Abstract
Introduction
Medication errors occur at any point of the medication management process and are a major cause of death and harm globally. The perioperative environment introduces challenges in identifying medication errors due to the frequent use of time-sensitive, high-alert medications in a dynamic and intricate setting. Pharmacists could potentially reduce the occurrence of these errors because of their training and expertise.
Aim
To provide the most up-to-date evidence on the roles and effects of pharmacist interventions on medication errors in perioperative settings.
Methods
PubMed, CINAHL, and Embase were searched from inception to September 2023. Studies were included if they tested a pharmacist-led intervention aimed at reducing medication errors in adult perioperative settings. The included studies were assessed for quality using the Crowe Critical Appraisal Tool. Data were extracted and synthesized using the DEPICT-2 (Descriptive Elements of Pharmacist Intervention Characterization Tool). Screening, quality assessment, and data extraction were performed by two independent researchers.
Results
Sixteen studies were eligible. All included studies incorporated multicomponent interventions, primarily medication reconciliation (n = 13), medicine-related recommendations (n = 12), staff education (n = 6), and patient counselling (n = 4). The development of implemented interventions was poorly reported across all papers. A diverse range of error reporting was observed, and none of the included studies provided definitions or basis for the categorization of errors. Although the studies showed that pharmacist interventions were associated with a reduction in overall medication errors rates, some studies showed inconsistent findings regarding error subtypes. The most common pharmacist intervention was medication optimization via holding or switching between agents.
Conclusion
While there is some evidence of positive impact of the pharmacist-led interventions on medication errors in perioperative setting, this evidence is generally of low quality and insufficient volume. Heterogeneity in study design, definitions, and case detection is common; hence, high-quality research that applies more stringent controls and uses clearer definitions is warranted.
Systematic review registration
PROSPERO CRD42023460812.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02710-1.
Key points
• There is some evidence for the outcomes of pharmacists’ intervention in perioperative settings, but this is generally of low quality and insufficient volume.
• Complex and multicomponent pharmacist interventions that span the whole perioperative journey are more likely to yield positive effects.
• There is lack of data on the development of the pharmacist-led interventions in terms of structure and processes, which might hinder the reproducibility of these interventions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02710-1.
Introduction
Medication errors are common events occurring throughout the spectrum of the medication utilization process [1]. According to the National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) in the USA, a medication error is “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer” [2]. Medication errors are one of the leading causes of harm to patients in hospitals. They have the potential to cause adverse outcomes such as temporary harm, permanent harm, prolonged hospitalization, and potential fatalities [3]. Annually in the USA, medication errors contribute to 7000 to 9000 fatalities and adversely impact hundreds of thousands of patients, resulting in unreported complications [4]. The economic burden of caring for individuals affected by these errors surpasses US $40 billion, affecting over 7 million patients [4]. Beyond the financial costs, patients endure psychological and physical distress due to medication errors. Moreover, these errors lead to diminished patient satisfaction and a declining trust in the healthcare system [5, 6].
Medication errors are prevalent both within and outside the perioperative context, presenting considerable difficulty in their detection within this specific setting [7]. The perioperative environment introduces a noteworthy challenge in identifying medication errors due to the frequent use of time-sensitive, high-alert medications in a dynamic, intricate, and stressful setting [8, 9]. Additionally, at various stages of the medication utilization process in the surgical setting, surgeons assume responsibility for tasks such as medication selection, preparation, administration, documentation, and, when required, subsequent monitoring. This process can occasionally bypass the involvement of pharmacists and other safety checkpoints that typically serve to minimize errors in settings such as medical facilities [10]. Several methods have been suggested to help reduce errors in perioperative settings such as the adoption of pre-filled syringes and pre-mixed infusions by pharmacy services, the implementation of barcode-assisted medication administration, the incorporation of audiovisual feedback systems, and the implementation of ward-based pharmacist [10–12].
The role of the pharmacist is in a constant state of expansion; pharmacists play a variety of roles aimed at improving patient care and creating a safe healthcare environment. The roles of a clinical pharmacist include providing patient review, patient counselling, medication reconciliation, and clinical decision-making [13, 14]. Clinical pharmacists provide a distinctive viewpoint on the interdisciplinary dynamics of perioperative teams and have a collaborative role within the surgical teams. They also have the capacity to methodically review patients’ medications and analyze their utilization throughout all phases of perioperative care [11, 15]. The role of clinical pharmacists in surgical units is relatively novel compared to other practice domains, such as medical wards. Although some systematic reviews and meta-analyses have demonstrated the positive impact of clinical pharmacist interventions in surgical settings—improving outcomes like chronic condition management, antimicrobial use, surgical site infection rates, length of stay, and readmission rates [16–18]—the influence of clinical pharmacy services on medication errors in perioperative settings remains inadequately evaluated [19].
This systematic review aims to provide the most up-to-date evidence on the roles and effect of pharmacist interventions on medication errors in perioperative settings. The review will help policymakers and clinicians to design effective pharmacist interventions to mitigate medication errors to improve overall healthcare outcomes in perioperative settings.
Methods
A systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Supplementary material S1) [20]. The review protocol was registered with PROSPERO (CRD4202346081).
Types of studies and eligibility criteria
Studies were included if they met the following criteria: (1) randomized controlled trials (RCTs), quasi-experimental, pre-post, prospective, and retrospective cohort, (2) evaluated a clinical pharmacist-led intervention, (3) conducted in perioperative settings, (4) had a control or comparison group (with healthcare professionals other than pharmacists), (5) reported the rate (or number) of overall medication errors or any of its subtypes, and (6) published in a peer-reviewed journal in English or Arabic language and available in full text. Case reports, expert opinions, systematic reviews, letters to editors, commentaries, correspondences, news articles, and qualitative studies were excluded from this review, as were conference abstracts if they were not available in full text. We also excluded studies focusing on pediatric patients.
For the purpose of this study, we adopted the NCCMERP definition of medication errors [2]. We also captured the definitions of medication errors used by individual studies.
