarditis. J Arrhythmia. 2025;41:e13181. 10.1002/joa3.13181
DATA AVAILABILITY STATEMENT
The authors of this study confirm that the data supporting the findings of this study are available within the article and is derived from the Sarcoid clinic database at this center.
REFERENCES
- 1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264–2274. 10.1161/CIRCULATIONAHA.111.019893 [DOI] [PubMed] [Google Scholar]
- 2. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. 10.1038/nrcardio.2015.2 [DOI] [PubMed] [Google Scholar]
- 3. Kusayama T, Furusho H, Kashiwagi H, Kato T, Murai H, Usui S, et al. Inflammation of left atrial epicardial adipose tissue is associated with paroxysmal atrial fibrillation. J Cardiol. 2016;68(5):406–411. 10.1016/j.jjcc.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 4. Larsen BT, Maleszewski JJ, Edwards WD, Cooper LT Jr, Sobonya RE, Thompson VE, et al. Atrial giant cell myocarditis: a distinctive clinicopathologic entity. Circulation. 2013;127(1):39–47. 10.1161/CIRCULATIONAHA.112.128900 [DOI] [PubMed] [Google Scholar]
- 5. Mccrea PC. Childers RW two unusual cases of giant cell myocarditis associated with mitral stenosis and with Wegener's syndrome. Br Heart J. 1964;26(4):490–498. 10.1136/hrt.26.4.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wohlgelernter D, Otis CN, Batsford WP, Cabin HS. Myocarditis presenting with silent atrium and left atrial thrombus. Am Heart J. 1984;108(6):1557–1558. 10.1016/0002-8703(84)90711-7 [DOI] [PubMed] [Google Scholar]
- 7. Gillie I, Fox H. Mitral stenosis together with a giant cell myocarditis limited to the left atrium. J Clin Pathol. 1968;21(6):750–752. 10.1136/jcp.21.6.750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thachil A, Christopher J, Sastry BK, Reddy KN, Tourani VK, Hassan A, et al. Monomorphic ventricular tachycardia and mediastinal adenopathy due to granulomatous infiltration in patients with preserved ventricular function. J Am Coll Cardiol. 2011;58(1):48–55. 10.1016/j.jacc.2011.02.044 [DOI] [PubMed] [Google Scholar]
- 9. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the Management of Patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125. 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 11. Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease–an evidence‐based guideline. Nat Rev Cardiol. 2012;9(5):297–309. 10.1038/nrcardio.2012.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 13. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–1184. 10.1161/01.cir.96.4.1180 [DOI] [PubMed] [Google Scholar]
- 14. Maixent JM, Paganelli F, Scaglione J, Lévy S. Antibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9(6):612–617. 10.1111/j.1540-8167.1998.tb00942.x [DOI] [PubMed] [Google Scholar]
- 15. Basso C, Corrado D, Rossi L, Thiene G. Ventricular preexcitation in children and young adults: atrial myocarditis as a possible trigger of sudden death. Circulation. 2001;103(2):269–275. 10.1161/01.cir.103.2.269 [DOI] [PubMed] [Google Scholar]
- 16. Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, et al. Myocardial imaging with 18F‐fluoro‐2‐deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35(5):933–941. 10.1007/s00259-007-0650-8 [DOI] [PubMed] [Google Scholar]
- 17. Watanabe E, Miyagawa M, Uetani T, Kinoshita M, Kitazawa R, Kurata M, et al. Positron emission tomography/computed tomography detection of increased 18F‐fluorodeoxyglucose uptake in the cardiac atria of patients with atrial fibrillation. Int J Cardiol. 2019;283:171–177. 10.1016/j.ijcard.2018.10.106 [DOI] [PubMed] [Google Scholar]
- 18. Frustaci A, Cameli S, Zeppilli P. Biopsy evidence of atrial myocarditis in an athlete developing transient sinoatrial disease. Chest. 1995;108(5):1460–1462. 10.1378/chest.108.5.1460 [DOI] [PubMed] [Google Scholar]
- 19. Yalagudri S, Zin Thu N, Devidutta S, Saggu D, Thachil A, Chennapragada S, et al. Tailored approach for management of ventricular tachycardia in cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2017;28(8):893–902. 10.1111/jce.13228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors of this study confirm that the data supporting the findings of this study are available within the article and is derived from the Sarcoid clinic database at this center.


