Abstract
Cerebral ischemia-reperfusion injury (CIRI) is clinically characterized by high rates of morbidity, disability, mortality, and recurrence as well as high economic burden. The clinical manifestations of CIRI are often accompanied by gastrointestinal symptoms such as intestinal bacterial dysbiosis and gastrointestinal bleeding. Gut microbiota plays an important role in the pathogenesis of CIRI, and its potential biological effects have received extensive attention. The gut microbiota not only affects intestinal barrier function but also regulates gastrointestinal immunity and host homeostasis. Traditional Chinese medicine (TCM), a multi-component and multi-targeted drug, has shown remarkable effects and few adverse reactions in the prevention and treatment of CIRI. Notably, the effect of TCM on CIRI by regulating gut microbiota and maintaining gastrointestinal homeostasis has gradually become a hot topic. This review summarizes the functional role of the gut microbiota in the development and progression of CIRI and the therapeutic effects of TCM on CIRI by improving gut microbiota dysbiosis, affecting gut microbiota metabolism, and maintaining host immunity. The active ingredients of TCM used for the treatment of CIRI in relevant studies were saponins, triterpenoids, phenolics, and alkaloids. In addition, the clinical effects of TCM used to treat CIRI were briefly discussed. This review established the clinical significance and development prospects of TCM-based CIRI treatments and provided the necessary theoretical support for the further development of TCM resources for the treatment of CIRI.
Keywords: cerebral ischemia-reperfusion injury, ischemic stroke, gut-brain axis, gut microbiota, traditional Chinese medicine
Introduction
Ischemic stroke is a common cerebrovascular disease with high morbidity, lethality, and disability, and is becoming increasingly younger.1,2 Globally, approximately 15 million patients suffer from ischemic stroke every year, resulting in more than half a million deaths and 5 million permanent disabilities.3 The incidence of ischemic stroke has gradually increased owing to demographic changes and the prevalence of diabetes and obesity, which places a heavy economic burden on families and society.4 Currently, treatment strategies for improving the clinical symptoms of ischemic stroke include thrombolysis, thrombectomy, and drugs.5,6 Recombinant tissue plasminogen activator is the only drug approved by the US Food and Drug Administration for the treatment of ischemic stroke.7 However, a few drawbacks of intravenous thrombolysis include partial recanalization, hemorrhagic transformation, delayed reperfusion, and secondary damage.8 In addition, ischemic stroke is often accompanied by vascular recanalization and blood flow reperfusion, which further aggravates cellular metabolic disorders and organ damage, ultimately resulting in severe neurological function deficiencies known as cerebral ischemia-reperfusion injury (CIRI). Notably, CIRI can aggravate brain injury and lead to high rates of disability and death.9 Unfortunately, the pathogenesis of CIRI remains unclear. Therefore, elucidating the pathogenesis of CIRI and developing safe and effective therapeutic agents are major research challenges in the field of ischemic stroke.
Increasing evidence has shown that the functional role of the intestinal microbiome in cerebrovascular diseases has recently attracted considerable attention from scholars at home and abroad.10 The intestinal microbiome is a diverse community of microorganisms that lives in the gastrointestinal tract,11 including bacteria, fungi, parasites, and viruses. The intestinal microbiome consists of six phyla: Proteobacteria, Actinomycetes, Fusobacterium, Verrucomicrobia, Firmicutes, and Bacteroides. Among them, Firmicutes and Bacteroides are the most abundant, accounting for 70–75% of healthy bacteria.12 Of note, the intestinal microbiome is frequently referred to as a super “organ”13 and is sometimes known as the “second brain”14 because of its involvement in several immunological, neurological, and endocrine reactions. Moreover, the intestinal microbiome is of vital importance to the central and intestinal, this relationship is named as the “microbiome-gut-brain” axis.15 Previous studies have found that gut microbiota dysbiosis is closely related to CIRI and is a key risk factor for cerebrovascular disease.16 CIRI can alter in the composition of the gut microbiota and lead to change the secretion of gastrointestinal hormones, which are important factors with implications for the pathology and treatments of CIRI.17,18 Numerous studies have demonstrated that gut microbiota disorders cause structural and functional abnormalities during the progression.19 Wen et al20 reported that 50% of patients with ischemic stroke developed gastrointestinal complications in the clinical setting, including dysphagia, gastrointestinal bleeding, constipation, and bowel incontinence. Notably, some studies have found that gut microbiota dysbiosis may serve as a new biomarker for the prevention and treatment of ischemic stroke.21–23 Lou et al24 proved that gut microbiota is closely related to the occurrence of high on-treatment platelet reactivity in patients with ischemic stroke. Meanwhile, Bacteroidetes have been shown to be highly correlated with infract size and neuroinflammation.25,26 Through neuronal networks, the brain and gut communicate bidirectionally to form a complex “gut-brain” axis. On the one hand, the brain affects the function or permeability of the gut by transmitting nerve impulses to reduce intestinal motility and secretion.27 On the other hand, the gut microbiota reversely makes an effect on the brain function by sending signals back into the brain through the neuro-immune and endocrine pathways.28 Furthermore, other studies have shown that gut microbiota-derived metabolites such as phenylacetylglutamine and white matter hyperintensity.29 Other gut microbiota metabolites may serve as therapeutic and prognostic biomarkers for CIRI, including trimethylamine-N-oxide (TMAO) and short-chain fatty acids (SCFAs).30,31 Intriguingly, an inverse correlation emerges between SCFA levels and the prognosis of reperfused stroke, particularly in elderly patients contending with pronounced stroke events. Lee et al32 reported that aged mice subjected to MCAO exhibited attenuated neuroinflammation and reduced neurological deficits when administered fecal transplants from young, SCFA-rich donors. Similarly, serum TMAO concentrations exhibit a positive correlation with the risk factors of stroke and the ensuing neurological deficits post-ischemic brain injuries.33 Lipopolysaccharide (LPS), a key component of the outer membrane of Gram-negative bacteria, can enter the brain through compromised gut and BBB, and thereby accelerating CIRI progression.34 Fecal microbiota transplantation from healthy donors exhibited a therapeutic effect on CIRI and other neurological disorders.35,36 Therefore, the disordered “microbiome-gut-brain” axis plays an important regulatory role in the pathogenesis of cerebrovascular diseases, including CIRI.37
Recently, traditional Chinese medicine (TCM) has received increasing attention from international medical researchers because of its favorable therapeutic effects and low toxicity.38 Preclinical experiments and clinical trials have demonstrated that TCM exerts therapeutic effects on CIRI owing to its antioxidant, gut microbiota modulation, free radical scavenging, antithrombotic, and neuroprotective properties.39–41 Modern pharmacology has confirmed that TCM is a promising resource with great chemical diversity and multi-target and multi-pathway characteristics.42 Increasing evidence has shown that TCM exerts an anti-CIRI effect by regulating the composition and metabolism of the gut microbiota.43,44 Moreover, the theory of the “gut-brain” axis has been documented in Chinese medicine since ancient times, and TCM has improved gut microbiota dysbiosis and metabolic syndrome in the treatment of CIRI.45 These findings suggest that gut microbiota may serve as a new therapeutic target for TCM in the prevention and treatment of CIRI.
This review summarizes the functional role of gut microbiota in the occurrence and progression of CIRI. In addition, the present review comprehensively summarizes the latest progress in TCM in curing CIRI through the “microbiome-gut-brain” axis, aiming to provide a theoretical basis for the development of new drugs against CIRI.
Functional Role of the “Microbiota-Gut-Brain” Axis in CIRI
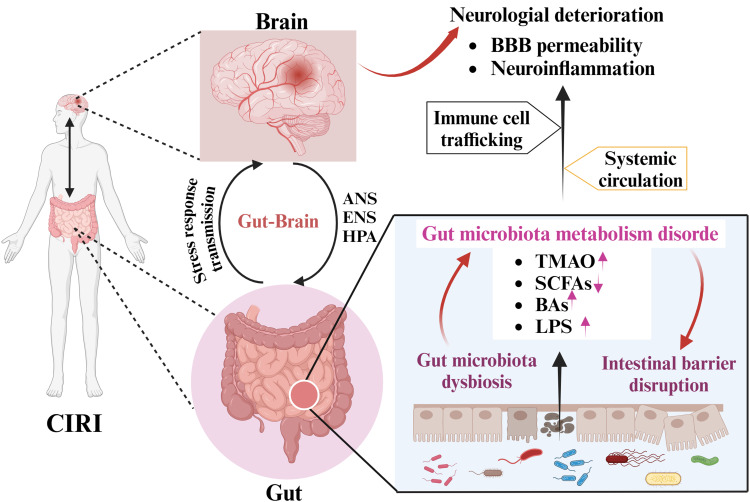
Gut microbiota plays an important role in human health and diseases.46 The “microbiota-gut-brain” axis is a bidirectional communication network between gut microbiota and their host.47 Dysbiosis of the gut microbiota is related to the development of common stroke risk factors, such as obesity, diabetes, hypertension, and atherosclerosis, which increase the risk of CIRI.48 Acute stress in ischemic stroke also changes the composition and abundance of the gut microbiota, which may affect the prognosis of CIRI.49 Moreover, numerous studies have shown that the gut microbiota can improve ischemic stroke prognosis by regulating neuroactive molecules and immune cell functions, enhancing neural network plasticity, and reducing neuroinflammation.18,50 These findings indicate that the pathogenesis of CIRI is closely related to an imbalance in gut microbiota. Herein, we summarize the functional role of the microbiota–gut–brain axis in the development and progression of CIRI (Figure 1) as well as the underlying mechanism.
Figure 1.
Schematic representation illustrating bidirectional interaction between CIRI and the gut microbiota via the gut-brain axis.
Note: Created in BioRender. Ren, (Y) (2024) https://BioRender.com/v74a311.
Abbreviations: CIRI, cerebral ischemia-reperfusion injury; ANS, autonomic nervous system; ENS, enteric nervous system; HPA, hypothalamic-pituitary-adrenal; BBB, blood-brain barrier; TMAO, trimethylamine N-oxide; SCFAs, short-chain fatty acids; BAs, bile acids; LPS, lipopolysaccharide.