Search strategy
A systematic literature search was performed on the following databases from index inception until September 2023: PubMed, Embase, and CINAHL. A search strategy was devised following discussion within the research team to yield relevant studies. The search strategy was kept deliberately broad to capture all outcomes of pharmacist-led interventions, which are medication errors, clinically important outcomes, antimicrobial stewardship, and management of chronic diseases. This review focuses merely on medication errors, and other outcomes are discussed elsewhere [16–18]. Keywords and medical subject headings used in the search comprised two categories: pharmacy, with keywords ‘Pharmacist [MeSH], ‘pharmacy’, ‘medication therapy management’, ‘pharmaceutical care’, and ‘medication counselling’ and peri-operative, with keywords ‘perioperative period [MeSH]’, ‘perioperative care [MeSH]’, ‘surgery’, and ‘procedure. Keywords in each category were searched using the operator OR and then combined between categories using the operator AND. We used Google Scholar as an additional citation tracking resource to search for any further studies not identified from the systematic search. A manual search of eligible articles’ reference lists was conducted to include relevant articles that were not identified through the database search. The full search strategies for each database are included in Supplementary material S2.
Study selection
Rayyan (Qatar Computing Research Institute), an online platform, was used for duplicate removal, independent screening of articles at the title and abstract stage, and subsequently at the full text stage [21]. Two authors (L. N., S. K.) reviewed titles and abstracts independently. Full texts of papers were subsequently examined independently by two authors (L. N. and S. K. or B. A. and M. A.) to determine if studies were eligible for inclusion in the review. Any uncertainty or disagreement about articles meeting the inclusion criteria was resolved after discussion among all authors to reach consensus.
Data extraction
A bespoke data extraction tool was developed based on the DEPICT-2 (Descriptive Elements of Pharmacist Intervention Characterization Tool) [22]. DEPICT-2 is a validated instrument for accurately describing and characterizing the details of pharmacist interventions. The tool consists of 93 items, subsumed into 11 domains: contact with recipient, setting, target population, clinical data sources, variables assessed, pharmacist intervention, timing of intervention, material that support intervention, repetition, communication with recipient, and changes in therapy and laboratory tests [22]. The final data extraction sheet included the following components:
General information: Author(s), year, country, study design, objectives, population, sample size, study duration, and surgical unit(s)
Description of intervention: Recipients, focus of intervention, setting, method of communication, clinical data source, pharmacist action, timing and frequency of action, and materials that support action
Key findings: The rate (or number) of medication errors or any of its subtypes before and after the intervention, types of errors, number of interventions, severity of errors, implicated medications, and acceptance rate.
The data extraction tool was piloted and agreed upon by the team prior to its use. An independent, duplicate data extraction of each study was undertaken (L. N., S. K., M. A., or B. A.).
Risk-of-bias assessment
The study team independently worked in pairs (L. N., S. K., M. A., B. A.) to assess the quality of selected articles using the validated Crowe Critical Appraisal Tool (CCAT) version 1.4 [23]. CCAT contains 8 categories applicable to all study designs, with the highest possible score being 40. The tool facilitates the recording of scores for each category so that the final score is not influenced by an overall opinion about the study [24]. The quality of studies was categorized as follows: high quality (36 and above), moderate quality [25–30], and low quality (29 and below). This was based on a consensus reached by the reviewers to group studies by quartiles, which was a similar approach adopted by Donnelly et al. and El-Awaisi et al. [31, 32]. The author of the CCAT tool was also contacted to ensure that this method of interpretation was valid.
Data analysis
Data synthesis was conducted qualitatively by grouping results into meaningful clusters. The DEPICT-2 tool was used to categorize the data for the description of pharmacist interventions, while meaningful clusters for the outcomes of these interventions were identified by recognizing common recurring events. Descriptive statistics including frequency and percentages were used to analyze the data.
Although meta-analysis was planned, it was deemed inappropriate due to the high levels of clinical and methodological heterogeneity. Heterogeneity was found in measures and definitions used for presenting the results (such as the denominator and numerator), as well as the surgical department of interest, demographic data, and components of the interventions.
Results
Identification and study selection
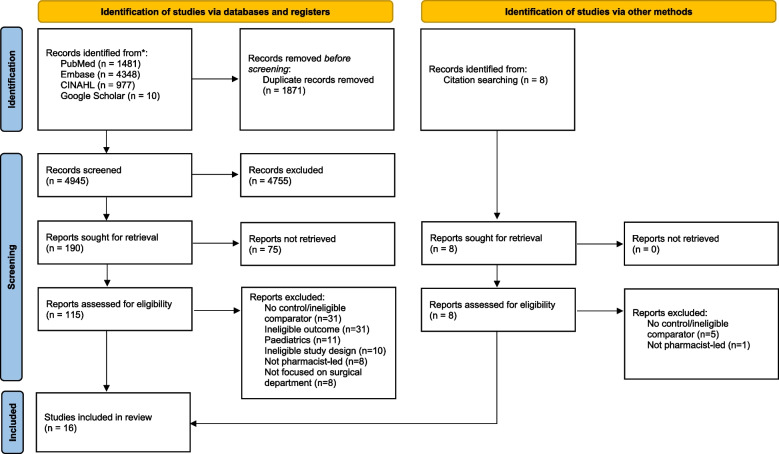
A total of 6816 records were identified from electronic databases and 8 records from the reference lists of retrieved articles. After removal of duplicates, 4945 records remained for title and abstract screening, resulting in the inclusion of 16 studies in the final analysis. It is worth noting that the most common reasons for exclusion were ineligible comparator and ineligible outcome (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram
Characteristics of included studies
The characteristics of included studies are presented in Table 1. Most studies were randomized interventional studies (n = 6) [25–27, 33–35], followed by before-and-after studies (n = 5) [28–30, 36, 37] and observational studies (n = 5) [38–42]. The studies were conducted in diverse parts of the world, including Asian countries [30, 33, 37, 39, 42], European countries [25, 26, 28, 29, 38], Americas [35, 36, 40, 41], and Australia [27, 34]. The publication dates spanned from 2007 all the way to 2023 with a total of 6325 included patients. Furthermore, surgical services varied among the studies comprising gastrointestinal/hepatobiliary surgical wards (n = 3) [30, 37, 38], bariatrics (n = 3) [36, 40, 41], orthopedic (n = 2) [28, 29], transplant (n = 1) [42], and other surgical wards (n = 6) [25–27, 33, 35, 39, 42], and only one study did not report the specific setting [34]. The duration of the studies was inconsistent, and in some studies, it was not reported, with a minimum duration of 1.5 months and a maximum duration of 24 months.
