Abstract
Background
Individuals with alcohol use disorder (AUD) often experience symptoms such as anxiety, depression, and decreased sleep quality. Although these are not diagnostic criteria, they may increase dependence risk and complicate treatment. This study aims to analyze comorbidities and their complex relationships in AUD patients through epidemiological surveys and network analysis.
Materials and methods
Using multi-stage stratified cluster random sampling, we selected 27,913 individuals and identified those with AUD for the study. All screened subjects were assessed with the General Health Questionnaire, Pittsburgh Sleep Quality Index, and Simple Coping Style Questionnaire, and diagnosed according to DSM-IV criteria. Network analysis and visualization were performed in R 4.4.0. The qgraph and bootnet packages in R were used to obtain partial correlation network analysis and node centrality of mental health, sleep quality, and coping styles in individuals with AUD through the estimateNetwork function. The bootnet package was used to assess the accuracy and stability of the network. The bnlearn package in R was used to construct directed acyclic graph (DAG) for individuals with AUD using the Bayesian hill-climbing algorithm.
Results
In the partial correlation network, among the three major comorbidity categories, ‘anxiety/depression’ was most strongly associated with ‘sleep quality’. ‘Anxiety/depression’ and ‘sleep quality’ had the highest node centrality, with ‘sleep latency’ also showing notable centrality. The DAG results indicated that ‘sleep latency’ had the highest probability priority, directly affecting ‘anxiety/depression’ and key sleep quality symptoms such as ‘subjective sleep quality’, ‘sleep disturbances’, ‘sleep duration’, and ‘sleep efficiency’, while also indirectly influencing other symptoms.
Conclusions
Among the comorbid symptoms of AUD, sleep latency appears to be a key factor in triggering other comorbid symptoms. This study provides a basis for interventions aimed at reducing the comorbid symptoms of AUD and promoting recovery.
Keywords: Alcohol use disorder, sleep quality, mental health, coping styles, epidemiological surveys, network analysis method
1. Introduction
Alcohol use disorder (AUD) is one of the most common chronic and recurrent mental disorders worldwide. It is characterized by a loss of control over alcohol use, leading to physical dependence, increased tolerance, and impairments in psychological, social, and physical health [1, 2]. This disorder is highly disabling and associated with multiple health issues, significantly increasing the risk of premature death in patients [3]. The 12-month prevalence of AUD in the United States is 13.9%. It is significantly associated with other substance use disorders and major depressive disorder [4]. In China, the prevalence of AUD is increasing. According to a survey conducted in four provinces from 2001 to 2005, the prevalence can reach up to 4% [5]. In a 2010 epidemiological survey of mental disorder patients over 18 in Shandong Province, the prevalence reached 5.55% [5]. These data reveal the high prevalence and complexity of AUD [6].
Anxiety, depression, and reduced sleep quality frequently occur in patients with AUD and significantly worsen the condition, posing challenges to treatment and recovery [7–10]. Although not part of the clinical diagnostic criteria for AUD, these conditions lead to decreased social functioning and encourage negative coping mechanisms, which directly increase the severity of alcohol use [11]. Jo-Anne Puddephatt’s 2021 study, complemented by later research, elucidates complex relationships between AUD and mental health challenges, particularly highlighting elevated drinking rates in individuals with mental health conditions [12–15]. Furthermore, studies link AUD not only to insomnia but also to a range of other sleep disorders [8, 9, 16], complicating the development of effective treatment strategies. Despite acknowledged interconnections, contemporary research rarely incorporates mental health, sleep, and coping strategies into its analyses. Past studies often have neglected the specific symptoms of individual comorbid conditions in AUD patients, as well as the dynamics and influence mechanisms among these symptoms. This oversight impedes the clinical management of AUD and highlights the urgent necessity for more comprehensive investigations.
Employing network analysis can provide visual and analytical insights into the relationships among comorbid symptoms, revealing potential connections and interactions that conventional research methods often miss. This method is effectively used in psychology and psychiatry to conceptualize mental constructs as dynamic systems of interacting elements [17]. Rather than viewing a construct as a single phenomenon measured by multiple variables, the network approach analyzes and visualizes the interactions among the variables themselves [18]. This approach enables researchers to explore the complex connections and dependencies among psychological variables more deeply. Investigating the structure and topology of a psychological variables network yields valuable insights into the organization and mechanisms of psychological constructs. For instance, central nodes in a network, highly connected to others, play a key role in sustaining the system by quickly spreading activation once triggered [17]. In psychopathology, central nodes are theoretically linked to a disorder’s prognosis [19, 20]. Assessing network structure allows researchers to explore node clustering into communities and the roles of specific nodes as bridges linking these communities [21]. In psychopathology networks, understanding community structures and identifying bridge nodes aids in examining comorbidity and enhances comprehension of how specific symptoms are pivotal to the network [22, 23]. Identifying these key nodes and their roles helps researchers better understand the complex interactions between different symptoms and their contribution to the overall mental health condition. Thus, adopting a network perspective in psychology research facilitates the identification of key variables that galvanize other variables or connect multiple constructs [24]. This approach not only deepens understanding of psychological constructs but also opens avenues for more targeted and effective interventions.
Therefore, considering the gaps in existing research and the advantages of network analysis, this study employs an epidemiological sampling survey focusing on individuals newly diagnosed with AUD. By integrating advanced network analysis techniques, we aim to thoroughly investigate the network dynamics among comorbid symptoms in AUD patients and develop a model to elucidate potential causal relationships among psychological, sleep, and coping symptoms. This study hypothesizes that various accompanying symptoms are interconnected and follow a specific sequential order. This innovative approach could enhance our understanding and management of AUD by unveiling complex symptom networks and facilitating more effective, personalized interventions, potentially leading to a paradigm shift in the perception and treatment of AUD.