Gut Microbiota Dysbiosis
Normal gut microbiota and the host are in a dynamic balance of mutual benefit and symbiosis. The “microbiome-gut-brain” axis consists of bidirectional communication between the brain and intestines, linking neural function, cognition, and emotion of the brain with peripheral intestinal functions.51 Unfortunately, the “gut-brain” axis is disrupted in CIRI,37 which contributes to alterations in the composition and abundance of gut microbiota. Wang et al52 reported that the ratio of Firmicutes to Bacteroidetes in CIRI mice was lower than that in normal mice, which usually serves as a sign of gut disturbance. Recent studies have reported that Enterobacteriaceae, Bifidobacterium, Lactobacillus, Desulfovibrio, Alloprevotella, Ruminococcus, and Escherichia are enriched in high-risk ischemic stroke patients.53,54 Another study found that patients with CIRI presented with an increased abundance of pathogenic bacteria in the intestinal tract, such as Enterobacter and Megasphaera, and a decreased abundance of commensal or beneficial genera, such as Bacteroides and Prevotella, and these gut microbiota dysbiosis was positively associated with the pathological progression of CIRI.55 Tan et al56 reported that dysbiosis of the gut microbiota in patients with acute ischemic stroke enhances the subsequent risk of poor functional outcomes. Moreover, systemic exposure to Porphyromonas gingivalis may increase the risk of ischemic stroke.57 Kawato et al58 found that continual gram-negative bacteria induced accelerated stroke onset in stroke-prone spontaneously hypertensive rats. Other studies have reported that the compositional and functional imbalance of the gut microbiota is an independent predictor of poor prognosis in ischemic stroke.18,59 A recent study has reported that Enterobacteriaceae may serve as a biomarker of cognitive impairment in CIRI.21 Dysbiosis of the gut microbiota has a positive effect on neural function of the brain, which in turn exacerbates brain infarction.50,60 Notably, prebiotic administration and fecal microbiota transplantation from healthy donors have been shown to inhibit CIRI progression and reduce brain infarct volume.61,62 In summary, gut microbiota plays a vital role in the occurrence and development of CIRI.
Intestinal Mucosal Barrier
Integrity of the intestinal mucosal barrier is the foundation of multiple functions of the intestinal tract to keep normal.63 Disruption of the intestinal mucosal barrier is an important pathophysiological process in ischemic stroke64 and CIRI,65 resulting in intestinal injury, secondary infection, and even death. A clinical study showed that dysbiosis of the gut microbiota is an independent risk factor for acute ischemic stroke-associated pneumonia.66 Another study showed that stroke increased intestinal barrier permeability and dysfunction, which enhanced the translocation and dissemination of bacteria originating from the gut microbiota.67 Chen et al68 found that cerebral ischemic stroke not only caused intestinal barrier disruption but also aggravated gut microbiota translocation. Notably, probiotics have been found to modulate host intestinal barrier function through their surface components and metabolites. For example, S-layer protein 2 of vaginal Lactobacillus crispatus 2029 promoted the growth of immature Caco-2 cells, production of vascular endothelial growth factor, decreased paracellular permeability, and enhanced the intestinal barrier function of Caco-2 monolayer cells.69 Hagihara et al70 proved that Clostridium butyricum MIYAIRI588 increased the abundance of beneficial genera, protected intestinal barrier function, and enhanced anti-inflammatory lipid metabolites. Another study showed that administration of Clostridium butyricum ameliorated CIRI progression via antioxidant and anti-apoptotic mechanisms.71 These results suggest that improving the function and integrity of the intestinal mucosal barrier is important in the treatment of CIRI.
Gut Microbiota Metabolism Disorder
Accumulating evidence has demonstrated that active metabolites produced by the gut microbiota play a crucial role in the maintenance of homeostasis, immune maturation, mucosal integrity, and energy metabolism.72,73 Intestinal microbiota-derived metabolites, such as SCFAs, TMAO, LPS, and bile acids (BAs), can exert beneficial or deleterious effects on CIRI and various extraintestinal organs.16 Among them, SCFAs are the main metabolites produced in the colon by bacterial fermentation of dietary fiber and resistant starch, including butyrate, acetate, and propionate,74 which may affect cerebral ischemic stroke outcomes by modulation of the “gut-brain” axis.75,76 For example, SCFAs produced by beneficial bacteria can promote motor function recovery after ischemic stroke by affecting the immune cells throughout the body.77 Chen et al62 reported that the intestinal levels of SCFAs in cerebral ischemic stroke model rats were lower than those in healthy rats, and that butyric acid showed the highest negative correlation with ischemic stroke. Lee et al32 proved that fecal microbiota transplantation promotes post-stroke recovery by modulating the gut microbiota and reducing inflammation and neurological deficits. TMAO is a key indicator for evaluating the prognosis of patients with cerebral ischemic stroke.78,79 A cross-sectional study showed that higher plasma TMAO level at admission was an independent predictor of stroke severity, infarct volume, and pro-inflammatory monocyte levels in patients with acute cerebral ischemia.80,81 Liu et al82 reported that trimethylamine produced by gut microbiota metabolism is converted to TMAO through hepatic endoflavin monooxygenase, which contributes to platelet hyperreactivity and thrombosis risk by augmenting Ca2+ release.83 Other studies have reported that circulating LPS can worsen clinical outcomes after cerebral ischemic stroke by increasing brain inflammation and blood-brain barrier (BBB) permeability.84–86 A recent study showed that elevated serum BAs levels contribute to improving poor functional outcomes after ischemic stroke.87 Functionally, gut microbiota-derived metabolites are involved in the physiological regulation of CIRI by regulating oxidative stress, inflammation, mitochondrial damage, apoptosis, ferroptosis, and neurological damage.43,88,89 Taken together, gut microbiota and their metabolites may serve as promising therapeutic targets for the diagnosis and treatment of CIRI.
Gut Microbiota and Host Immunity
Based on current knowledge, the gut microbiota is not only essential for immune homeostasis but also influences the host’s response to immune-mediated diseases and the function of the gastrointestinal immune system.90 The gastrointestinal tract is considered to be the largest immune organ in the human body and contains more than 70% of the entire immune system in terms of the number of immune cells.17 Previous studies have found that patients with cerebral ischemic stroke are susceptible to immunocompromised.91,92 Notably, CIRI-induced gut microbiota dysbiosis can trigger immune responses via T cell activation.93 Benakis et al94 showed that dysbiosis of the gut microbiota weakened intestinal immune homeostasis and altered dendritic cell activity in a cerebral ischemic stroke mouse model, as evidenced by increased regulatory T cells and reduced interleukin (IL)-17-positive γδ T cells. Hu et al89 summarized that gut microbiota dysbiosis activates the intestinal immune system and promoted pro-inflammatory cytokine levels, which in turn leads to CIRI. Other studies proved that CIRI causes persistent host gut microbiota dysbiosis and promoted pro-inflammatory cytokine secretion.95,96 Moreover, intestinal dysbiosis facilitates ischemic stroke pathology and prognosis by regulating immunological pathways, such as the NF-κB pathway,97 the type I interferon pathway,98 and the inflammasome pathway.99 Increasing evidence has demonstrated that gut dysbiosis reduces Treg cells and systemic anti-inflammatory cytokines100,101 such as IL-10 and transforming growth factor-beta. Gut microbiota metabolites (such as SCFAs) promote Treg cell proliferation and serve as ligands for G-protein-coupled receptors.102 SCFAs exhibit protective effects against CIRI progression by regulating host immune homeostasis and inhibiting inflammation.103,104 Collectively, gut microbiota affects CIRI prognosis by regulating immune and inflammatory responses via the “gut-brain” axis.
Effect of TCM on Gut Microbiota in CIRI
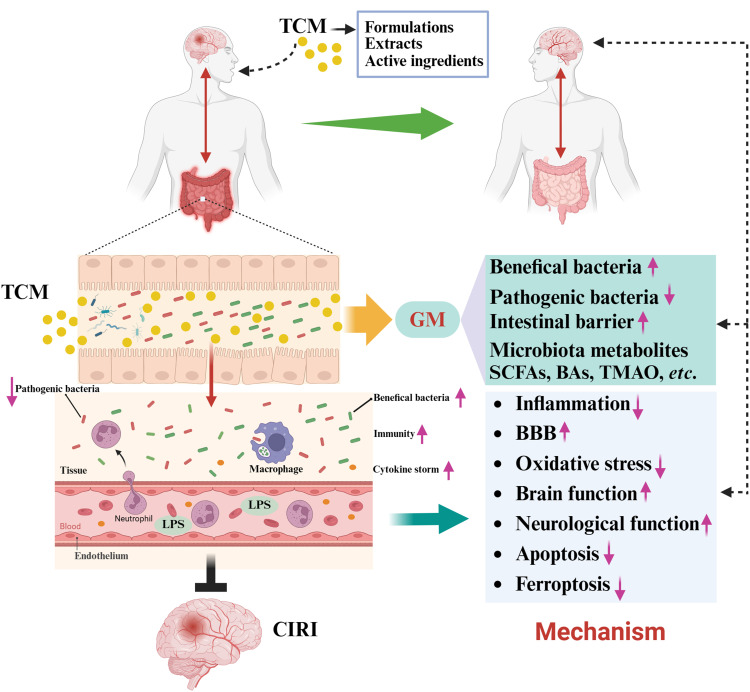
CIRI belongs to the category of “stroke disease” in Chinese medicine, and its pathogenesis is rooted in the deficiency of the liver and kidney.105 Currently, TCMs serves as a promising resource for the treatment of CIRI by activating blood circulation and removing blood stasis, modulating gut microbiota, clearing heat and removing toxins, benefiting qi and tonifying blood, nourishing yin, and activating blood.40,106,107 Numerous studies have shown that TCM exerts anti-CIRI prevention and therapeutic effects by regulating the gut microbiota.108,109 On the one hand, TCMs improved intestinal disorders caused by CIRI, as well as modulated the composition and abundance of gut microbiota.110 In contrast, TCMs alleviate the organ dysfunction syndrome caused by pathogenic bacterial translocation and harmful gut microbiota metabolites in cerebral ischemic stroke.111,112 In addition, TCM treatment improved the intestinal mucosal barrier and maintained intestinal homeostasis by regulating the composition of the intestinal microbiome.113 The functional role of TCM in CIRI by regulating the gut microbiota is summarized in Figure 2 and Table 1.