Table 1.
Characteristics of included studies
| Author (year) | Country | Objective | Study design | Sample size | Study duration | Surgical unit | Patient characteristics |
|---|---|---|---|---|---|---|---|
| AbuRuz et al. [33] | Jordan | Explore the value of a pharmaceutical care service in general surgery to identify and reduce DRPs compared with standard care | Unblinded randomized controlled trial |
123 patients ▪ 60 controls ▪ 63 interventions |
NR | Multiple | Patients who were hospitalized with an expected duration of stay > than 2 days |
| Bansal et al. [38] | UK | Assess implementation of an Enhanced Surgical Medicines Optimization Service (ESMOS) and evaluate its impact on postoperative care outcomes at 12 months after its implementation | Retrospective observational study | 246 patients | 12 months | General (hepato‐pancreato‐biliary (HPB), upper gastrointestinal (GI), lower GI, and vascular) | Patients who underwent major elective general surgical procedures with an American Society of Anesthesiologists (ASA) score for physiological status of ≥ 2 (i.e., a patient with a mild systemic disease) |
| Chen et al. [39] | China | Evaluate the role of the clinical pharmacist in the rational use of proton pump inhibitors (PPIs) in a general surgery department | Prospective observation |
1074 patients ▪ 536 controls ▪ 538 interventions |
6 months | Multiple | Patients who were treated by the hepatobiliary and pancreatic, gastrointestinal, and thyroid and breast surgery groups |
| Falconer et al. [40] | USA | Evaluate the feasibility and effectiveness of a pharmacy-led initiative to facilitate discharge medicine reconciliation after bariatric surgery | Retrospective observational study |
353 patients ▪ 158 pre ▪ 195 post |
NR | Bariatric | Patients aged 18 years or older who underwent primary or revisional laparoscopic or robotic weight loss surgery |
| Fitzpatrick et al. [28] | UK | Investigate the significance of pharmacist input, the impact on reducing prescribing errors, postoperative patient outcomes, and patient and staff satisfaction with the service | Retrospective pre-/post-intervention implementation |
209 patients ▪ 80 pre ▪ 129 post |
20 weeks | Orthopedic | Patients undergoing arthroplasty (THR/TKR/UKR) |
| Hale et al. [34] | Australia | Evaluate a new model of service for the Australia healthcare system, of inpatient medication prescribing by a pharmacist in an elective surgery preadmission clinic (PAC) against usual care | RCT |
384 patients ▪ 190 controls 194 interventions |
3 months | NR | Patients undergoing elective surgery |
| Han et al. [41] | USA | Evaluate the impact of a bariatric clinic-based pharmacist on inpatient length of stay, medication errors, and patient experience | Retrospective observational study |
135 patients ▪ 67 controls ▪ 68 interventions |
NR | Bariatric | Patients that were admitted for primary bariatric surgery |
| Kwan et al. [35] | Canada | Evaluate structured pharmacist medication history interviews with assessments in a surgical preadmission clinic and use of a postoperative medication order form reduces number of patients with ≥ 1 postoperative medication discrepancy related to home medications | RCT |
416 patients ▪ 214 controls ▪ 202 interventions |
1.5 months | Multiple | Patients who had a surgical preadmission clinic visit before undergoing surgical procedures |
| Léguillon et al. [29] | France | Assess if clinical pharmacist (CP) intervention in a geriatric perioperative care unit decreases the number of potentially inappropriate prescriptions compared to the comprehensive medication reconciliation performed by ortho-geriatric teams without a CP intervention | Before-after-control-impact study |
209 patients ▪ 150 controls ▪ 59 interventions |
8 months | Orthopedic | Patients 75 and above admitted under geriatric perioperative care units with hip fracture |
| Luo et al. [30] | China | Evaluate the impact and cost–benefit of clinical pharmacist interventions on inappropriate use of prophylactic acid suppressant | Retro-prospective intervention study |
448 patients ▪ 218 pre ▪ 230 post |
3 months | Hepatobiliary | Patients undergoing elective hepatobiliary surgery and have no systemic diseases |
| Marotti et al. [25] | UK | Determine if the number of missed doses of regular medication was significantly different between usual care (control), preoperative pharmacist medication history only, and preoperative pharmacist medication history and supplementary prescribing surgery day | RCT |
357 patients ▪ 118 usual cares ▪ 119 pharmacist medication history 120 history and prescribing |
5 months | Multiple | NR |
| Nguyen et al. [27] | Australia | Evaluate the impact of a PeRiopErative and Prescribing (PREP) pharmacist on postoperative medication management | Randomized prospective interventional study |
104 patients ▪ 51 controls ▪ 53 interventions |
3 months | Multiple (general, vascular, orthopedic, gynecological, and urology) | Patients for elective surgery at high risk for medication misadventure |
| SUREPILL Study Group [26] | Netherlands | Evaluate ward-based pharmacy interventions to reduce medication-related harm in surgical patients | RCT |
1094 patients ▪ 547 controls ▪ 547 interventions |
2 years | Multiple | Patients admitted for elective surgery with expected hospital stay longer than 48 h |
| Van Prooyen et al. [36] | USA | Evaluate the impact of an inpatient pharmacy consult on discharge medication doses, classes, and formulations prescribed for patients after bariatric surgery | Retrospective-prospective intervention study |
252 patients ▪ 167 controls ▪ 85 interventions |
6 months | Bariatric | Patients 18 years or older and admitted to the hospital for weight loss bariatric surgery with either the SG or RYGB procedures |
| Yang et al. [42] | China | Assess the impact of pharmacist led post‐transplant medication management for kidney transplant recipients | Retrospective cohort study |
204 patients ▪ 84 pre ▪ 120 post |
2 years | Transplant | Patients receiving living‐ donor or deceased‐donor kidney transplants |
| Zhang et al. [37] | China | Evaluate the clinical effects of a clinical pharmacist intervention on inappropriate PPI prescriptions in a tertiary general hospital hepatobiliary surgery ward | Retrospective pre-/postintervention study |
717 patients ▪ 420 pre ▪ 297 post |
6 months | Hepatobiliary | Patients receiving PPI |
Risk-of-bias assessment
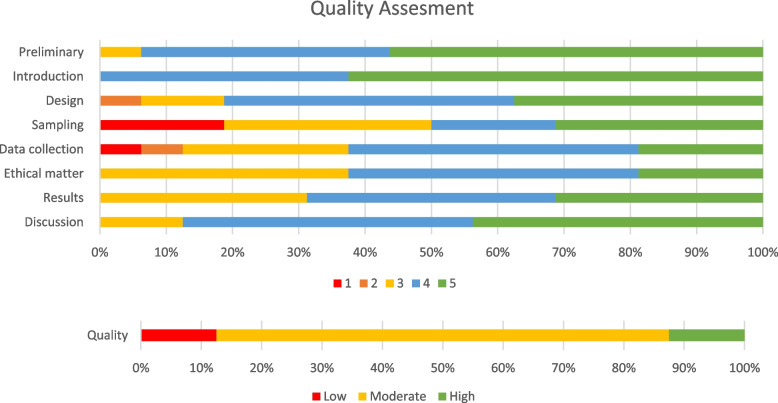
Total scores ranged between 25 and 38, with a mean score of 32.4. Twelve studies were adjudicated to have moderate overall quality on the CCAT assessment tool [25, 28–30, 33–38, 40, 42], whereas two studies each were of high quality [26, 39] and low quality [27, 41]. Significant weaknesses affecting the quality of included studies pertained to the study designs, sampling methods, and data collection practices (Fig. 2).