2. Method
2.1. Subject
This survey targeted residents aged 18 and older in Shandong Province, forming a key component of the fourth epidemiological survey of mental disorders in the region. Using a multi-stage stratified cluster random sampling method, a sampling framework was created for each of the 16 cities in the province. Based on this framework, the population of each county (city, district) was sorted, and 62 townships and 34 streets were selected using a random number table. In each selected township (street), administrative villages (neighborhood committees) were sorted, and one village or neighborhood committee was randomly chosen. From the registered households, 300 were selected using a mechanical sampling method. All adults (aged 18 and above) who had lived in these households for more than six months were numbered, and the respondents were determined using random numbers.
In this epidemiological survey, besides investigating the overall prevalence of mental disorders, it was necessary to obtain reliable data on the prevalence of major mental illnesses such as depression and schizophrenia. Therefore, the sample size was estimated based on the lower prevalence of schizophrenia. We referenced the prevalence of schizophrenia (p = 6‰) to ensure a robust statistical foundation [24, 25]. Based on this data, with an allowable error of δ = 0.001 and a type I error probability of α = 0.05, the estimated sample size was 22,911 individuals. To accommodate potential non-responses and ensure comprehensive coverage, the sample size was increased by 20%, resulting in a total of 28,000 estimated participants. In the survey, 27,913 participants (96.92%) completed the screening, and 26,161 provided valid responses. Additional screening was conducted according to DSM-IV criteria to identify patients newly diagnosed with AUD and those previously diagnosed but still meeting the diagnostic criteria; these patients were included in the study. All participants provided written informed consent. This study was conducted in full accordance with the Declaration of Helsinki and received approval from the Ethics Committee of the Shandong Mental Health Center (ethical approval: 2014 Ethics Review No. R03).
2.2. Measures
2.2.1. Basic information
This self-designed form collects sociodemographic information from respondents, including details such as name, gender, age, years of education, marital status, and current occupation.
2.2.2. General health questionnaire (GHQ-12)
David Goldberg designed this self-report questionnaire in 1972 to assess various psychological states. It is widely used in epidemiological studies [26, 27]. The questionnaire uses a four-point rating scale, with scores ranging from 0 to 48, and includes 6 positive and 6 negative items. To provide a more nuanced analysis, this study uses Graetz’s three-factor model to classify the questionnaire into three dimensions: anxiety and depression, social dysfunction, and loss of confidence [28]. This model has been validated in numerous large-scale studies [29].
2.2.3. Pittsburgh sleep quality index (PSQI)
The Pittsburgh Sleep Quality Index (PSQI) includes 19 self-rated items and 5 observer-rated items. Notably, the 19th self-rated item and all observer-rated items are excluded from the total score calculation, resulting in a scoring system based on 18 self-rated items. The scoring covers various aspects of sleep, including subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of hypnotic medication, and daytime dysfunction [30].
2.2.4. Simplified coping style questionnaire (SCSQ)
Xie and Zhang developed the Simple Coping Style Questionnaire by integrating coping style scales from both domestic and international sources, considering the practical application and Chinese cultural characteristics. The questionnaire has shown high validity and reliability through testing. It is divided into two parts: positive and negative coping, and includes 20 items, rated on a four-point scale [31]. The positive coping score is the average of items 1–12, ranging from 0 to 3. The negative coping score is the average of items 13 to 20, also ranging from 0 to 3. The coping tendency score is obtained by subtracting the negative from the positive coping score. A positive value indicates a tendency towards positive coping, while a negative value indicates a tendency towards negative coping.
2.2.5. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV)
The DSM-IV was used as the diagnostic standard for mental disorders [32]. To assess Axis I disorders according to DSM-IV-TR criteria, the SCID-I/P (Structured Clinical Interview for DSM-IV Axis I Disorders, patient edition) was employed. This clinical diagnostic scale allows psychiatric personnel to evaluate patients’ mental states item by item during face-to-face interviews.
2.3. Training of survey personnel
To ensure survey efficiency and accuracy, we assembled 16 professional field teams by recruiting medical personnel from each city. Each team was responsible for survey tasks in their respective city, including organization and coordination, quality control, data management, and field surveys. Additionally, all team members underwent specialized training to improve the accuracy and efficiency of data collection.
2.4. Field survey
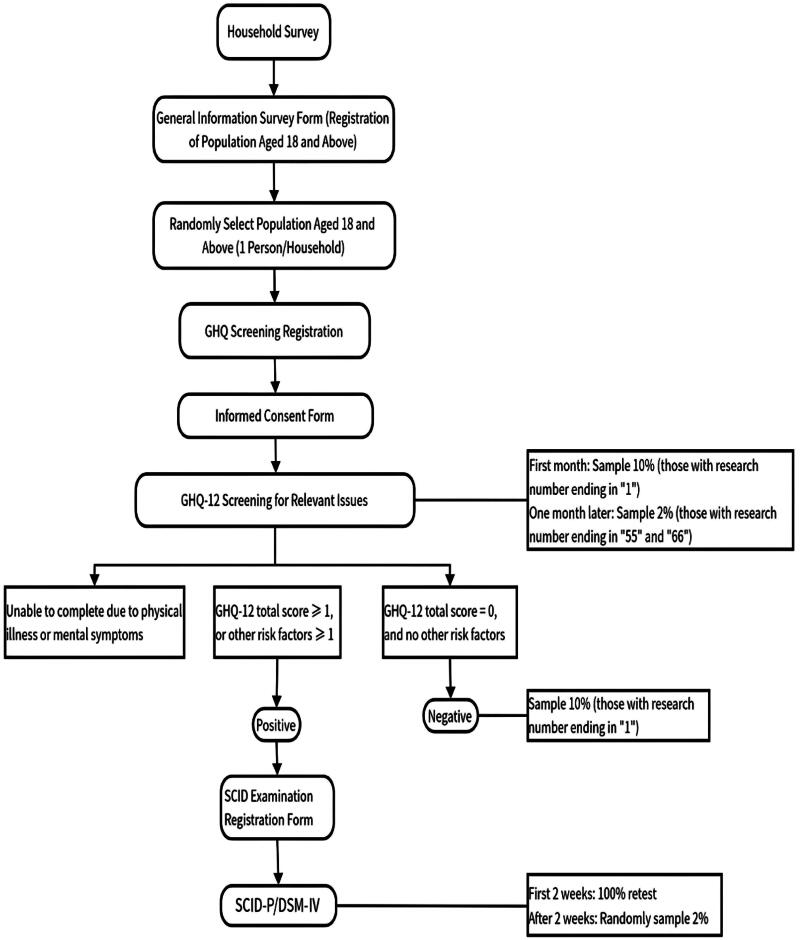
Trained investigators conducted field surveys with local village doctors and personnel from street offices. The survey had two stages: first, screening using GHQ-12; second, diagnosis using SCID-I/P for high-risk (positive) individuals identified in the screening, those unable to complete the screening due to physical illness, and 10% of low-risk (negative) individuals randomly selected. Information was collected on general conditions, sleep quality, and coping styles of individuals meeting the diagnostic criteria. The detailed survey process is shown in Figure 1.