Figure 2.
Traditional Chinese medicine in the treatment of CIRI via the “gut-brain” axis.
Note: Created in BioRender. Ren, (Y) (2024) https://BioRender.com/s71m955.
Abbreviations: TCM, traditional Chinese medicine; GM, gut microbiota.
Table 1.
Summary of Traditional Chinese Medicine in Treating CIRI by Regulating Gut Microbiota
| Name | Microbiota Affected | Efficacy | Reference |
|---|---|---|---|
| Prescription | |||
| Buqi-Huoxue-Tongnao decoction | The abundance of Turicibacter and Faecalibaculum↑ | Infarct volume, neurological damage, and intestinal bacterial translocation↓ Intestinal barrier integrity and indole lactic acid levels↑ |
[114] |
| Dan-deng-tong-nao capsule | The abundance of Firmicutes and Actinobacteria↑ The abundance of Proteobacteria and Verrucomicrobiales↓ |
Infract volume and neurological deficit↓ | [115] |
| Tao Hong Si Wu decoction | The abundance of Firmicutes and Actinobacteria↑ The abundance of Proteobacteria↓ |
Neurological damage, intestinal barrier destruction, and levels of IL-1β and TNF-α↓ Expression of LPS, DAO, and D-lactic acid↓ TLR-4/NF-κB pathway↓ |
[116] |
| Sanhua decoction | The abundance of Clostridia, Lachnospiraceae, Ruminococcaceae, and Coprococcus↑ The abundance of Enterobacteriaceae, Desulfovibrio, and Lactobacillus↓ |
Infract volume and brain injury↓ Serum levels of butyric acid, isovaleric acid, valeric acid, and caproic acid↓ Fecal butyric acid↑ |
[117] |
| Naotaifang III | The abundance of Firmicutes and Clostridium↑ The abundance of Bacteroides and prevotella_9↓ |
Neurological deficit, brain damage, and infract volume↓ Levels of LPS and IL-1β↓ LPS/TLR-4/NF-κB pathway↓ |
[118] |
| Eleutherococcus senticosus | The abundance of Lactobacillus reuteri and Clostridium butyricum↑ The abundance of Proteobacteria, Enterobacter, Oscillibacter, and Escherichia Shigella↓ |
Oxidative stress and inflammation↓ Contents of 5-HT and GABA↑ Contents of ASP and Glu↓ |
[119] |
| San Hua Tang | The abundance of Lactobacillales, Olsenella, and Bifidobacterium↑ The abundance of Bacteroidetes↓ |
Neurological damage, infract volume, and inflammatory response↓ Intestinal mucosal barrier↑ Levels of acetic acid, butyric acid, and propionic acid↑ |
[120] |
| Shuanglu Tongnao | The abundance of Paracoccus↑ The abundance of Bacteroidetes↓ |
Neurological deficit and infract volume↓ Intestinal barrier function↑ TLR-4/NF-κB pathway↓ |
[121] |
| Puerariae lobatae Radix | The abundance of Firmicutes, Bifidobacterium, Akkermansia, Romboutsia, and Butyricicoccus↑ The abundance of Bacteroidetes, Alistipes, Klebsiella, Fusobacterium, and Faecalibacterium↓ |
Neurological impairment, infract size, dyslipidemia, and gut barrier disruption↓ Levels of glycine, L-phenylalanine, enkephalin L, and melatonin↑ |
[122] |
| Pushen capsule | The abundance of Alistipes and Ruminiclostridium↑ The abundance of Lactobacillus, Bifidobacterium, Ruminococcus_2, and Pseudomonas↓ |
Infarct volume↓ BBB, brain plasticity, and cognitive repair↑ Adora2a expression↓ |
[123] |
| Buzhong Yiqi decoction | The abundance of Prevotellaceae_NK3B31_group and Akkermansia↑ The abundance of Bacteroidetes, Patescibateria, Tenericutes, and Cyanobacteria↓ |
Neurological deficit and infract size↓ Cell apoptosis in the cortex↓ |
[124] |
| Zhilong Huoxue Tongyu capsule | The abundance of Bacteroidetes, Actinobacteria, and Prevotella↑ The abundance of Firmicutes, Proteobacteria, and Tenericutes↓ |
Infract size and intestinal barrier damage↓ Metabolic disturbances↓ |
[125] |
| Huangqi-Honghua | The abundance of Blautia, Lachnospiraceae, Oscillibacter, and Bifidobacterium↑ The abundance of Ruminococcaceae, Bacteroides, Phascolarctobacterium, and Desulfovibrionaceae↓ |
Neurological deficit, infarct volume, and rate of necrotic neurons↓ Intestinal barrier integrity and levels of allolithocholic acid, isolithocholic acid, and cholic acid↑ |
[44] |
| Angong Niuhuang pill | The abundance of Lachnoclostridium, Enterorhabdus, Roseburia, Lachnospiraceae_UCG-006, and Colidextribacter↑ The abundance of Alloprevotella↓ |
Infarct volume, neurological deficit, and neuronal death↓ Nissl bodies and levels of uridine, inosine, and guanosine↑ |
[43] |
| Xingnaojing injection | The abundance of Akkermansia↑ The abundance of Flavobacteriaceae, Deferribacteraceae, and Deferribacteres↓ |
Infract volume, brain tissue damage, and inflammation↓ Intestinal mucosal barrier and levels of short-chain fatty acids, propionate, valerate, isobutyrate, and isovalerate↑ TLR-4/NF-κB pathway↓ |
[126] |
| NaoMaiTong | The abundance of Coprococcus and Blautia↑ The abundance of Verrucomicrobiota and Escherichia Shigella↓ |
Infract volume and neurological deficit↓ Oxidative stress and inflammation↓ |
[127] |
| Xinglou Chengqi decoction | The abundance of Verrucomicrobia and Akkermansia↑ The abundance of Paraprevotella, Roseburia, Streptophyta, and Enterococcus↓ |
Cerebral infarction, neuronal apoptosis, and inflammation↓ Neurological function and levels of levels of short chain fatty acids and butyric acid↑ |
[128] |
| Qishiwei Zhenzhu pill | The abundance of Firmicutes↑ The abundance of Proteobacteria and Escherichia Shigella↓ |
Infract volume and neurological deficit↓ Intestinal integrity↑ Inflammatory response↓ |
[129] |
| Dioscorea polystachya | The abundance of Lactobacillus, Ruminococcus, and Clostridium↑ The abundance of Bacteroidetes↓ |
Cognitive dysfunction and neurological deficit↓ LPS content, oxidative stress, and inflammation↓ The content of short-chain fatty acids, GABA, and 5-HT↑ |
[109] |
| Tong-Qiao-Huo-Xue decoction | The abundance of Allobaculum, Lactobacillus, and Bifidobacterium↑ The abundance of Bacteroidetes↓ |
Infract volume and neurological deficit↓ Intestinal epithelial barrier and FOXP3↑ γδT cell proportion and IL-17 expression↓ |
[130] |
| Puerariae lobatae Radix with Chuanxiong Rhizoma | The abundance of Alloprevotella, Ruminococcaceae_UCG_005, Ruminococcaceae_NK4A214_group, Ruminococcaceae_UCG_004, Oscillospira, Lachnospiraceae_NK4B4_group, Akkermansia, and Megasphaera↑ The abundance of Escherichia Shigella↓ |
Neurological deficit and infract size↓ Blood lipid levels and thrombotic risk↓ Intestinal permeability and gut microbiota translocation↓ |
[68] |
| Extract | |||
| Water extract of Rheum tanguticum | The abundance of Enterorhabdus, Defluviitaleaceae, Christensenellaceae, and Lachnospira↑ The abundance of Fournierella and Bilophila↓ |
Infarct volume and neurological deficit↓ Levels of isoleucine, lactate, valine, N6-acetyllysine, methionine, choline, myo-inositol, lipid, and 3-aminoisobutyric acid↓ Levels of propylene glycol, N, N-dimethylglycine, trimethylamine N-oxide, glucose, and betaine↑ |
[131] |
| Acorus tatarinowii oil | The abundance of Prevotella_copri↑ The abundance of Akkermansia and Verrucomicrobiales↓ |
Infarct volume, neurological deficit, and inflammation↓ M2 phenotypic polarization of microglia↑ |
[132] |
| Rhubarb anthraquinone glycosides | / | Contents of 5-HT, 5-HIAA, and GABA↑ Contents of ASP and Glu↓ |
[19] |
| Active ingredient | |||
| Notoginsenoside R1 | The abundance of Bacteroidota and Muribaculaceae↑ The abundance of Enterobacterales↓ |
Infarct volume, neurological deficit, neuronal apoptosis, inflammation, and intestinal permeability↓ TLR4/MyD88/NF-κB pathway↓ |
[133] |
| Berberine | The abundance of Akkermansia, Escherichia-Shigella, Bacteroides, and Parasutterella↑ The abundance of Odoribacter, Alistipes, Clostridium_sensu_stricto_1, and Helicobacter↓ |
Brain infarct volume, the expressions of two synaptic-associated proteins (PSD95 and SYP), neurological deficit, and neuroinflammation↓ Glial cell activation and NLRP3 expression↓ The production of butyric acid↑ |
[134] |
| Cornuside | The abundance of Lachnospiraceae, Treponema, Prevotellaceae_NK3B31_group, Lactobacillus, and Ruminococcaceae↑ The abundance of Bacteroides↓ |
Infarct volume and neurological deficit↓ Intestinal permeability, neuroinflammation, and intestinal inflammation↓ IL-17A/TRAF6/NF-κB pathway↓ |
[135] |
| Escin | / | Infarct volume, neuroinflammation, and intestinal dysfunction and permeability↓ Neurological function↑ LPS/TLR4/NF-κB pathway↓ |
[136] |
| Indole-3-propionic acid | The abundance of Lactobacillus and Lachnospiraceae_NK4A136_group↑ The abundance of Akkermansia, Alistipes, and Mucispirillum↓ |
Neurological deficit and infarct volume↓ Intestinal epithelial barrier and Th17 numbers↑ Tregs, neuroinflammation, and neuron apoptosis↓ |
[137] |
| Astragaloside IV | The abundance of Holdemanella and Clostridium↑The abundance of Bifidobacterium and Escherichia-Shigella↓ | Levels of ROS and MDA↓ The T-AOC, SOD, and GSH↑ Autophagy↓ |
[138] |
Abbreviations: ASP, aspartic acid; GABA, γ-aminobutyric acid; Glu, glutamic acid; GSH, glutathione; MDA, malondialdehyde; NLRP3, NLR family pyrin domain containing 3; ROS, reactive oxygen species; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxy indole acetic acid.