Fig. 2.
Risk-of-bias graph
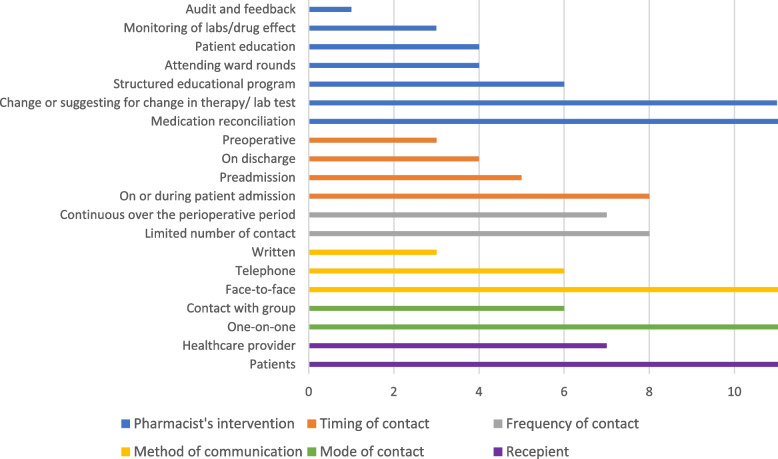
Characteristics of pharmacist interventions
Table 2 and Fig. 3 detail the characteristics of the pharmacist-led interventions across the included studies. All included studies incorporated multicomponent interventions. The most frequently identified intervention was history taking and medication reconciliation [25–29, 33–36, 38, 40–42], followed by 12 studies that clearly identified therapeutic change recommendations or suggestions [25, 26, 28, 30, 34, 35, 37–42]. Education and counselling interventions directed toward patients were described by four records [26, 36, 40, 42], while six records focused on educational activities provided to other healthcare professionals [30, 33, 37, 39, 41, 42].
Table 2.
Description of pharmacist interventions
| Author (year) | Pharmacist intervention | Focus of the intervention | Recipients | Mode of contact | Methods of communication | Settings | Pharmacy contact (frequency) | Data source | Source guide for intervention | Supporting materials |
|---|---|---|---|---|---|---|---|---|---|---|
| AbuRuz et al. [33] |
▪ Attending and discussing during medical rounds ▪ Follow-up of patient daily to resolve or prevent DRPs ▪ Discharge medication counselling |
N/A | Patients | Contact with group (medical staff and patients) | ▪ Face to face | ▪ Hospital bedside | On or during patient admission, on discharge (continuous) | EMR | Up-to-date guidelines | None |
| Bansal et al. [38] |
ESMOS services ▪ Patients are reviewed in a virtual pharmacist clinic whereby patients’ pre‐existing medical comorbidities are recorded along with any high‐risk medication the patient is taking ▪ In the postoperative phase, close monitoring on the ward with the focus being on medicines optimization to minimize the incidence of any postoperative complications occurring ▪ Collaboratively work in multidisciplinary teams |
N/A | Patients | One on one |
▪ Preadmission telephone ▪ Postadmission face to face |
▪ Preadmission recipient home ▪ Postadmission Hospital bedside |
One time before admission and throughout the admission (continuous) | EMR | Always events toolkit by the NHS | None |
| Chen et al. [39] |
▪ Quarterly lecture on rational use of PPIs to medical staff ▪ Rounds attendance and medication recommendations ▪ Interception of irrational PPI use ▪ Daily prescription audits and communicated with doctors and provides feedback ▪ Enlisting all essential monitoring drugs and checked medical records every month ▪ Monitoring of PPI-related ADR |
PPI | Patients, medical staff | One on one (patient), contact with group (surgical team) | ▪ Face to face, written | ▪ Hospital bedside | On or during patient admission (continuous) | EMR | The PPIs Review Guidelines of the Second Affiliated Hospital of Fujian Medical University | None |
| Falconer et al. [40] |
▪ Patient identification by surgery team ▪ Consultation to pharmacy (medication history documentation, documentation of patient preferred pharmacy, reconciliation of home and inpatient medications for hospital admission, recommend changes for medications as indicated, and provide patient education regarding proposed changes) ▪ Assigned clinical pharmacist to perform face-to-face inpatient consultation ▪ Standardized documentation and communication of recommendations with surgery team |
N/A | Patients | Contact with group (medical staff and patients) | ▪ Face to face | ▪ Hospital bedside | On or during patient admission, on discharge (continuous) | EMR | Primary literature review, institutional expert opinion, drug information from databases | None |
| Fitzpatrick et al. [28] |
▪ Review of electronic notes ▪ Phone call with patients to confirm history, demographics, answer patient’s questions, and involve patient in shared decision-making ▪ Discussion with the surgical team to highlight or resolve perioperative medical issues ▪ Individualized discharge prescription written, emailed, dispensed, and supplied to wards before patient admission |
VTE prophylaxis, NSIADs, QTc prolonging medications | Patients | One on one (patient), contact with group (surgical team) | ▪ Face to face, telephone, written | ▪ Recipient home | 1–2 weeks before admission, 7–10 days post discharge (twice) | NHS (VPN), (ARISE) dataset in Scotland | Evidence-based guideline produced in collaboration with surgical MDT team, health board guidance for VTE risk assessment and procedures | None |
| Hale et al. [34] | ▪ Usual pharmacy care in addition to prescribing (continuing, discontinuing, and initiating medications with co-signature of physician | N/A | Patients and physicians | One on one | Face to face | Hospital (bedside and outpatient clinic) | Pre-operative (once) | Medication chart | Clinical guidelines and hospital VTE prophylaxis guidelines | None |
| Han et al. [41] |
▪ A clinical pharmacist integration into the bariatric surgery clinic (as part of every patient’s preoperative clinic evaluation) ▪ A onetime 30- to 60-min meeting with the pharmacist prior to meeting with the surgeon ▪ Obtained medication histories and provided recommendations to patients and the team on perioperative medication management ▪ Resolved any potential DRP (e.g., medication absorption after bariatric surgery) ▪ Provided medication education to the patient |
N/A | Patients | One on one | Face to face | Hospital (clinic) | Before admission (only once) | NR | NR | None |
| Kwan et al. [35] |