Figure 1.
Epidemiological survey framework.
2.5. Statistical analyses
Data entry and verification were performed using Epidata 3.02 to ensure accuracy and completeness. In accordance with recent guidelines on the application of network analysis in clinical psychology [33], we utilized the R package ‘huge’ to apply the nonparanormal transformation [34]. This transformation was essential to satisfy the assumptions of multivariate normality, which is critical for the validity of subsequent network analyses [35]. Subsequently, R 4.4.0 was employed to perform network analysis and directed acyclic graph (DAG) analysis on general mental health, sleep quality, and coping styles of individuals with AUD. The aim was to explore potential relationships and causal mechanisms among various variables.
2.5.1. Network analysis
Network analysis was conducted using the qgraph and bootnet packages in R 4.4.0, utilizing the estimateNetwork function [36]. In this model, each node represents an item or symptom, and each edge represents the partial correlation between two nodes. The color and thickness of the edges indicate the nature and strength of the correlations: green indicates a positive partial correlation, red indicates a negative partial correlation, and line thickness indicates the strength of the correlation [33]. Utilizing partial correlations mitigates the impact of confounding factors by accounting for the effects of other variables within the network. This approach isolates the unique contribution of each variable, thus offering a more precise depiction of direct relationships among variables. Consequently, it provides a clearer representation of the true associations among symptoms, facilitating a more accurate understanding of the complex interactions within the network. The network comprises 12 nodes, corresponding to the three mental health dimensions of the GHQ scale (anxiety/depression, social dysfunction, and loss of confidence), the seven sleep symptoms of the PSQI (sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleep medication, and daytime dysfunction), and the coping styles of the SCSQ.
2.5.2. Node centrality analysis
In this analysis, the bootnet package in R was used to calculate the centrality measure of nodes, known as expected influence (EI) [37]. This measure considers both positive and negative connections between nodes, providing an assessment of each node’s overall influence within the network. Compared to traditional strength centrality, EI offers a more precise method for evaluating node importance, revealing their roles in the network more comprehensively [38]. The higher the EI value of a node, the greater its influence and contribution to the network’s structure and function [39].
2.5.3. Network accuracy and stability
The bootnet package in R was used to assess the network’s accuracy and stability [37]. First, a non-parametric bootstrap method (performed 1000 times) was employed to calculate the 95% confidence intervals (CI) of the edge weights, evaluating their accuracy. The narrowness of the CI indicates the accuracy of the estimates, with narrower CIs representing more reliable results. Second, a case-dropping bootstrap method was used to assess node centrality stability to calculate the correlation stability (CS) coefficient [18]. Generally, a CS coefficient greater than 0.7 indicates high reliability of network centrality.
2.5.4. DAG analysis
The bnlearn package in R was used to construct DAGs for individuals with AUD, using the Bayesian hill-climbing algorithm [40, 41]. This algorithm adds, removes, and changes edge directions to estimate a network structure that optimizes the Bayesian Information Criterion (BIC) [40]. Then, 10,000 bootstrapped networks were calculated to evaluate the stability of the DAG using a statistically driven method (optimal cut point) that retained edges with high sensitivity and specificity [42].
Additionally, to facilitate the interpretation of the DAG, arrow thickness was used to represent the change in the BIC value when the arrow was removed. The thicker the arrow, the greater the contribution of that edge to the model structure [22].
3. Results
3.1. Sample characteristics
The initial screening identified 1324 patients, including those newly diagnosed with AUD and those previously diagnosed but still meeting the AUD diagnostic criteria. After excluding those who did not complete the questionnaire, 1239 AUD patients remained in the study, comprising 25 (2%) females and 1214 (98%) males. The average general mental health score was 20.86 ± 2.86, ranging from 12 to 36. The average sleep quality score was 3.34 ± 3.10, ranging from 0 to 18. The average coping style tendency score was 0.001 ± 1.04, ranging from −3.88 to 3.54. The comorbid symptoms and sample characteristics of individuals with AUD are detailed in Table 1.
Table 1.
Sample characteristics of comorbid symptoms with AUD.