Effect of TCM on Gut Microbiota Dysbiosis
Currently, TCM is used both as the main therapeutic agent and an adjuvant therapy for the prevention and treatment of several neurological diseases, including CIRI.139 Numerous studies using TCM have found that the gut microbiota serves as a key regulatory mechanism in the treatment of cerebrovascular diseases.140,141 For example, Shengmai San,142 NaoMaiTong,143 Angong Niuhuang pill,43 San Hua Tang,120 Dan-deng-tong-nao capsule,115 and other TCM formulas have been used to treat CIRI by modulating gut microbiota. Chen et al68 reported that the Gegen and Chuanxiong combination treatment increased the abundance of beneficial bacteria and relieved the complications of CIRI, which was similar to the results of Wang et al.44 Moreover, several Chinese herbs exhibited potent anti-CIRI therapeutic efficacy by modulating intestinal homeostasis, including Gastrodia elata Blume,110 rhubarb,144 and Dioscorea polystachya.109 Furthermore, the active ingredients of TCM, including hydroxycinnamic acids,145 rhubarb anthraquinone glycosides,146 escin,136 and notoginsenoside R1.133 Xu et al138 found that Astragaloside IV reduces autophagy and oxidative stress induced by gut microbiota dysbiosis in mouse models of acute ischemic stroke. Collectively, TCM can prevent the development and progression of CIRI by improving the dysbiosis of the gut microbiota.
Effect of TCM on Intestinal Mucosal Barrier and Host Immunity
TCMs have been reported to improve the integrity and permeability of the intestinal mucosal barrier by regulating the composition and abundance of gut microbiota and, in turn, reducing the immune-inflammatory response triggered by the migration of harmful bacteria and toxic metabolites of gut microbiota to other organs and the circulatory system.45,147 For example, Qishiwei Zhenzhu pill treatment ameliorated CIRI progression by improving intestinal integrity and gut microbiota disorder and inhibiting inflammation.129 Zhang et al130 demonstrated that Tong-Qiao-Huo-Xue decoction had a protective effect against CIRI by improving intestinal mucosal barrier destruction and reducing the activation and migration of intestinal γδT cells. Treatment with Panax notoginsenoside extract exerts a neuroprotective effect on CIRI by upregulating brain-derived neurotrophic factor levels and inflammation by enhancing the abundance of beneficial bacteria (such as Bifidobacterium longum).148 Moreover, Chinese herbal monomers exert a therapeutic effect on CIRI by improving the intestinal mucosal barrier and suppressing inflammation. For example, Dou et al149 showed that resveratrol alleviates CIRI by inhibiting intestinal pro-inflammatory immunity, neuroinflammation, and inflammation-induced BBB disruption by modulating gut microbiota-mediated Th17/Tregs and Th1/Th2 polarity shifts. Similarly, cornuside restricts CIRI progression by inactivating the IL-17F/TRAF6/NF-κB pathway via maintaining intestinal barrier function, and reducing intestinal inflammation and neuroinflammation.135 Collectively, TCM alleviated CIRI by inhibiting inflammation and promoting intestinal mucosal barrier repair induced by dysbiosis of the gut microbiota.
Effect of TCM on Intestinal Metabolism
Recent studies have found that the gut microbiota can participate in the progression of CIRI by releasing endotoxins and their harmful metabolites to mediate systemic and neuroinflammation.86,150 Many gut microbiota metabolites have been identified,151,152 such as SCFAs, BA, TMAO, LPS, indole-3-propionic acid, choline and its metabolites, ethanol, imidazole propionate, and endotoxins have been etc. TCM has been shown to alleviate CIRI progression by regulating the gut microbiota metabolites. For example, Tanhuo decoction mitigated acute ischemic stroke by reducing the levels of LPS and TMAO by modulating the “gut-brain” axis.153 The Ang Niuhuang pill ameliorated CIRI by modulating microbial metabolites related to inflammation and neuroprotection,43 such as uridine, guanosine, and inosine. Administration of San Hua Tang enhanced SCFA levels and inhibited inflammation in rats with CIRI.120 Moreover, other TCMs ameliorated CIRI progression by regulating enteric metabolism. Huangqi-Honghua combination treatment reduced neurological deficits, cerebral infarct volume, and necrotic neuron rate in the CIRI rat model by maintaining gut homeostasis and affecting BA metabolism,44 which is similar to the protective effect of rhubarb on CIRI.154 NaoMaiTong treatment not only improved gut microbiota disturbances but also adjusted plasma metabolic disorders.127 Liu et al131 showed that Dahuang prevents CIRI progression by improving gut microbiota disorders and regulating amino acid and energy metabolism. Several studies have shown that gastrointestinal hormones play an important role in CIRI progression by maintaining intestinal and immune homeostasis,155 which have become a therapeutic target for the treatment of CIRI with TCM.156,157 Collectively, TCM treatment attenuated CIRI by regulating microbiota metabolites.
Clinical Trials of TCM for CIRI Management
Preclinical studies have confirmed that TCM has a wide range of pharmacological effects on CIRI, and clinical trials are being conducted. For example, Guo et al153 showed that acute ischemic stroke patients treated with Tanhuo decoction had increased abundance of beneficial bacteria (such as Bifidobacteria, Streptococci, and Ruminococci), decreased abundance of pathogenic bacteria (such as Bacteroidetes, Anaerobacter, and Enterococci faecalis), and reduced levels of aseptic inflammation and microbial metabolites (LPS and TMAO). Guo et al140 showed that TCM treatment increased the microbial diversity and abundance of beneficial bacteria in patients with CIRI and the effect of approaching healthy people’s gut microbiota. Other studies have shown that TCM therapy improves neurological function and limb motor impairment, and enhances the quality of life of patients with CIRI.158,159 Moreover, ongoing clinical studies are exploring the safety and efficacy of TCM for the treatment of CIRI (Table 2). For example, a Singaporean substudy reported that Neuroaid (MLC601) was a safe TCM for ischemic stroke patients receiving a 3-month treatment.160,161 Recently, a multicenter, randomized, and placebo-controlled trial found that Naoxintong capsule was a safe drug for treating cerebral ischemic stroke and reduced the 2-year stroke recurrence rate.162 Another clinical study pointed out that the efficacy and safety of TCM for the treatment of CIRI are superior to those of Western medicine.163 However, the specific active components of TCM against CIRI and their mechanisms remain unknown, and the combination of molecular docking studies, molecular dynamics simulations, network pharmacology, and genomics/transcriptomics analyses may deepen our understanding of the multi-targeted effects and potential efficacy of TCM in treating CIRI. Based on these findings, TCM is a promising alternative for clinical treatment of CIRI. Importantly, there is an urgent need to establish screening models of compatibility and screening targets for TCM in various diseases, including CIRI.
Table 2.
Clinical Trials of Traditional Chinese Medicine in Cerebral Ischemic Stroke
| Category | Year of Registration | Sponsor | Recruiting Status | Clinical Trial ID |
|---|---|---|---|---|
| Taohong Tongluo Xiaoban | 2024 | Beijing Electric Power Hospital, China | Not yet recruiting | NCT06549582 |
| Suhexiang pill | 2023 | Dongzhimen Hospital, China | Recruiting | NCT05833932 |
| DengzhanShengmai capsule | 2007 | Guangzhou University of Traditional Chinese Medicine, China | Completed | NCT00548223 |
| Zhongfeng Huichun pill | 2024 | Dongzhimen Hospital Beijing University of Chinese medicine, China | Recruiting | ChiCTR2400083136 |
| Naoshuantong capsule | 2023 | The First Affiliated Hospital of Henan University of Chinese medicine, China | Not yet recruiting | ChiCTR2300075877 |
| Chuanzhitongluo pill | 2023 | The Second Affiliated Hospital of Chongqing Medical University, China | Recruiting | ChiCTR2300074147 |
| Ruyi Zhenbao tablet | 2023 | The Second People’s Hospital of Yuhuan, China | Not yet recruiting | ChiCTR2300073074 |
| Breviscapine dripping pill | 2023 | The Third Affiliated Hospital of Beijing University of Chinese Medicine, China | Not yet recruiting | ChiCTR2300067750 |
| Shenjingfuyuanfang granule | 2020 | Shanghai Municipal Hospital of Traditional Chinese Medicine, China | Not yet recruiting | ChiCTR2000040010 |
| Ruyi Zhenbao tablet | 2020 | Xuanwu Hospital of Capital Medical University, China | Completed | ChiCTR2000036691 |
| Sanchitongtshu capsule | 2019 | West China School of Medicine, Sichuan University, China | Not yet recruiting | ChiCTR1900022495 |
| Xuesaitong soft capsule | 2018 | Xuanwu Hospital Capital Medical University, China | Completed | ChiCTR1800016363 |
Discussion and Perspective
Gut microbiota has become a research hotspot and is emerging as an important new therapeutic target for the treatment of CIRI. The symptoms of CIRI are characterized by gut microbiota imbalance, intestinal and BBB dysfunction, and peripheral and central inflammations. Meanwhile, the imbalance in intestinal homeostasis facilitated the development and progression of CIRI, and probiotics or fecal microbial transplantation from healthy donors contributed to improving CIRI by modulating the composition and abundance of the gut microbiota. Moreover, gut microbiota dysbiosis may lead to the production of harmful metabolites and the disruption of gastrointestinal hormone secretion. Currently, both clinical and animal studies have shown that TCM has a corrective effect on disordered gut microbiota, such as increasing the abundance of beneficial bacteria, decreasing the abundance of pathogenic bacteria, and maintaining intestinal homeostasis. Increasing evidence has shown that TCM is effective in the prevention and treatment of CIRI by improving gut microbiota dysbiosis and intestinal barrier dysfunction, and altering gut microbiota metabolism. Therefore, elucidating the functional role and mechanisms of TCM in regulating gut microbiota by the “gut-brain” axis may provide a theoretical basis for new treatment strategy for CIRI.