Surgical Pharmacist in Preadmission Clinic Evaluation (SPPACE) ▪ Conducted a standardized comprehensive medication history interview and assessment focusing on the patient’s current home medication regimen in the preadmission clinic ▪ Description of any issue was written in the medical record to be considered by the surgeon ▪ Conducted telephone interviews with patients they were unable to see in the clinic ▪ After postoperative admission, verified with the patient if any medication changes had been made since the clinic assessment |
N/A | Patients | One on one | Face to face or telephone if patient did not attend the clinic | Hospital (clinic), recipient home (by telephone) if patient did not attend the clinic | Before admission and postoperative admission, if possible (once or twice) | Patient history taking, if needed the pharmacist contacted community pharmacy or family physician | Not reported | Preprinted postoperative medication order form, EMR |
| Léguillon et al. [29] |
▪ Medication’s review (within 72 h of admission) ▪ Pharmaceutical synthesis (summarized pharmaceutical plan with proposals) ▪ Dedicated meetings with geriatricians |
N/A | Patients, geriatrician | One on one (patient, geriatrician) | Face to face | Hospital bedside | On or during patient admission (continuous) | EMR | STOPP/START, Medication appropriateness Index, French guideline | None |
| Luo et al. [30] |
▪ Provide educational sessions and handouts about SUP for medical teams ▪ Collect information about the patient from the EMR and HIS ▪ Judge appropriateness of prophylactic acid suppressant use on: indication, selection, dose, duration of prophylaxis, combination, and replacement depending on the criteria ▪ Communicates immediately with the prescriber with their recommendation if any ▪ Report to the hospital administration every week |
Omeprazole, lansoprazole | Physicians | One on one | Face to face | Hospital | On or during patient admission (as needed) | EMR | Hospital protocol | None |
| Marotti et al. [25] | ▪ Document medication history or document medication history and prescribe | Beta-blockers, statins, antiplatelets, anticoagulant | Patients | One on one | EMR, telephone, fax | Hospital | Pre-operative (once) | EMR | Hospital protocol | None |
| Nguyen et al. [27] |
▪ PREP pharmacist contacted patients via telephone 1 week prior to surgery to obtain BPMH and reconcile medications ▪ After surgery, a surgical pharmacist was provided with a handover from the PREP pharmacist for continuation of care ▪ The surgical pharmacist would verify the BPMH ▪ Confirmed MRF is generated by the pharmacist ▪ At discharge, the PREP pharmacist prepared discharge prescriptions for the patients, which is then checked and signed by the doctor |
OTC products, cardiovascular medications, and analgesics | Patients | One on one | Telephone |
▪ First contact: recipient home ▪ Second and third contact: hospital bedside |
Before admission by the PREP pharmacist, on admission, and before discharge by the surgical pharmacist (three times) | NR | NR | Medication reconciliation form, EMR, handover form |
| SUREPILL Study Group [26] |
▪ Medication reconciliation ▪ Consultation with the patient using a standard questionnaire ▪ Reviewing medication chart and optimizing medications when needed ▪ Performing interventions with liaison with the physician ▪ Weekly patient meetings (when possible) ▪ Reviewing discharge medications ▪ Patient counselling about the medications |
N/A | Patients and physicians | One on one | Face to face | ▪ Hospital | On or during patient admission, on discharge (continuous) | EMR & patient history taking | Hospital protocol | None |
| Van Prooyen et al. [36] |
▪ Completion and documentation of a medication history ▪ Review of the home medication list for needed postsurgical medication changes ▪ Creation of a discharge medication plan based on a defined protocol ▪ Documentation of the recommended discharge medication plan in a consult note ▪ Patient education outlining the discharge medication plan |
Extended release or other noncrushable formulations, NSAIDs, loop diuretics | Patients, surgeons | One on one (patient, Doctor) | Face to face, written, telephone | Hospital bedside | Post-operative day 1 (once, patient and as needed for physicians) | EMR | Institutional protocol based on guideline recommendation, primary literature review, drug information databases, and the team’s expertise | None |
| Yang et al. [42] |
▪ Direct patient care and medication management during hospitalization ▪ Reviewing medication regimens, resolving DRPs and medication reconciliation ▪ Answering drug information questions ▪ Therapeutic drug monitoring (TDM) ▪ Making therapeutic recommendations ▪ Patient education |
N/A | Patients | One on one (patient), contact with group (medical team) | Face to face | Hospital bedside | On or during patient admission (continuous) | Not reported | Not reported | None |
| Zhang et al. [37] |
▪ Participation in daily medical rounds and clinical duties ▪ Targeted educational interventions for medical staff |
PPI | Patients, medical staff | Contact with group (medical staff and patients) | Face to face | Hospital bedside | On or during patient admission (continuous) | EMR | Martindale: The Complete Drug Reference (39th), New Materia Medica, drug instructions, American Society of Health-System Pharmacists criteria and Expert consensus on the application of PPIs | None |
Fig. 3.