| Sample (n = 1239) | GHQ-12 |
PSQI |
SCSQ |
||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Urbanicity | |||||||
| Urban | 427 | 20.82 | 2.69 | 2.86 | 2.91 | 0.12 | 0.97 |
| Rural | 812 | 20.88 | 2.94 | 3.59 | 3.17 | −0.06 | 1.07 |
| Sex | |||||||
| Female | 25 | 21.88 | 3.31 | 4.80 | 4.70 | −0.24 | 0.92 |
| Male | 1214 | 20.83 | 2.84 | 3.31 | 3.06 | 0.01 | 1.04 |
| Age, y | |||||||
| 18–30 | 55 | 20.02 | 2.84 | 1.73 | 1.99 | 0.31 | 1.11 |
| 31–44 | 293 | 20.81 | 2.96 | 2.64 | 2.73 | 0.32 | 1.1 |
| 45–59 | 333 | 20.96 | 2.96 | 3.25 | 2.98 | −0.1 | 0.97 |
| 60–74 | 479 | 20.86 | 2.78 | 3.87 | 3.32 | −0.16 | 0.98 |
| ≥75 | 79 | 21.14 | 2.41 | 4.15 | 3.26 | 0.02 | 1.05 |
| Years of education | |||||||
| <1 | 127 | 21.39 | 3.21 | 4.41 | 3.72 | −0.17 | 1.02 |
| 1–6 | 419 | 20.99 | 2.87 | 3.79 | 3.15 | −0.18 | 0.96 |
| 7–9 | 467 | 20.67 | 2.81 | 3.01 | 2.99 | 0.11 | 1.06 |
| 10–12 | 156 | 20.73 | 2.63 | 2.71 | 2.66 | 0.23 | 1.1 |
| ≥13 | 70 | 20.61 | 2.83 | 2.26 | 2.26 | 0.21 | 1.03 |
| Marital status | |||||||
| Never married | 39 | 20.59 | 2.81 | 2.97 | 3.06 | −0.37 | 1.27 |
| Married | 1103 | 20.85 | 2.84 | 3.27 | 3.09 | 0.03 | 1.03 |
| Other | 97 | 20.99 | 3.04 | 4.25 | 3.12 | −0.23 | 1.03 |
| Occupation | |||||||
| Farmer/fisherman | 743 | 20.9 | 2.92 | 3.75 | 3.33 | −0.11 | 1.02 |
| Employed | 337 | 20.53 | 2.62 | 2.33 | 2.37 | 0.29 | 1.05 |
| Retired | 104 | 20.84 | 2.53 | 3.27 | 2.65 | −0.07 | 0.94 |
| Unemployed | 54 | 22.30 | 3.51 | 4.17 | 3.30 | −0.11 | 1.06 |
| Student | 1 | 20.86 | 0.00 | 0.44 | |||
Abbreviations: GHQ-12: General Health Questionnaire; PSQI: Pittsburgh Sleep Quality Index; SCSQ: Simplified Coping Style Questionnaire.
3.2. Network structure and centrality measure analysis
Figure 2 reveals the extensive correlations between psychological states, sleep quality, and coping styles with AUD. Among mental health symptoms, A1 ‘anxiety/depression’ had the strongest correlation with A3 ‘loss of confidence’. Among sleep quality symptoms, B3 ‘sleep duration’ was most closely related to B4 ‘sleep efficiency’. Among the three categories of comorbid symptoms, A1 ‘anxiety/depression’ had the strongest association with B1 ‘sleep quality’. Other important associations include A1 ‘anxiety/depression’ with B5 ‘sleep disturbances’ and B2 ‘sleep latency’ with B7 ‘daytime dysfunction’. Additionally, the associations of A3 ‘loss of confidence’ with B5 ‘sleep disturbances’ and C ‘coping styles’ are also noteworthy.
Figure 2.
Network structure of comorbid symptoms with AUD. Green lines indicate positive relationships. The thicker the edge, the greater the correlation between the two variables; the thinner the edge, the smaller the correlation; A1: anxiety/depression; A2: social dysfunction; A3: loss of confidence; B1: sleep quality; B2: sleep latency; B3: sleep duration; B4: sleep efficiency; B5: sleep disturbances; B6: sleep medication; B7: daytime dysfunction; C: coping styles.
The standardized estimate of the EI centrality measure is shown in Figure 3. EI centrality estimates varied widely across nodes. A1 ‘anxiety/depression’ and B1 ‘sleep quality’ were the most influential factors; B5 ‘sleep disturbances’, B3 ‘sleep duration’ and B2 ‘sleep latency’ were also found to be highly central. C ‘coping styles’ was the least influential factor.
Figure 3.
The expected influence measure for the network structure among AUD patients (Z). A1: anxiety/depression; A2: social dysfunction; A3: loss of confidence; B1: sleep quality; B2: sleep latency; B3: sleep duration; B4: sleep efficiency; B5: sleep disturbances; B6: sleep medication; B7: daytime dysfunction; C: coping styles.
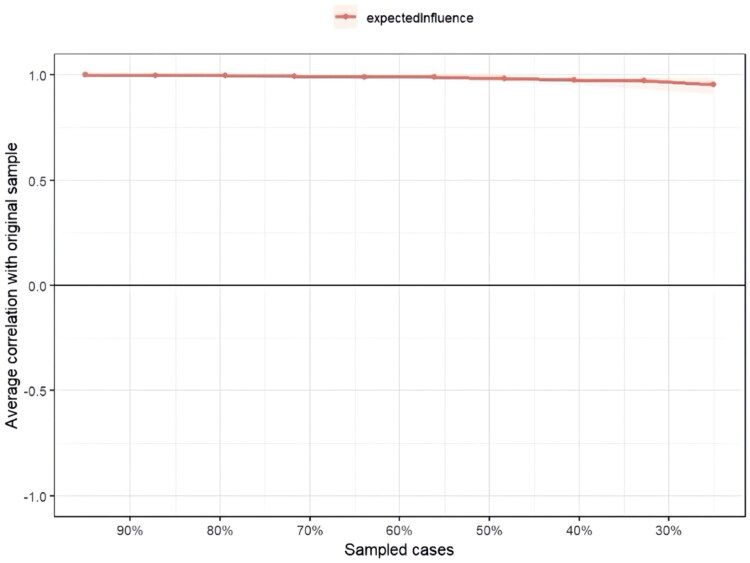
The case-dropping subset bootstrap procedure in Figure 4 showed that the values of EI remained stable even after dropping large portions of the sample, indicating the robustness of the EI estimates. Additionally, Figure 5 assesses the accuracy of the edge weights using the bootstrap method. The bootstrapped 95% confidence intervals (CIs) for the estimated edge weights were narrow, indicating that these estimates were reliable and accurate.
Figure 4.
Stability of centrality indices by case dropping subset bootstrap. Red lines indicate the average correlation between the expected influence in the original sample and the expected influence in the subsample.
Figure 5.