However, the treatment of CIRI using TCM still faces challenges that must be addressed. (1) The metabolism, toxicity, and pharmacokinetic profile of TCM in clinical trials of CIRI should be further explored. (2) Research on the active ingredients of TCM is limited by its unstable chemical structure, low bioavailability, and susceptibility to oxidation. The quality, safety and effectiveness of TCM can be controlled by the use of well-defined compounds. (3) The greatest challenge in TCM drug delivery into the brain is bypassing the BBB, which prevents the entry of many potential therapeutic agents. Currently, there exist primarily three approaches for achieving drug delivery to the brain through BBB targeting, such as receptor-mediated transcytosis, carrier-mediated transcytosis, and absorptive-mediated transcytosis.164 (4) Nearly 25,000 Chinese herbals will become extinct, severely limiting the clinical use of TCM for the treatment of CIRI. (5) There is a lack of sufficient clinical data on the efficacy of TCM against CIRI. (6) The detailed molecular mechanisms of TCM anti-CIRI by regulating the “gut-brain” axis are not clear.
Conclusion
TCMs have excellent anti-CIRI effects and are important agents for the treatment of cerebrovascular diseases. This review analyzed the functional role of gut microbiota in the pathogenesis of CIRI and systematically summarized recent advancements in research on TCM for the prevention and treatment of CIRI, along with clinical evidence, which provides a scientific and comprehensive reference for the use of TCM in the treatment of CIRI and promotes the utilization and development of TCM resources.
Funding Statement
There is no funding to report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Nakamura A, Otani K, Shichita T. Lipid mediators and sterile inflammation in ischemic stroke. Int Immunol. 2020;32(11):719–725. doi: 10.1093/intimm/dxaa027 [DOI] [PubMed] [Google Scholar]
- 2.Putaala J. Ischemic stroke in young adults. Continuum. 2020;26(2):386–414. doi: 10.1212/con.0000000000000833 [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917 [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Stark BA, Johnson CO; Global, regional, and national burden of stroke and its risk factors, 1990-2019. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/s1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feske SK. Ischemic stroke. Am J Med. 2021;134(12):1457–1464. doi: 10.1016/j.amjmed.2021.07.027 [DOI] [PubMed] [Google Scholar]
- 6.Naik Bukke SP, Gopalakrishnaiah T, Onohuean H, et al. Drug utilization analysis of analgesics and adjuvants used in pain management. Arch Pharm Prac. 2024;15(2):4. doi: 10.51847/wHhW6w9i1C [DOI] [Google Scholar]
- 7.Chapman SN, Mehndiratta P, Johansen MC, et al. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 2014;10:75–87. doi: 10.2147/vhrm.S39213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandelwal P, Yavagal DR, Sacco RL. Acute ischemic stroke intervention. J Am Coll Cardiol. 2016;67(22):2631–2644. doi: 10.1016/j.jacc.2016.03.555 [DOI] [PubMed] [Google Scholar]
- 9.Yang K, Zeng L, Ge A, et al. A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury. Front Immunol. 2022;13:930171. doi: 10.3389/fimmu.2022.930171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X, Wang L, Xiao L, et al. Gut microbes in cerebrovascular diseases: gut flora imbalance, potential impact mechanisms and promising treatment strategies. Front Immunol. 2022;13:975921. doi: 10.3389/fimmu.2022.975921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aya V, Flórez A, Perez L, et al. Association between physical activity and changes in intestinal microbiota composition: a systematic review. PLoS One. 2021;16(2):e0247039. doi: 10.1371/journal.pone.0247039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuziel GA, Rakoff-Nahoum S. The gut microbiome. Curr Biol. 2022;32(6):R257–r264. doi: 10.1016/j.cub.2022.02.023 [DOI] [PubMed] [Google Scholar]
- 14.Ochoa-Repáraz J, Kasper LH. The second brain: is the gut microbiota a link between obesity and central nervous system disorders? Curr Obes Rep. 2016;5(1):51–64. doi: 10.1007/s13679-016-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang X, Fu Y, Cao WT, et al. Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis. Transl Neurodegener. 2022;11(1):49. doi: 10.1186/s40035-022-00323-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Dong XY, Huang R. Gut microbiota in ischemic stroke: role of gut bacteria-derived metabolites. Transl Stroke Res. 2023;14(6):811–828. doi: 10.1007/s12975-022-01096-3 [DOI] [PubMed] [Google Scholar]
- 17.Li N, Wang X, Sun C, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19(1):191. doi: 10.1186/s12866-019-1552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluta R, Januszewski S, Czuczwar SJ. The role of gut microbiota in an ischemic stroke. Int J Mol Sci. 2021;22(2):915. doi: 10.3390/ijms22020915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Li Q, Yu X, et al. Rhubarb anthraquinone glycosides protect against cerebral ischemia-reperfusion injury in rats by regulating brain-gut neurotransmitters. Biomed Chromatogr. 2021;35(5):e5058. doi: 10.1002/bmc.5058 [DOI] [PubMed] [Google Scholar]
- 20.Wen SW, Wong CHY. An unexplored brain-gut microbiota axis in stroke. Gut Microbes. 2017;8(6):601–606. doi: 10.1080/19490976.2017.1344809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling Y, Gong T, Zhang J, et al. Gut microbiome signatures are biomarkers for cognitive impairment in patients with ischemic stroke. Front Aging Neurosci. 2020;12:511562. doi: 10.3389/fnagi.2020.511562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang L, Lou Y, Liu L, et al. Gut microbiotic features aiding the diagnosis of acute ischemic stroke. Front Cell Infect Microbiol. 2020;10:587284. doi: 10.3389/fcimb.2020.587284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SY, Lee SP, Kim D, et al. Gut dysbiosis: a new avenue for stroke prevention and therapeutics. Biomedicines. 2023;11(9):2352. doi: 10.3390/biomedicines11092352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou Z, Ouyang H, Chen G, et al. Gut microbiota as predictors of the occurrence of high on-treatment platelet reactivity in acute ischemic stroke patients. Front Cell Infect Microbiol. 2023;13:1257317. doi: 10.3389/fcimb.2023.1257317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benakis C, Poon C, Lane D, et al. Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke. 2020;51(6):1844–1854. doi: 10.1161/strokeaha.120.029262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N, Liu C, Yang Y, et al. Xiao-Xu-Ming decoction prevented hemorrhagic transformation induced by acute hyperglycemia through inhibiting AGE-RAGE-mediated neuroinflammation. Pharmacol Res. 2021;169:105650. doi: 10.1016/j.phrs.2021.105650 [DOI] [PubMed] [Google Scholar]
- 27.Cervi AL, Lukewich MK, Lomax AE. Neural regulation of gastrointestinal inflammation: role of the sympathetic nervous system. Auton Neurosci. 2014;182:83–88. doi: 10.1016/j.autneu.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 28.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- 29.Yu F, Feng X, Li X, et al. Gut-derived metabolite phenylacetylglutamine and White matter hyperintensities in patients with acute ischemic stroke. Front Aging Neurosci. 2021;13:675158. doi: 10.3389/fnagi.2021.675158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Qu J, Xu J, et al. Trimethylamine-N-oxide: a potential biomarker and therapeutic target in ischemic stroke. Front Neurol. 2023;14:1156879. doi: 10.3389/fneur.2023.1156879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou PS, Yang IH, Kuo CM, et al. The prognostic biomarkers of plasma trimethylamine N-oxide and short-chain fatty acids for recanalization therapy in acute ischemic stroke. Int J Mol Sci. 2023;24(13):10796. doi: 10.3390/ijms241310796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, d’Aigle J, Atadja L, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127(4):453–465. doi: 10.1161/circresaha.119.316448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rexidamu M, Li H, Jin H, et al. Serum levels of Trimethylamine-N-oxide in patients with ischemic stroke. Biosci Rep. 2019;39(6):BSR20190515. doi: 10.1042/bsr20190515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darbandi ZK, Amirahmadi S, Goudarzi I, et al. Folic acid improved memory and learning function in a rat model of neuroinflammation induced by lipopolysaccharide. Inflammopharmacology. 2024;32(2):1401–1411. doi: 10.1007/s10787-023-01314-w [DOI] [PubMed] [Google Scholar]
- 35.Wei J, Wang G, Lai M, et al. Faecal microbiota transplantation alleviates ferroptosis after ischaemic stroke. Neuroscience. 2024;541:91–100. doi: 10.1016/j.neuroscience.2024.01.021 [DOI] [PubMed] [Google Scholar]