Summary of pharmacist interventions characteristics according to DEPICT 2 tool
Outcomes related to the impact of pharmacist interventions on overall medication errors
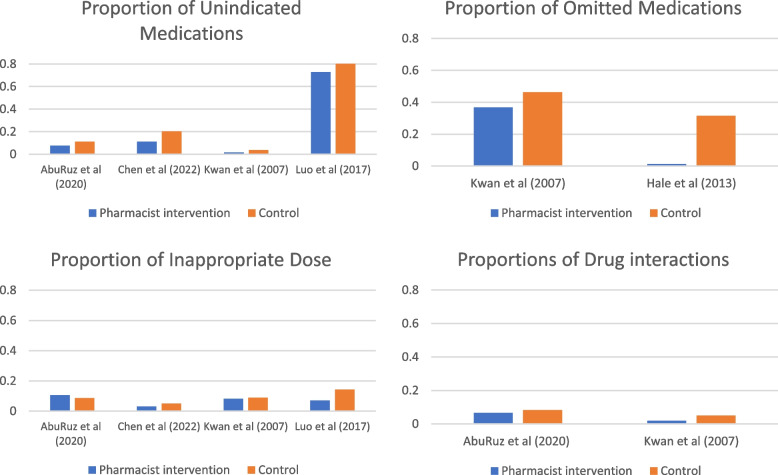
Significant heterogeneity was noted in medication errors reporting across studies. While some studies reported proportions of overall medications errors across different levels of care, others focused on errors occurring at specific times in the perioperative journey (such as inpatient or at discharge). Additionally, some studies focused on specific classes of medication or types of errors (e.g., medication discrepancies) (Table 3, Fig. 4).
Table 3.
Description of outcomes of pharmacist interventions
| Author (year) | Medication error | Severity of errors | Type of intervention(s) | Number of intervention (s) | Acceptance rate | Follow-up duration |
|---|---|---|---|---|---|---|
| AbuRuz et al. [33] |
▪ Total DRPs: 1062 (517 control, 545 intervention) ▪ Reduction in DRPs in the intervention group at discharge was significantly higher than in the control group [MD − 2.63 (− 1.3 to − 3.94); P < 0.0001] |
NR |
▪ Unnecessary drug therapy (11.2% vs 7.7%) ▪ Untreated condition (10.8% vs 8.1%), need for additional/combination therapy (7.2% vs 10.3%), and need of more effective drug (6.9% vs 6.1%) ▪ Efficacy-related issues (16.6% vs 21.3%), efficacy drug interaction issue (1% vs 0.9%), and safety drug interaction issue (7.2% vs 5.7%) ▪ Low (1.5% vs 4.0%) or high dose (7.2% vs 6.6%) ▪ Safety-related issues (20.5% vs 20.0%), drug is contraindicated (2.1% vs 1.5%) ▪ The patient is at high risk for developing adverse drug reaction and requires prophylaxis or intervention (3.5% vs 5.3%) or allergic reaction or undesirable effect (0.6% vs 0.9%) ▪ Inappropriate knowledge about medications or diseases (14.7% vs 13.21%) ▪ Inappropriate medicine adherence (8.1% vs 7.52%) ▪ Need for additional/more frequent drug monitoring (20.7% vs 19.45%) |
NR | 90% | 6 months |
| Bansal et al. [38] | None | NR |
▪ Medicines optimization: 186 ▪ Peri‐operative drug management: 234 |
420 | NR | 12 months |
| Chen et al. [39] | ▪ Irrational use of PPI (64.11% vs 31.55%; P < 0.001) | NR |
▪ Medication unindicated: 20.2% vs 11.2%, P = 0.018 ▪ Composition ratios of unindicated drugs (63 vs 26), unsuitable drug selection (71 vs 8), and unsuitable drug formulation (37 vs 12): P < 0.05 ▪ Repeated administration (6 vs 2), inappropriate usage and dosage (27 vs 17), and incompatibility (0 vs 0) were nonsignificant: P > 0.05 |
NR | Not reported | Not reported |
| Falconer et al. [40] | NR | NR |
▪ Number of new or changed medications (3.7 ± 1.2 vs 4.2 ± 1.8; P = 0.003) ▪ Number of stopped medications (1.2 ± 1.5 vs 1.5 ± 1.9; P = 0.09) ▪ Antihypertensive medications were decreased or stopped (44.7% vs 85.4%; P < 0.001) ▪ Antidiabetic medications were decreased or stopped (65.9% vs. 66.7%; P = 0.43) ▪ Discrepancies among the final medication list (28.5% vs 20.0%; P = 0.59) |
NR | 77% | 30 days |
| Fitzpatrick et al. [28] | ▪ Prescriptions with ≥ 1 prescribing difference: 38.8% patient | NR |
Pre-intervention group ▪ Postoperative thromboprophylaxis prescribed: 22.5% of patients (18.75% of patients were given aspirin as thromboprophylaxis despite (BMI) > 30 (classified as at increased VTE risk and should be prescribed alternative thromboprophylaxis) ▪ NSAID prescription despite caution, contraindication, or existing NSAID prescription: 15% ▪ QT-prolonging medication despite a borderline or prolonged QTc on preoperative ECG: 6.3% |
▪ 115 | NR | 9 days |
| Hale et al. [34] | NR | NR |
▪ Medication omission (31.5% vs 1.2%, P < 0.001) ▪ Prescribing errors involving selection of drug, dose, or frequency (6.3% vs 0.2%, P < 0.001) Orders with at least one component of the prescription missing, incorrect, or unclear (43% vs 23%, P < 0.001) |
▪ NR | NR | Hospital duration |
| Han et al. [41] | None | NR |
▪ Prior-to-admission corrections: Adding missing medications (93%), removing old medications (52%), updating missing or incorrect medication details (48%), removing duplicate medications (5%) ▪ Pharmacist interventions pre-op: Dose change (16%), taper (13%), other interventions (12%), monitoring (10%) ▪ Pharmacist interventions post-op: New medication counselling (100%), admin instruction change (85%), hold medication until follow-up (78%), agent change (66%), discontinue medication (60%) |
▪ Prior-to-admission corrections: 61 patients (90%) in the intervention group. The pharmacist made a median of four corrections per patient ▪ Pre-op interventions: Median of 13 interventions per patient in the preoperative clinic consultation |
NR | NR |
| Kwan et al. [35] | Number of patients with at least one postoperative medication discrepancy: intervention: 86/214 (40.2%) vs 41/202 (20.3%), P < 0.001 (odds ratio, 0.38; 95% confidence interval, 0.24–0.59) |
Causing discomfort and/or clinical deterioration ▪ Probable (20% vs 33.8%) ▪ Possible (38.3% vs 32.5%) ▪ Unlikely (41.7% vs 33.7%) |