Bootstrapped confidence intervals of estimated edge weights. Red lines indicate the edge weights in the study sample, black lines represent the average edge weights estimated by the bootstrap, and the gray area represents the confidence intervals derived from the bootstrap.
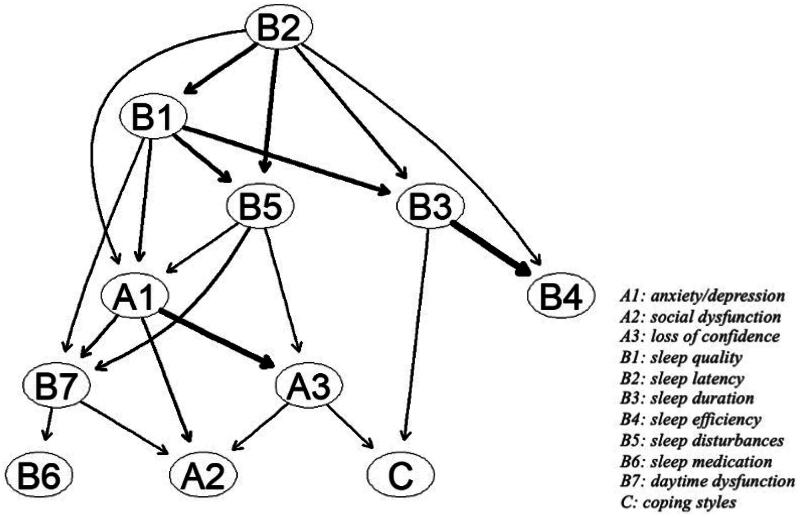
3.3. DAG of comorbid symptoms with AUD
DAG illustrates the potential causal relationships between variables, aiding in the inference and in-depth analysis of these relationships, thereby providing a deeper understanding of complex behaviors and psychological phenomena. Figure 6 shows the DAG based on 10,000 bootstrap samples, retaining only significant edges. The thickness of the arrows represents the change in the BIC value when the arrow is removed from the DAG. The thicker the arrow, the greater its impact on the model. Important connections include: from B3 ‘sleep duration’ to B4 ‘sleep efficiency’, from B2 ‘sleep latency’ to B1 ‘sleep quality’ and from A1 ‘anxiety/depression’ to A3 ‘loss of confidence’. Other significant connections impacting the DAG include: from B1 ‘sleep quality’ to A1 ‘anxiety/depression’, from A1 ‘anxiety/depression’ to B7 ‘daytime dysfunction’ and from A3 ‘loss of confidence’ to C ‘coping styles’. B2 ‘sleep latency’ is a key node in the DAG, as it directly influences A1 ‘anxiety/depression’ and major sleep quality symptoms (such as B1 ‘sleep quality’, B5 ‘sleep disturbances’, B3 ‘sleep duration’ and B4 ‘sleep efficiency’) and indirectly affects other symptoms. In this model, A2 ‘social dysfunction’, B6 ‘sleep medication’ and C ‘coping styles’ are considered outcome symptoms, influenced directly or indirectly by other symptoms.
Figure 6.
DAG of common comorbid symptoms with AUD. The edge thickness represents the significance of model fit; A1: anxiety/depression; A2: social dysfunction; A3: loss of confidence; B1: sleep quality; B2: sleep latency; B3: sleep duration; B4: sleep efficiency; B5: sleep disturbances; B6: sleep medication; B7: daytime dysfunction; C: coping styles.
4. Discussion
This study is the first to use both undirected and directed network structures to describe the relationships between mental health, sleep symptoms, and coping strategies in individuals with AUD. The network structure depicting partial correlations between symptom pairs allows for bidirectional edges, providing greater clarity on how symptoms are interrelated. Bayesian analysis has the advantage of suggesting explicit directions for causal relationships [22]. When examining both undirected and directed network structures, it is evident that in AUD, the activation of sleep latency triggers the activation of other parts of the network. Moreover, anxiety and depression serve as central nodes, connecting multiple other variables within the network. These findings are crucial for understanding the behavioral patterns and recovery processes of individuals with AUD.
Sleep problems in individuals with AUD are closely related to their psychological states and coping styles, consistent with previous research findings [43, 44]. More than half of individuals with AUD experience sleep problems that severely impact their daily functioning and can persist for years [8, 45]. During alcohol withdrawal, these sleep problems become more severe, delaying the recovery process and potentially leading to relapse [46, 47]. This study further confirms the importance of addressing sleep latency among various sleep issues.
Sleep latency acts as an activation source in the network, directly triggering subjective sleep quality, sleep duration, sleep efficiency, sleep disturbances, and anxiety/depression, and indirectly influencing other related symptoms. Extended sleep latency is the strongest predictor of poor subjective sleep quality [48] and may lead to sleep apnea among sleep disturbances [49]. Studies have found that alcohol consumption is proportional to sleep latency [50]. Alcohol is often used as an informal sleep aid [51]. For individuals not diagnosed with AUD, drinking may temporarily shorten sleep latency and improve the quality of non-rapid eye movement (NREM) sleep in the first half of the night [46]. However, for individuals with AUD, long-term drinking leads to tolerance of the effects that promote NREM sleep, eventually reducing the sedative effects [52]. This makes it harder for them to fall asleep and leads to over-reliance on hypnotic drugs and excessive drinking [46, 51, 53]. When individuals increase their alcohol consumption to alleviate insomnia symptoms, this behavior exacerbates the complex interaction between AUD and sleep problems, further deteriorating psychological and behavioral states and increasing the risk of relapse. Additionally, drinking time usually overlaps with bedtime, disrupting the first half of the sleep cycle [54]. This reduces slow-wave sleep and increases rapid eye movement (REM) sleep [52, 55], thus disrupting normal sleep architecture [54].