- 36.Hediyal TA, Vichitra C, Anand N, et al. Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: an update. Front Immunol. 2024;15:1324018. doi: 10.3389/fimmu.2024.1324018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Yang H, Hou S, et al. Influence of the brain‑gut axis on neuroinflammation in cerebral ischemia‑reperfusion injury (Review). Int J Mol Med. 2024;53(3):30. doi: 10.3892/ijmm.2024.5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Q, Calduch RM. On traditional Chinese medicine regulation in China: how quality and safety of use are insured. Pharmacol Res. 2017;119:371–372. doi: 10.1016/j.phrs.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 39.Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5(1):8–24. doi: 10.1016/j.apsb.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng X, Hu J, Liu X, et al. Therapeutic targets by traditional Chinese medicine for ischemia-reperfusion injury induced apoptosis on cardiovascular and cerebrovascular diseases. Front Pharmacol. 2022;13:934256. doi: 10.3389/fphar.2022.934256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, Sun F, Zhang W, et al. Novel insight into the therapeutical potential of flavonoids from traditional Chinese medicine against cerebral ischemia/reperfusion injury. Front Pharmacol. 2024;15:1352760. doi: 10.3389/fphar.2024.1352760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Liu Z, Liao J, et al. Network pharmacology approaches for research of traditional Chinese medicines. Chin J Nat Med. 2023;21(5):323–332. doi: 10.1016/s1875-5364(23)60429-7 [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Hui X, Wang Y, et al. Angong niuhuang pill ameliorates cerebral ischemia/reperfusion injury in mice partly by restoring gut microbiota dysbiosis. Front Pharmacol. 2022;13:1001422. doi: 10.3389/fphar.2022.1001422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K, Chen Y, Cao J, et al. Mechanism of Huangqi-Honghua combination regulating the gut microbiota to affect bile acid metabolism towards preventing cerebral ischaemia-reperfusion injury in rats. Pharm Biol. 2022;60(1):2189–2199. doi: 10.1080/13880209.2022.2136209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao L, Xia X, Shuai Y, et al. Gut microbiota, a hidden protagonist of traditional Chinese medicine for acute ischemic stroke. Front Pharmacol. 2023;14:1164150. doi: 10.3389/fphar.2023.1164150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113(12):2019–2040. doi: 10.1007/s10482-020-01474-7 [DOI] [PubMed] [Google Scholar]
- 47.Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17(12):94. doi: 10.1007/s11910-017-0802-6 [DOI] [PubMed] [Google Scholar]
- 48.Lai Y, Dhingra R, Zhang Z, et al. Toward elucidating the human gut microbiota-brain axis: molecules, biochemistry, and implications for health and diseases. Biochemistry. 2022;61(24):2806–2821. doi: 10.1021/acs.biochem.1c00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhuo Z, Wang H, Zhang S, et al. Selenium supplementation provides potent neuroprotection following cerebral ischemia in mice. J Cereb Blood Flow Metab. 2023;43(7):1060–1076. doi: 10.1177/0271678x231156981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu K, Gao X, Xia G, et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021;8:gutjnl–2020–323263. doi: 10.1136/gutjnl-2020-323263 [DOI] [PubMed] [Google Scholar]
- 51.Mayer EA, Nance K, Chen S. The gut-brain axis. Annu Rev Med. 2022;73(1):439–453. doi: 10.1146/annurev-med-042320-014032 [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Ren S, Lv H, et al. Gut microbiota from mice with cerebral ischemia-reperfusion injury affects the brain in healthy mice. Aging. 2021;13(7):10058–10074. doi: 10.18632/aging.202763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng X, Gao X, Peng Y, et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front Cell Infect Microbiol. 2019;9:4. doi: 10.3389/fcimb.2019.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen YZ, Huang ZY, Zhou WW, et al. Uncovering the characteristics of the gut microbiota in patients with ischemic stroke and hemorrhagic stroke. Sci Rep. 2024;14(1):11776. doi: 10.1038/s41598-024-62606-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin J, Liao SX, He Y, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery Atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11):e002699. doi: 10.1161/jaha.115.002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan C, Wu Q, Wang H, et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr. 2021;45(3):518–529. doi: 10.1002/jpen.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pussinen PJ, Alfthan G, Jousilahti P, et al. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis. 2007;193(1):222–228. doi: 10.1016/j.atherosclerosis.2006.06.027 [DOI] [PubMed] [Google Scholar]
- 58.Kawato T, Tanaka H, Tabuchi M, et al. Continual Gram-negative bacterial challenge accelerates stroke onset in stroke-prone spontaneously hypertensive rats. Clin Exp Hypertens. 2013;35(1):28–34. doi: 10.3109/10641963.2012.689042 [DOI] [PubMed] [Google Scholar]
- 59.Xia GH, You C, Gao XX, et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol. 2019;10:397. doi: 10.3389/fneur.2019.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh V, Roth S, Llovera G, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36(28):7428–7440. doi: 10.1523/jneurosci.1114-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akhoundzadeh K, Vakili A, Shadnoush M, et al. Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J Med Sci. 2018;43(1):32–40. doi: 10.4161/gmic.29232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen R, Xu Y, Wu P, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:104403. doi: 10.1016/j.phrs.2019.104403 [DOI] [PubMed] [Google Scholar]
- 63.Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24(6):503–512. doi: 10.1111/j.1365-2982.2012.01921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prame Kumar K, McKay LD, Nguyen H, et al. Sympathetic-mediated intestinal cell death contributes to gut barrier impairment after stroke. Transl Stroke Res. 2023. doi: 10.1007/s12975-023-01211-y [DOI] [PubMed] [Google Scholar]
- 65.Pan P, Song Y, Du X, et al. Intestinal barrier dysfunction following traumatic brain injury. Neurol Sci. 2019;40(6):1105–1110. doi: 10.1007/s10072-019-03739-0 [DOI] [PubMed] [Google Scholar]
- 66.Xia GH, Zhang MS, Wu QH, et al. Dysbiosis of gut microbiota is an independent risk factor of stroke-associated pneumonia: a Chinese pilot study. Front Cell Infect Microbiol. 2021;11:715475. doi: 10.3389/fcimb.2021.715475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley D, Mason LJ, Mackin KE, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22(11):1277–1284. doi: 10.1038/nm.4194 [DOI] [PubMed] [Google Scholar]
- 68.Chen R, Wu P, Cai Z, et al. Puerariae lobatae radix with chuanxiong rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem. 2019;65:101–114. doi: 10.1016/j.jnutbio.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 69.Abramov VM, Kosarev IV, Priputnevich TV, et al. S-layer protein 2 of vaginal Lactobacillus crispatus 2029 enhances growth, differentiation, VEGF production and barrier functions in intestinal epithelial cell line Caco-2. Int J Biol Macromol. 2021;189:410–419. doi: 10.1016/j.ijbiomac.2021.08.150 [DOI] [PubMed] [Google Scholar]
- 70.Hagihara M, Kuroki Y, Ariyoshi T, et al. Clostridium butyricum modulates the microbiome to protect intestinal barrier function in mice with antibiotic-induced dysbiosis. iScience. 2020;23(1):100772. doi: 10.1016/j.isci.2019.100772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J, Ling Z, Wang F, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. 2016;613:30–35. doi: 10.1016/j.neulet.2015.12.047 [DOI] [PubMed] [Google Scholar]
- 72.Debnath N, Kumar R, Kumar A, et al. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol Genet Eng Rev. 2021;37(2):105–153. doi: 10.1080/02648725.2021.1989847 [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Zhu N, Su X, et al. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12(5):793. doi: 10.3390/cells12050793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry N, Frank J, McLouth C, et al. Short chain fatty acids taken at time of thrombectomy in acute ischemic stroke patients are independent of stroke severity but associated with inflammatory markers and worse symptoms at discharge. Front Immunol. 2021;12:797302. doi: 10.3389/fimmu.2021.797302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang T, Pan C, Xie C, et al. Microbiota metabolites and immune regulation affect ischemic stroke occurrence, development, and prognosis. Mol Neurobiol. 2023;60(11):6176–6187. doi: 10.1007/s12035-023-03473-x [DOI] [PubMed] [Google Scholar]
- 77.Sadler R, Cramer JV, Heindl S, et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40(5):1162–1173. doi: 10.1523/jneurosci.1359-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu D, Gu S, Zhou Z, et al. Associations of plasma TMAO and its precursors with stroke risk in the general population: a nested case-control study. J Intern Med. 2023;293(1):110–120. doi: 10.1111/joim.13572 [DOI] [PubMed] [Google Scholar]
- 79.Zhang P, Wang R, Qu Y, et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and stroke outcome: a systematic review. Front Mol Neurosci. 2023;16:1165398. doi: 10.3389/fnmol.2023.1165398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu C, Xue F, Lian Y, et al. Relationship between elevated plasma trimethylamine N-oxide levels and increased stroke injury. Neurology. 2020;94(7):e667–e677. doi: 10.1212/wnl.0000000000008862 [DOI] [PubMed] [Google Scholar]
- 81.Haghikia A, Li XS, Liman TG, et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol. 2018;38(9):2225–2235. doi: 10.1161/atvbaha.118.311023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu C, Li Z, Song Z, et al. Choline and butyrate beneficially modulate the gut microbiome without affecting atherosclerosis in APOE*3-Leiden.CETP mice. Atherosclerosis. 2022;362:47–55. doi: 10.1016/j.atherosclerosis.2022.10.009 [DOI] [PubMed] [Google Scholar]
- 83.Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daulatzai MA. Fundamental role of pan-inflammation and oxidative-nitrosative pathways in neuropathogenesis of Alzheimer’s disease in focal cerebral ischemic rats. Am J Neurodegener Dis. 2016;5(2):102–130. [PMC free article] [PubMed] [Google Scholar]