▪ Drug omission (36.7% vs 46.5%), incorrect or omitted dose (8.3% vs 8.9%), drug formulation (1.7% vs 5.7%) and frequency (0% vs 4.5%), and inappropriate route (5% vs 1.3%) ▪ Orders requesting pharmacy to clarify medications (no medication orders or incomplete medication orders written) (6.7% vs 17.8%) or illegible order (0% vs 0.6%) ▪ No indication (1.7% vs 3.8%) ▪ Slow to restart (15.0% vs 3.2%) or too fast to restart drug therapy (1.7% vs 0.6%) ▪ Drug interactions (5% vs 1.9%) ▪ Omission of drug name (3.3% vs 1.3%) or misspelled drug name (1.7% vs 0.6%) ▪ Therapeutic duplication (5% vs 0.6%) ▪ Allergy or intolerance (0% vs 0.6%) ▪ Miscellaneous (8.3% vs 1.9%) |
▪ NR | NR | Hospital duration |
| Léguillon et al. [29] |
▪ Number of PIPs: − 2.46 PIPs (95% CI: − 2.63; − 2.24), P < 0.001 ▪ Number of overall PIMs: − 1.13 [− 1.27; − 0.98], P < 0.001 ▪ -Number of overall PPOs (omission/underuse): − 1.35 [− 1.52; − 1.18] < 0.001 ▪ Number of patients with one or more PIPs at hospital discharge: 95% vs 29%, P < 0.001 No patient in the intervention group had three or more PIPs at hospital discharge, compared to 61% in the control group (P < 0.001) |
N/A | ▪ N/A | ▪ N/A | N/A | Not reported |
| Luo et al. [30] | ▪ NR | NR |
▪ No indication (82.41% vs 72.78%), P = 0.023 ▪ Inappropriate choice of acid suppressant (2.32% vs 0%), P = 0.046, dose (14.35% vs 7.10%), P = 0.025, and route (61.11% vs 37.27), P < 0.001 ▪ Repeated medication (1.39% vs 0%), P > 0.05 ▪ Unnecessary replacement of drugs (25.93% vs 10.06%), P < 0.001 ▪ Unnecessary prolonged duration of prophylaxis (65.28% vs 37.87%), P < 0.001 |
▪ NR | NR | Hospital duration |
| Marotti et al. [25] | NR | NR |
Control group vs. pharmacist taking history vs. pharmacist prescribing (means) ▪ Doses missed during inpatient stay (3.21 vs 3.30 vs 1.07; P < 0.001) ▪ Medications charted at an incorrect dose: 0.48 vs 0.12 vs 0.02, P < 0.001 ▪ Medications charted at an incorrect frequency: 0.29 vs 0.07 vs 0.015, P < 0.001 |
▪ NR | N/A | Hospital duration |
| Nguyen et al. [27] |
▪ Proportion of patients ≥ 1 med error [96% vs 9%, P < 0.001] ▪ Preadmission errors/patient [5.25 vs 0.21, P < 0.001] ▪ Proportion of discharge prescriptions with ≥ 1 error [78% vs 25%, P < 0.001] ▪ Time to charting the first regular home medication [21 h vs 16.8 h, P = 0.605] ▪ Quality of inpatient charting (errors per patient) [1.31 vs 0.64, P = 0.047] ▪ Proportion of patients who received a discharge summary with a completed medication list [33% vs 75%, P = 0.002] |
Preadmission: Low-risk errors (46.4% vs 72.7%), moderate high, and extreme error (53.6% vs 27.3%) Discharge: Moderate error (44.2% vs 44.4%) |
▪ Omission: Preadmission (2.84 vs 0.21), inpatient (1.12 vs 0.66), discharge (1.38 vs 0.92) ▪ Incorrect medication listed: Preadmission (0.55 vs 0), inpatient (0.04 vs 0.04), discharge (0.27 vs 0.06) ▪ Incorrect strength: Preadmission (0.65 vs 0), inpatient (0.04 vs 0), discharge (0.08 vs 0.03) ▪ Incorrect frequency: Preadmission (0.53 vs 0), inpatient (0.02 vs 0.04), discharge (0.14 vs 0.03) ▪ Incorrect dose: Preadmission (0.39 vs 0), inpatient (0.06 vs 0), discharge (0.11 vs 0.17) ▪ Incorrect dosage form: Preadmission (0.18 vs 0), inpatient (0.04 vs 0), discharge (0.14 vs 0.03) ▪ Incorrect instructions: Preadmission (0.12 vs 0), inpatient (0 vs 0.02), discharge (0.05 vs 0.03) |
NR | NR | Hospital duration |
| SUREPIL Study Group [26] |
Mean number of preventable ADE: incidence of 3.84% (2.49 to 5.91%) vs 2.74% (1.65 to 4.57%), P = 0.324 Incidence rate ratio of 0.71 (95% CI (0.37–1.39) |
NR | ▪ NR | 880 | NR | Hospital duration |
| Van Prooyen et al. [36] | NA | NR |
▪ Interventions: Anti-HTN adjustment, discontinue (non-insulin injectable, NSAIDs, loop diuretics, oral diabetic), insulin dose adjustment ▪ The prescription at discharge: Extended-release medication 19.3% reduction, P = 0.0005. Capsules unable to be opened P = 0.27. Noncrushable tablets 7 (4.2%) vs 1 (1.2%), P = 0.27. Enteric- or film-coated tablets: 2 (1.2%) vs 3(3.5%), P = 0.34. NSAIDs: 15 (9%) vs 3 (3.5%), P = 0.11, and loop diuretics: taking at baseline 13 (7.8%) vs 10 (11.8%), P = 0.30, continued at discharge: 6 (46.2%) vs 2 (20%), P = 0.38 |
130 | 85.40% | 30 days |
| Yang et al. [42] | None | NR |
▪ Change in drug treatment (n = 396) ▪ Dose adjustment (n = 61) ▪ Discontinuation of a drug (n = 121) ▪ Order entry error (n = 34) |
630 | 97.10% | 30 days |
| Zhang et al. [37] |
▪ Inappropriate PPI use (48.9% vs. 22.7%; P < 0.001) ▪ Unindicated PPIs prescriptions (32.2% vs 14.2%; P < 0.001) ▪ Inappropriate PPI daily dose (782 vs 96, P < 0.001), duration (611 vs 45, P < 0.001), and route of administration (223 vs 89, P < 0.001) |
NR |
▪ Starting new therapy (n = 15) ▪ Discontinuation of inappropriate therapy (n = 136) ▪ Dose adjustment (n = 78), change route (n = 124) ▪ Inappropriate PPI daily dose (782 vs 96, P < 0.001), duration (611 vs 45, P < 0.001), and route of administration (223 vs 89, P < 0.001) between the pre- and post-intervention groups |
356 | 88% | Hospital duration |
Fig. 4.