Anxiety/depression shows high levels of EI, indicating that they are key nodes in the network and are highly interconnected with other variables, complicating the comorbid symptoms of AUD. In individuals with AUD, anxiety and depression often co-occur and exacerbate the maintenance and relapse of AUD [56]. Similarly, anxiety and depression are also triggers for alcohol cravings [57]. By combining undirected and directed structure diagrams, it was found that anxiety and depression are mainly caused by sleep problems. Specifically, they are directly activated by sleep latency and are also indirectly affected by sleep quality due to sleep latency. The prevalence of sleep-onset insomnia is high among individuals with AUD [58], resulting in poor subjective sleep quality. Poor sleep quality complicates emotion regulation [59], particularly in relation to anxiety and depression [60, 61]. Furthermore, the weakening of positive emotions is often associated with the loss of NREM slow-wave sleep. Difficulty falling asleep reduces NREM sleep [58], further complicating the emotional regulation process. Thus, addressing sleep latency and improving sleep quality can be crucial steps in managing anxiety and depression in individuals with AUD.
At the same time, anxiety/depression also directly activates the nodes of social dysfunction, daytime dysfunction, and loss of confidence. Cognitive functions, especially problem-solving and decision-making abilities, decline due to alcohol-related damage to the frontal lobe [62, 63]. This decline in cognitive abilities restricts daily life and work activities, further aggravating anxiety and depression in individuals with AUD. As a result, self-efficacy decreases, leading to a loss of confidence and a tendency toward more negative coping strategies. Moreover, increased anxiety and depression further affect attention and memory, worsening both daytime and social functioning [1]. This results in significant daytime and social dysfunction.
This study provides essential data for policymakers, mental health professionals, and psychiatrists, facilitating a deeper understanding of the specific needs and effective treatment approaches for individuals with AUD. Beyond addressing the core issues of AUD, it is equally critical to consider its associated symptoms. Using network structure analysis, this study elucidates the complex interconnections and interactions between comorbid symptoms, further uncovering their sequential relationships. These findings can help identify specific intervention points in clinical practice, ultimately improving the effectiveness of treatment strategies. However, this study has some limitations. First, the subjects were mainly elderly residents in rural areas, and their average age may limit the generalizability of the results. Although a weighted design was added to the statistical analysis to reduce this bias, the specificity of this population structure may still impact the study results. Future research should include a broader population, especially individuals from urban areas and different age groups, to enhance the representativeness and applicability of the results. Second, because this study is part of epidemiological survey data, it did not assess the severity of AUD or the stage of progression in individuals, such as their daily alcohol consumption and the age at which they began drinking. Refining the classification and staging of AUD will help to more accurately and deeply study the interactive relationships among accompanying symptoms of different manifestations in patients with AUD. Third, although the DAG approach can bring cross-sectional data closer to a causal interpretation, the cross-sectional nature of this study makes the estimation of some network characteristics more tentative and exploratory. Prospective designs are needed to test the generated hypotheses and to consider combining traditional regression models with recently developed graphical learning methods.
5. Conclusions
Among the many comorbid symptoms of AUD, sleep problems are particularly prominent and profoundly affect patients’ mental health and coping styles. Specifically, sleep latency acts as an activation source among comorbid symptoms in individuals with AUD, prompting the onset and exacerbation of other symptoms. It severely affects the anxiety/depression and sleep quality of AUD patients. Additionally, anxiety/depression are also related to most networks, further aggravating comorbid symptoms and severely hindering the recovery of individuals with AUD. Therefore, treating AUD should not be limited to reducing drinking frequency; attention must also be given to managing sleep problems. Improving sleep quality, especially by shortening sleep latency, is crucial.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Authors contributions
Xin Yu: Conceptualization (equal); methodology (lead); data curation (lead); Formal analysis (lead); visualization (equal), writing-original draft (lead). Wen Zhang: Methodology (equal); validation (equal); investigation (equal); writing-review and editing (equal). Can Wang: Resources (equal); investigation (equal); writing-review and editing (equal). Guolin Mi: Resources (equal); investigation (equal); writing-review and editing (equal). Xiuzhe Chen: Resources (equal); investigation (equal); writing-review and editing (equal). Yanhu Wang: Resources (equal); investigation (equal); writing-review and editing (equal). Xu Chen: Supervision (lead); project administration (lead); writing-review and editing (lead).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Kendler KS, Ohlsson H, Sundquist J, et al. Alcohol use disorder and mortality across the lifespan: a longitudinal cohort and co-relative analysis. JAMA Psychiatry. 2016;73(6):575–581. doi: 10.1001/jamapsychiatry.2016.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayo-Smith MF, Lawrence D.. Treatment of alcohol use disorder in hospitalized patients: some sobering findings. Ann Intern Med. 2023;176(8):1129–1130. doi: 10.7326/M23-1419. [DOI] [PubMed] [Google Scholar]