- 85.Catorce MN, Gevorkian G. Evaluation of anti-inflammatory nutraceuticals in LPS-induced mouse neuroinflammation model: an update. Curr Neuropharmacol. 2020;18(7):636–654. doi: 10.2174/1570159x18666200114125628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang Z, Li L, Liu L, et al. Ischemic stroke and dysbiosis of gut microbiota: changes to LPS levels and effects on functional outcomes. Altern Ther Health Med. 2023;29(5):284–292. [PubMed] [Google Scholar]
- 87.Wang Z, Li J, Xu Y, et al. Elevated gut microbiota metabolite bile acids confer protective effects on clinical prognosis in ischemic stroke patients. Front Neurosci. 2024;18:1388748. doi: 10.3389/fnins.2024.1388748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Geng J, Hong Y, et al. Orally Administered crocin protects against cerebral ischemia/reperfusion injury through the metabolic transformation of crocetin by gut microbiota. Front Pharmacol. 2019;10:440. doi: 10.3389/fphar.2019.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu W, Kong X, Wang H, et al. Ischemic stroke and intestinal flora: an insight into brain-gut axis. Eur J Med Res. 2022;27(1):73. doi: 10.1186/s40001-022-00691-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koch HJ, Uyanik G, Bogdahn U, et al. Relation between laterality and immune response after acute cerebral ischemia. Neuroimmunomodulation. 2006;13(1):8–12. doi: 10.1159/000092108 [DOI] [PubMed] [Google Scholar]
- 92.Ellis JP, Kalata N, Joekes EC, et al. Ischemic stroke as a complication of cryptococcal meningitis and immune reconstitution inflammatory syndrome: a case report. BMC Infect Dis. 2018;18(1):520. doi: 10.1186/s12879-018-3386-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi J, Kim BR, Akuzum B, et al. T(REG)king from gut to brain: the control of regulatory T cells along the gut-brain axis. Front Immunol. 2022;13:916066. doi: 10.3389/fimmu.2022.916066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22(5):516–523. doi: 10.1038/nm.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y, Liang J, Ouyang F, et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front Neurol. 2019;10:661. doi: 10.3389/fneur.2019.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Q, Deng P, Chen S, et al. Electroacupuncture and human iPSC-derived small extracellular vesicles regulate the gut microbiota in ischemic stroke via the brain-gut axis. Front Immunol. 2023;14:1107559. doi: 10.3389/fimmu.2023.1107559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Egashira Y, Suzuki Y, Azuma Y, et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J Neuroinflammation. 2013;10(1):105. doi: 10.1186/1742-2094-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giles EM, Stagg AJ. Type 1 Interferon in the human intestine-a co-ordinator of the immune response to the microbiota. Inflamm Bowel Dis. 2017;23(4):524–533. doi: 10.1097/mib.0000000000001078 [DOI] [PubMed] [Google Scholar]
- 99.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6(1):6734. doi: 10.1038/ncomms7734 [DOI] [PubMed] [Google Scholar]
- 100.Yan J, Greer JM, Etherington K, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009;206(1–2):112–117. doi: 10.1016/j.jneuroim.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 101.Liesz A, Hu X, Kleinschnitz C, et al. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke. 2015;46(5):1422–1430. doi: 10.1161/strokeaha.114.008608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80–93. doi: 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonçalves P, Araújo JR, Di Santo JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(3):558–572. doi: 10.1093/ibd/izx029 [DOI] [PubMed] [Google Scholar]
- 104.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y, Fang XM. The pan-liver network theory: from traditional Chinese medicine to western medicine. Chin J Physiol. 2023;66(6):401–436. doi: 10.4103/cjop.CJOP-D-22-00131 [DOI] [PubMed] [Google Scholar]
- 106.Hou Y, Qieni X, Li N, et al. Longzhibu disease and its therapeutic effects by traditional Tibetan medicine: ershi-wei Chenxiang pills. J Ethnopharmacol. 2020;249:112426. doi: 10.1016/j.jep.2019.112426 [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Liu X, Zhang W, et al. Synergy of “Yiqi” and “Huoxue” components of QishenYiqi formula in ischemic stroke protection via lysosomal/inflammatory mechanisms. J Ethnopharmacol. 2022;293:115301. doi: 10.1016/j.jep.2022.115301 [DOI] [PubMed] [Google Scholar]
- 108.Sun J, Wang F, Ling Z, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042 [DOI] [PubMed] [Google Scholar]
- 109.Pang SQ, Luo ZT, Wang CC, et al. Effects of Dioscorea polystachya ‘yam gruel’ on the cognitive function of diabetic rats with focal cerebral ischemia-reperfusion injury via the gut-brain axis. J Integr Neurosci. 2020;19(2):273–283. doi: 10.31083/j.jin.2020.02.69 [DOI] [PubMed] [Google Scholar]
- 110.Ding X, Liu Z, Liu Y, et al. Comprehensive evaluation of the mechanism of gastrodia elata Blume in ameliorating cerebral ischemia-reperfusion injury based on integrating fecal metabonomics and 16S rDNA sequencing. Front Cell Infect Microbiol. 2022;12:1026627. doi: 10.3389/fcimb.2022.1026627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong Q, Lin X, Shen L, et al. The protective effect of herbal polysaccharides on ischemia-reperfusion injury. Int J Biol Macromol. 2016;92:431–440. doi: 10.1016/j.ijbiomac.2016.07.052 [DOI] [PubMed] [Google Scholar]
- 112.Yang B, Zhang LY, Chen Y, et al. Melatonin alleviates intestinal injury, neuroinflammation and cognitive dysfunction caused by intestinal ischemia/reperfusion. Int Immunopharmacol. 2020;85:106596. doi: 10.1016/j.intimp.2020.106596 [DOI] [PubMed] [Google Scholar]
- 113.Su LJ, Ren YC, Chen Z, et al. Ginsenoside Rb1 improves brain, lung, and intestinal barrier damage in middle cerebral artery occlusion/reperfusion (MCAO/R) micevia the PPARγ signaling pathway. Chin J Nat Med. 2022;20(8):561–571. doi: 10.1016/s1875-5364(22)60204-8 [DOI] [PubMed] [Google Scholar]
- 114.Liu Y, Zhao P, Cai Z, et al. Buqi-Huoxue-Tongnao decoction drives gut microbiota-derived indole lactic acid to attenuate ischemic stroke via the gut-brain axis. Chin Med. 2024;19(1):126. doi: 10.1186/s13020-024-00991-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi Y, Du Q, Li Z, et al. Multiomics profiling of the therapeutic effect of Dan-deng-tong-nao capsule on cerebral ischemia-reperfusion injury. Phytomedicine. 2024;128:155335. doi: 10.1016/j.phymed.2023.155335 [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, Xue S, Fei C, et al. Protective effect of Tao Hong Si Wu Decoction against inflammatory injury caused by intestinal flora disorders in an ischemic stroke mouse model. BMC Complement Med Ther. 2024;24(1):124. doi: 10.1186/s12906-024-04417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ni Y, Cai L, Gou X, et al. Therapeutic effect of Sanhua decoction on rats with middle cerebral artery occlusion and the associated changes in gut microbiota and short-chain fatty acids. PLoS One. 2024;19(2):e0298148. doi: 10.1371/journal.pone.0298148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nie H, Ge J, Yang K, et al. Naotaifang III protects against cerebral ischemia injury through LPS/TLR4 signaling pathway in the microbiota-gut-brain axis. Drug Des Devel Ther. 2023;17:3571–3588. doi: 10.2147/dddt.S421658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang R, Sun Y, Wang M, et al. Therapeutic effect of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. leaves on ischemic stroke via the microbiota–gut–brain axis. Phytother Res. 2023;37(10):4801–4818. doi: 10.1002/ptr.7947 [DOI] [PubMed] [Google Scholar]
- 120.Luo S, Chen Y, Zhao R, et al. Application of omics technology to investigate the mechanism underlying the role of San Hua Tang in regulating microglia polarization and blood-brain barrier protection following ischemic stroke. J Ethnopharmacol. 2023;314:116640. doi: 10.1016/j.jep.2023.116640 [DOI] [PubMed] [Google Scholar]
- 121.Zhai Y, Luo Y, Mo X, et al. Zhuang medicine Shuanglu Tongnao compound recipe treats stroke by affecting the intestinal flora regulated by the TLR4/NF-κB signaling pathway. Ann Transl Med. 2023;11(4):174. doi: 10.21037/atm-23-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lian Z, Xu Y, Wang C, et al. Gut microbiota-derived melatonin from puerariae lobatae radix-resistant starch supplementation attenuates ischemic stroke injury via a positive microbial co-occurrence pattern. Pharmacol Res. 2023;190:106714. doi: 10.1016/j.phrs.2023.106714 [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Shen L, Xie J, et al. Pushen capsule treatment promotes functional recovery after ischemic stroke. Phytomedicine. 2023;111:154664. doi: 10.1016/j.phymed.2023.154664 [DOI] [PubMed] [Google Scholar]
- 124.Li Q, Cao M, Wei Z, et al. The protective effect of Buzhong Yiqi decoction on ischemic stroke mice and the mechanism of gut microbiota. Front Neurosci. 2022;16:956620. doi: 10.3389/fnins.2022.956620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang R, Liu M, Ren G, et al. Zhilong Huoxue Tongyu Capsules’ effects on ischemic stroke: an assessment using fecal 16S rRNA gene sequencing and untargeted serum metabolomics. Front Pharmacol. 2022;13:1052110. doi: 10.3389/fphar.2022.1052110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu G, Lin J, Zhang L, et al. Uncovering the mechanism of the xingnaojing injection against ischemic stroke using a combined network pharmacology approach and gut microbiota analysis. Evid Based Complement Alternat Med. 2022;2022:5886698. doi: 10.1155/2022/5886698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xian M, Shen L, Zhan S, et al. Integrated 16S rRNA gene sequencing and LC/MS-based metabolomics ascertained synergistic influences of the combination of acupuncture and NaoMaiTong on ischemic stroke. J Ethnopharmacol. 2022;293:115281. doi: 10.1016/j.jep.2022.115281 [DOI] [PubMed] [Google Scholar]