- 3.Tucker JA, Chandler SD, Witkiewitz K.. Epidemiology of recovery from alcohol use disorder. Alcohol Res. 2020;40(3):02. doi: 10.35946/arcr.v40.3.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatr. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips MR, Zhang JX, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet. 2009;373(9680):2041–2053. doi: 10.1016/S0140-6736(09)60660-7. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatr. 2019;6(3):211–224. doi: 10.1016/S2215-0366(19)30074-4. [DOI] [PubMed] [Google Scholar]
- 7.Boden JM, Fergusson DM.. Alcohol and depression. Addiction. 2011;106(5):906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 8.Chakravorty S, Kember RL, Mazzotti DR, et al. The relationship between alcohol- and sleep-related traits: results from polygenic risk score and Mendelian randomization analyses. Drug Alcohol Depend. 2023;251:110912. doi: 10.1016/j.drugalcdep.2023.110912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inkelis SM, Hasler BP, Baker FC.. Sleep and alcohol use in women. Alcohol Res. 2020;40(2):13. doi: 10.35946/arcr.v40.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHugh RK, Weiss RD.. Alcohol use disorder and depressive disorders. Alcohol Res. 2019;40(1):40. doi: 10.35946/arcr.v40.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson H, Persson E, Perini I, et al. Acute effects of alcohol on social and personal decision making. Neuropsychopharmacology. 2022;47(4):824–831. doi: 10.1038/s41386-021-01218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puddephatt JA, Jones A, Gage SH, et al. Associations of alcohol use, mental health and socioeconomic status in England: findings from a representative population survey. Drug Alcohol Depend. 2021;219:108463. doi: 10.1016/j.drugalcdep.2020.108463. [DOI] [PubMed] [Google Scholar]
- 13.Zegel M, Lebeaut A, Healy N, et al. Mental health correlates of probable posttraumatic stress disorder, probable. Behav Modif. 2022;46(2):395–421. doi: 10.1177/01454455211033517. [DOI] [PubMed] [Google Scholar]
- 14.Lespine LF, Bramness JG, Pignon B, et al. Gender-related associations between psychiatric disorders and alcohol use. Arch Women’s Mental Health. 2022;25:895–902. doi: 10.1007/s00737-022-01253-5. [DOI] [PubMed] [Google Scholar]
- 15.Kang SJ, Pei CZ, Lee DH, et al. A pilot randomized clinical trial of biomedical link with mental health in art. PLoS One. 2023;18(5):e0284344. doi: 10.1371/journal.pone.0284344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Colrain IM.. Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology. 2020;45(1):141–165. doi: 10.1038/s41386-019-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costantini G, Epskamp S, Borsboom D, et al. State of the aRt personality research: a tutorial on network analysis of personality data in R. J Res Personal. 2015;54:13–29. doi: 10.1016/j.jrp.2014.07.003. [DOI] [Google Scholar]
- 18.Borsboom D, Robinaugh DJ, Rhemtulla MC, et al. Robustness and replicability of psychopathology networks. World Psychiatr. 2018;17(2):143–144. doi: 10.1002/wps.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott H, Jones PJ, Schmidt U.. Central symptoms predict posttreatment outcomes and clinical impairment in anorexia nervosa: a network analysis. Clin Psychol Sci. 2020;8(1):139–154. doi: 10.1177/2167702619865958. [DOI] [Google Scholar]
- 20.Papini S, Rubin M, Telch MJ, et al. Pretreatment posttraumatic stress disorder symptom network metrics predict the strength of the association between node change and network change during treatment. J Trauma Stress. 2020;33(1):64–71. doi: 10.1002/jts.22379. [DOI] [PubMed] [Google Scholar]
- 21.Jones PJ, Ma R, McNally RJ.. Bridge centrality: a network approach to understanding comorbidity. Multivariate Behav Res. 2021;56(2):353–367. doi: 10.1080/00273171.2019.1614898. [DOI] [PubMed] [Google Scholar]
- 22.McNally RJ, Mair P, Mugno BL, et al. Co-morbid obsessive-compulsive disorder and depression: a Bayesian network approach. Psychol Med. 2017;47(7):1204–1214. doi: 10.1017/S0033291716003287. [DOI] [PubMed] [Google Scholar]
- 23.Mikolajczak M, Roskam I.. A theoretical and clinical framework for parental burnout: the balance between risks and resources (BR2). Front Psychol. 2018;9:886. doi: 10.3389/fpsyg.2018.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui L, Su K, Cui Z, et al. Prevalence,demographic characteristics and function status of the schizophrenia in Hebei province. Chin J Nervous Mental Dis. 2007;33:155–158. [Google Scholar]
- 25.Dong M, Zhang J, Lu C, et al. A case-control study on the quality of life and the way of response among patients with anxiety disorder in Shandong province. Chin J Epidemiol. 2013;34:953–957. [PubMed] [Google Scholar]
- 26.Hankins M. The reliability of the twelve-item general health questionnaire (GHQ-12) under realistic assumptions. BMC Public Health. 2008;8(1):355. doi: 10.1186/1471-2458-8-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werneke U, Goldberg DP, Yalcin I, et al. The stability of the factor structure of the General Health Questionnaire. Psychol Med. 2000;30(4):823–829. doi: 10.1017/S0033291799002287. [DOI] [PubMed] [Google Scholar]
- 28.Graetz B. Multidimensional properties of the General Health Questionnaire. Soc Psychiatry Psychiatr Epidemiol. 1991;26(3):132–138. doi: 10.1007/BF00782952. [DOI] [PubMed] [Google Scholar]
- 29.Yimin L, Yongxin L.. The factor structure of the 12-item general health questionnaire: the multi-group analyses. Psychol Explor. 2015;35:355–359. [Google Scholar]
- 30.Liu X, Tang M, Hu L, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatr. 1996;02:103–107. [Google Scholar]
- 31.Xie Y. Preliminary study on the reliability and validity of the simplified coping style questionnaire. Chin J Clin Psychol. 1998;02:53–54. [Google Scholar]
- 32.Diagnostic criteria from DSM-IV . American Psychiatric Association; 1994. [Google Scholar]
- 33.Epskamp S, Fried EI.. A tutorial on regularized partial correlation networks. Psychol Methods. 2018;23(4):617–634. doi: 10.1037/met0000167. [DOI] [PubMed] [Google Scholar]
- 34.Zhao T, Liu H, Roeder K, et al. The huge package for high-dimensional undirected graph estimation in R. J Mach Learn Res. 2012;13:1059–1062. [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Fei X, Liu H, et al. Package ‘Huge.’ R Package Version. 2020:1. [Google Scholar]
- 36.Epskamp S, Cramer AOJ, Waldorp LJ, et al. qgraph: network Visualizations of Relationships in Psychometric Data. J Stat Soft. 2012;48(4):1–18. doi: 10.18637/jss.v048.i04. [DOI] [Google Scholar]