- 128.Gao Q, Han ZY, Tian DF, et al. Xinglou Chengqi Decoction improves neurological function in experimental stroke mice as evidenced by gut microbiota analysis and network pharmacology. Chin J Nat Med. 2021;19(12):881–899. doi: 10.1016/s1875-5364(21)60079-1 [DOI] [PubMed] [Google Scholar]
- 129.Fu K, Zhang D, Song Y, et al. Tibetan medicine Qishiwei Zhenzhu Pills can reduce cerebral ischemia-reperfusion injury by regulating gut microbiota and inhibiting inflammation. Evid Based Complement Alternat Med. 2021;2021:2251679. doi: 10.1155/2021/2251679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang F, Zhai M, Wu Q, et al. Protective effect of Tong-Qiao-Huo-Xue decoction on inflammatory injury caused by intestinal microbial disorders in stroke rats. Biol Pharm Bull. 2020;43(5):788–800. doi: 10.1248/bpb.b19-00847 [DOI] [PubMed] [Google Scholar]
- 131.Liu X, Wang Y, Tian Y, et al. The water extract of rhubarb prevents ischemic stroke by regulating gut bacteria and metabolic pathways. Metabolites. 2024;14(4):216. doi: 10.3390/metabo14040216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang Y, Li Y, Guan D, et al. Acorus tatarinowii oils exert protective effects on microglia-mediated inflammatory injury via restoring gut microbiota composition in experimental stroke rats. Brain Res Bull. 2024;213:110990. doi: 10.1016/j.brainresbull.2024.110990 [DOI] [PubMed] [Google Scholar]
- 133.Zhang S, Chen Q, Jin M, et al. Notoginsenoside R1 alleviates cerebral ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway through microbiota-gut-brain axis. Phytomedicine. 2024;128:155530. doi: 10.1016/j.phymed.2024.155530 [DOI] [PubMed] [Google Scholar]
- 134.Duan H, Hu J, Deng Y, et al. Berberine mediates the production of butyrate to ameliorate cerebral ischemia via the gut microbiota in mice. Nutrients. 2023;16(1):9. doi: 10.3390/nu16010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan C, Liu Z, Xie W, et al. Cornuside protects against ischemic stroke in rats by suppressing the IL-17F/TRAF6/NF-κB pathway via the brain-gut axis. Exp Neurol. 2024;373:114672. doi: 10.1016/j.expneurol.2023.114672 [DOI] [PubMed] [Google Scholar]
- 136.Li M, Wang S, Zhang C, et al. Escin alleviates stress-induced intestinal dysfunction to protect brain injury by regulating the gut-brain axis in ischemic stroke rats. Int Immunopharmacol. 2023;115:109659. doi: 10.1016/j.intimp.2022.109659 [DOI] [PubMed] [Google Scholar]
- 137.Xie Y, Zou X, Han J, et al. Indole-3-propionic acid alleviates ischemic brain injury in a mouse middle cerebral artery occlusion model. Exp Neurol. 2022;353:114081. doi: 10.1016/j.expneurol.2022.114081 [DOI] [PubMed] [Google Scholar]
- 138.Xu N, Kan P, Yao X, et al. Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J Microbiol. 2018;56(11):838–846. doi: 10.1007/s12275-018-8327-5 [DOI] [PubMed] [Google Scholar]
- 139.Zhao N, Gao Y, Jia H, et al. Anti-apoptosis effect of traditional Chinese medicine in the treatment of cerebral ischemia-reperfusion injury. Apoptosis. 2023;28(5–6):702–729. doi: 10.1007/s10495-023-01824-6 [DOI] [PubMed] [Google Scholar]
- 140.Guo Q, Ni C, Li L, et al. Integrated traditional Chinese medicine improves functional outcome in acute ischemic stroke: from clinic to mechanism exploration with gut microbiota. Front Cell Infect Microbiol. 2022;12:827129. doi: 10.3389/fcimb.2022.827129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu T, Xing Y, Gao Q, et al. Ginkgo biloba extract drives gut flora and microbial metabolism variation in a mouse model of Alzheimer’s disease. Pharmaceutics. 2023;15(12):2746. doi: 10.3390/pharmaceutics15122746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xuejiang W, Magara T, Konishi T. Prevention and repair of cerebral ischemia-reperfusion injury by Chinese herbal medicine, shengmai san, in rats. Free Radic Res. 1999;31(5):449–455. doi: 10.1080/10715769900301011 [DOI] [PubMed] [Google Scholar]
- 143.Lin H, Chen S, Shen L, et al. Integrated analysis of the cecal microbiome and plasma metabolomics to explore NaoMaiTong and its potential role in changing the intestinal flora and their metabolites in ischemic stroke. Front Pharmacol. 2021;12:773722. doi: 10.3389/fphar.2021.773722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mao M, Cao X, Liang Y, et al. Neuroprotection of rhubarb extract against cerebral ischaemia-reperfusion injury via the gut-brain axis pathway. Phytomedicine. 2024;126:155254. doi: 10.1016/j.phymed.2023.155254 [DOI] [PubMed] [Google Scholar]
- 145.Shen H, Tong X, Yang J, et al. Biotransformation of natural hydroxycinnamic acids by gut microbiota from normal and cerebral ischemia-reperfusion injured rats: a comparative study. Food Funct. 2020;11(6):5389–5395. doi: 10.1039/d0fo00775g [DOI] [PubMed] [Google Scholar]
- 146.Li Q, Guo Y, Yu X, et al. Protective mechanism of rhubarb anthraquinone glycosides in rats with cerebral ischaemia-reperfusion injury: interactions between medicine and intestinal flora. Chin Med. 2020;15(1):60. doi: 10.1186/s13020-020-00341-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang M, Fu R, Xu D, et al. Traditional Chinese Medicine: a promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology. J Ethnopharmacol. 2024;318(Pt A):116879. doi: 10.1016/j.jep.2023.116879 [DOI] [PubMed] [Google Scholar]
- 148.Li H, Xiao J, Li X, et al. Low cerebral exposure cannot hinder the neuroprotective effects of Panax notoginsenosides. Drug Metab Dispos. 2018;46(1):53–65. doi: 10.1124/dmd.117.078436 [DOI] [PubMed] [Google Scholar]
- 149.Dou Z, Rong X, Zhao E, et al. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gut-brain axis. Cell Mol Neurobiol. 2019;39(6):883–898. doi: 10.1007/s10571-019-00687-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kurita N, Yamashiro K, Kuroki T, et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J Cereb Blood Flow Metab. 2020;40(12):2505–2520. doi: 10.1177/0271678x19899577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ahmed H, Leyrolle Q, Koistinen V, et al. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. 2022;14(1):2102878. doi: 10.1080/19490976.2022.2102878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li S, Zhao X, Lin F, et al. Gut flora mediates the rapid tolerance of electroacupuncture on ischemic stroke by activating melatonin receptor through regulating indole-3-propionic acid. Am J Chin Med. 2022;50(4):979–1006. doi: 10.1142/s0192415x22500409 [DOI] [PubMed] [Google Scholar]
- 153.Guo Q, Jiang X, Ni C, et al. Gut microbiota-related effects of Tanhuo decoction in acute ischemic stroke. Oxid Med Cell Longev. 2021;2021(1):5596924. doi: 10.1155/2021/5596924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xian M, Ma Z, Zhan S, et al. Network analysis of microbiome and metabolome to explore the mechanism of raw rhubarb in the protection against ischemic stroke via microbiota-gut-brain axis. Fitoterapia. 2024;175:105969. doi: 10.1016/j.fitote.2024.105969 [DOI] [PubMed] [Google Scholar]
- 155.Ghosh S, Pramanik S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch Microbiol. 2021;203(9):5281–5308. doi: 10.1007/s00203-021-02516-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He X, Cai Q, Li J, et al. Involvement of brain-gut axis in treatment of cerebral infarction by β-asaron and paeonol. Neurosci Lett. 2018;666:78–84. doi: 10.1016/j.neulet.2017.12.036 [DOI] [PubMed] [Google Scholar]
- 157.Xia ZY, Luo C, Liu BW, et al. Shengui Sansheng Pulvis maintains blood-brain barrier integrity by vasoactive intestinal peptide after ischemic stroke. Phytomedicine. 2020;67:153158. doi: 10.1016/j.phymed.2019.153158 [DOI] [PubMed] [Google Scholar]
- 158.Zhao QY, Tang RH, Lu GX, et al. Efficacy of Getong Tongluo capsule for convalescent-phase of ischemic stroke and primary hypertension: a multicenter, randomized, double-blind, controlled trial. Chin J Integr Med. 2021;27(4):252–258. doi: 10.1007/s11655-020-3320-3 [DOI] [PubMed] [Google Scholar]
- 159.Yu Y, Tang L, Cui F, et al. Effect of Qizhitongluo capsule on lower limb rehabilitation after stroke: a randomized clinical trial. Pharmacol Res. 2021;165:105464. doi: 10.1016/j.phrs.2021.105464 [DOI] [PubMed] [Google Scholar]
- 160.Venketasubramanian N, Chen CL, Gan RN, et al. A double-blind, placebo-controlled, randomized, multicenter study to investigate Chinese medicine neuroaid efficacy on stroke recovery (CHIMES Study). Int J Stroke. 2009;4(1):54–60. doi: 10.1111/j.1747-4949.2009.00237.x [DOI] [PubMed] [Google Scholar]
- 161.Young SH, Zhao Y, Koh A, et al. Safety profile of MLC601 (Neuroaid) in acute ischemic stroke patients: a Singaporean substudy of the Chinese medicine neuroaid efficacy on stroke recovery study. Cerebrovasc Dis. 2010;30(1):1–6. doi: 10.1159/000313398 [DOI] [PubMed] [Google Scholar]
- 162.Yu XF, Zhu XY, Yuan CX, et al. Naoxintong capsule for secondary prevention of ischemic stroke: a multicenter, randomized, and placebo-controlled trial. Chin J Integr Med. 2022;28(12):1063–1071. doi: 10.1007/s11655-022-3586-8 [DOI] [PubMed] [Google Scholar]
- 163.Xie RM, Chen HX, Xie YM. Effects of integrative medicine protocols on the improvement of neural function deficit and disability outcomes in patients with acute ischemic cerebral stroke. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(9):1175–1180. [PubMed] [Google Scholar]
- 164.Sarma KN, Thalluri C, Mandhadi JR. Nanofibers in drug delivery systems: a comprehensive scientific review of recent approaches. Int J Pharm Investig. 2024;14(3):633. doi: 10.5530/ijpi.14.3.75 [DOI] [Google Scholar]