- 37.Epskamp S, Borsboom D, Fried EI.. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50(1):195–212. doi: 10.3758/s13428-017-0862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarov A, Suarez-Jimenez B, Levi O, et al. Symptom structure of PTSD and co-morbid depressive symptoms – a network analysis of combat veteran patients. Psychol Med. 2020;50(13):2154–2170. doi: 10.1017/S0033291719002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinaugh DJ, Millner AJ, McNally RJ.. Identifying highly influential nodes in the complicated grief network. J Abnorm Psychol. 2016;125(6):747–757. doi: 10.1037/abn0000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briganti G, Scutari M, McNally RJ.. A tutorial on Bayesian networks for psychopathology researchers. Psychol Methods. 2023;28(4):947–961. doi: 10.1037/met0000479. [DOI] [PubMed] [Google Scholar]
- 41.Haber NA, Wood ME, Wieten S, et al. DAG with omitted objects displayed (DAGWOOD): a framework for revealing causal assumptions in DAGs. Ann Epidemiol. 2022;68:64–71. doi: 10.1016/j.annepidem.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Scutari M, Nagarajan R.. Identifying significant edges in graphical models of molecular networks. Artif Intell Med. 2013;57(3):207–217. doi: 10.1016/j.artmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong L, Xie Y, Zou X.. Association between sleep duration and depression in US adults: a cross-sectional study. J Affect Disord. 2022;296:183–188. doi: 10.1016/j.jad.2021.09.075. [DOI] [PubMed] [Google Scholar]
- 44.Lan W, Ling S, Ran W, et al. Investigation on alcohol abuse or dependence of male workers in Gaocheng District of Shijiazhuang city of Hebei province. Chin J Nervous Mental Dis. 2021;47:141–148. [Google Scholar]
- 45.Stalder T, Hucklebridge F, Evans P, et al. Use of a single case study design to examine state variation in the cortisol awakening response: relationship with time of awakening. Psychoneuroendocrinology. 2009;34(4):607–614. doi: 10.1016/j.psyneuen.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Sharma R, Parikh M, Mishra V, et al. Sleep, sleep homeostasis and arousal disturbances in alcoholism. Brain Res Bull. 2022;182:30–43. doi: 10.1016/j.brainresbull.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Waldrop AE, Back SE, Sensenig A, et al. Sleep disturbances associated with posttraumatic stress disorder and alcohol dependence. Addict Behav. 2008;33(2):328–335. doi: 10.1016/j.addbeh.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christoph A. Associations of subjective sleep quality with depression score, anxiety, physical symptoms and sleep onset latency in young students. Central Eur J Public Health. 2011;19:115–117. doi: 10.21101/cejph.a3647. [DOI] [PubMed] [Google Scholar]
- 49.Morrone E, Lupo ND, Trentin R, et al. Microsleep as a marker of sleepiness in obstructive sleep apnea patients. J Sleep Res. 2020;29(2):e12882. doi: 10.1111/jsr.12882. [DOI] [PubMed] [Google Scholar]
- 50.Popovici I, French MT.. Binge drinking and sleep problems among young adults. Drug Alcohol Depend. 2013;132(1-2):207–215. doi: 10.1016/j.drugalcdep.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thakkar MM, Sharma R, Sahota P.. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. doi: 10.1016/j.alcohol.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Reen E, Rupp TL, Acebo C, et al. Biphasic effects of alcohol as a function of circadian phase. Sleep. 2013;36(1):137–145. doi: 10.5665/sleep.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lydon DM, Ram N, Conroy DE, et al. The within-person association between alcohol use and sleep duration and quality in situ: an experience sampling study. Addict Behav. 2016;61:68–73. doi: 10.1016/j.addbeh.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCullar KS, Barker DH, McGeary JE, et al. Altered sleep architecture following consecutive nights of presleep alcohol. Sleep. 2024;47(4):zsae003. doi: 10.1093/sleep/zsae003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyata S, Noda A, Ito N, et al. REM sleep is impaired by a small amount of alcohol in young women sensitive to alcohol. Intern Med. 2004;43(8):679–684. doi: 10.2169/internalmedicine.43.679. [DOI] [PubMed] [Google Scholar]
- 56.Ribadier A, Varescon I.. Anxiety and depression in alcohol use disorder individuals: the role of personality and coping strategies. Subst Use Misuse. 2019;54(9):1475–1484. doi: 10.1080/10826084.2019.1586950. [DOI] [PubMed] [Google Scholar]
- 57.Cheng B, Coates JM, Gullo MJ, et al. Relationship between alcohol craving dimensions and features of comorbid mental health in an alcohol dependent sample. Addict Behav. 2022;124:107106. doi: 10.1016/j.addbeh.2021.107106. [DOI] [PubMed] [Google Scholar]
- 58.Laniepce A, Cabé N, André C, et al. The effect of alcohol withdrawal syndrome severity on sleep, brain and cognition. Brain Commun. 2020;2(2):fcaa123. doi: 10.1093/braincomms/fcaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickett SM, Barbaro N, Mello D.. The relationship between subjective sleep disturbance, sleep quality, and emotion regulation difficulties in a sample of college students reporting trauma exposure. Psychol Trauma. 2016;8(1):25–33. doi: 10.1037/tra0000064. [DOI] [PubMed] [Google Scholar]
- 60.Fisher RS, Dattilo TM, Sharkey CM, et al. Sleep patterns related to emotion dysregulation among adolescents and young adults. J Pediatr Psychol. 2022;47(1):111–120. doi: 10.1093/jpepsy/jsab084. [DOI] [PubMed] [Google Scholar]
- 61.Gémes K, Forsell Y, Janszky I, et al. Moderate alcohol consumption and depression – a longitudinal population-based study in Sweden. Acta Psychiatr Scand. 2019;139(6):526–535. doi: 10.1111/acps.13034. [DOI] [PubMed] [Google Scholar]
- 62.Haynie DL, Lewin D, Luk JW, et al. Beyond sleep duration: bidirectional associations among chronotype, social jetlag, and drinking behaviors in a longitudinal sample of US high school students. Sleep. 2018;41(2):zsx202. doi: 10.1093/sleep/zsx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson DA, Sullivan RM, Smiley JF, et al. Developmental alcohol exposure is exhausting: sleep and the enduring consequences of alcohol exposure during development. Neurosci Biobehav Rev. 2024;158:105567. doi: 10.1016/j.neubiorev.2024.105567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.