Bar charts depicting the proportion of errors in intervention and control arms
Medication errors throughout the perioperative period
The overall medication errors throughout the perioperative period were reported by three studies. Nguyen et al. [41] reported that the proportion of patients with at least one error was 96% and 9% in control and intervention groups respectively (P < 0.001). Fitzpatrick et al. [28] reported that pharmacist interventions led to at least one prescribing difference in 38.8% of the patients. Similarly, Léguillon et al. [29] demonstrated a statistically significant reduction (P < 0.001) in the number of potentially inappropriate prescriptions (PIPs) and potentially inappropriate medications (PIMs).
Medication errors prior to admission
Only one study reported medication errors prior to admission. Nguyen et al. [27] involved perioperative pharmacy services (the PREP pharmacist group) that contacted patients via telephone approximately 1 week prior to scheduled surgery. Findings revealed that PREP group achieved an overall reduction in errors from 5.25 to 0.21 per patient (P < 0.001).
Medication errors during hospitalization
Admission reconciliations and inpatient charting were also investigated by Nguyen et al. [27], who also reported a decrease in errors from 1.32 to 0.76 per patient during hospitalization.
Chen et al. [39] and Zhang et al. [37] reported on the inpatient use of proton pump inhibitors (PPIs). The irrational and inappropriate prescription of PPIs significantly decreased after the involvement of a pharmacist intervention (P < 0.001). Furthermore, medication discrepancies postoperatively were assessed by Kwan et al. [35] demonstrating a 19.9% reduction in medication discrepancies between home medications and postoperative medications when pharmacists collected histories and participated in the patient care (OR: 0.38; 95% CI: 0.24–0.59; P < 0.001). Lastly, SUREPILL Study Group [26] reported a nonsignificant reduction in the incidence of preventable drug-related problem (DRPs) per 100 admissions.
Medication errors on discharge
Three studies reported medication errors in the discharge prescription. A France-based before-and-after study showed a decrease in the proportion of patients with one or more PIPs at hospital discharge from 95 to 29% (P < 0.001). It also showed that none of the patients in the intervention group had three or more PIPs at hospital discharge, compared to 61% in the control group (P < 0.001) [29]. AbuRuz et al. investigated DRPs at hospital discharge and found a mean reduced difference of 2.63 (P < 0.0001) [33]. Additionally, a study that included patients at high risk for medication disadvantages reported a substantial decline in medication errors on discharge, from 78 to 25% (P < 0.001) [27].
Outcomes related to the impact of pharmacist interventions on types of medication errors
A diverse error reporting has been observed across the included studies. Additionally, none of the included studies provided definitions or basis for the categorization of errors. Consequently, types of errors were classified in two broad categories of either errors of omission or commission.
Omission errors
Errors resulting from failure to follow correct procedures or from not taking the appropriate actions have been categorized as omission errors. A total of eight articles reported on omission errors or pharmacist interventions aimed at addressing these errors, employing various outcome definitions. The included studies showed inconsistent findings in which pharmacist interventions showed favorable findings in some but not all of the investigated endpoints. It is pivotal to note that most of the studies were not statistically powered to draw a robust conclusion, as these errors were investigated as a secondary outcome. For instance, Nguyen et al. [27] reported on omission errors across all levels of care, demonstrating a decrease in errors with the pharmacist intervention compared to usual care: preadmission (2.84 vs 0.21), inpatient (1.12 vs 0.66), and discharge (1.38 vs 0.92). Conversely, AbuRuz et al. [33] showed a decrease in the incidence of errors in comparison with standard medical care for untreated condition (10.8% vs 8.1%) and recommendation for a more effective drug (6.9% vs 6.1%); however, the incidence was increased for efficacy-related issues (16.6% vs 21.3%), need for additional therapy (7.2% vs 10.3%), and low dose (1.5% vs 4.0%).
Meanwhile, some studies reported statistical significance rather than numerical incidence. For example, Falconer et al. [40] reported a statistically significant reduction of omission errors after implementing the pharmacist-led intervention compared to the pre-intervention period (3.7 ± 1.2 vs 4.2 ± 1.8; P = 0.003); however, no significant difference was observed in addressing discrepancies among the final medication lists (28.5% vs 20.0%; P = 0.59). Two RCTs reported significant improvements in missed doses during inpatient stays (3.21 vs 3.30 vs 1.07, P < 0.001) and in the unintended omissions of medications (31.5% vs 1.2%, P < 0.001), respectively, compared to the control arm [25, 34]. Finally, Kwan et al. [35] reported reductions in drug omissions with pharmacist medication assessments in a surgical preadmission clinic compared to the standardized care arm (46.5% vs 36.7%) and delays in restarting drug therapy (15.0% vs 3.2%).
Commission errors
Errors resulting from doing something wrong were extensively reported in the included studies, with a total of 12 studies focusing on commission errors. These studies exhibited considerable variation in their definitions, methods of reporting, and data categorization. Nguyen et al. [27] presented finding on multiple commission errors at preadmission, inpatient, and discharge. Findings showed reductions in nearly all investigated error subtypes across different levels of care compared to usual care, including lack of clear instructions, incorrect medication lists, incorrect strengths, frequencies, and dosages. However, exceptions were noted, including an increase in incorrect frequency during the inpatient period (from 0.02 error/patient in control to 0.04 error/patient in intervention), incorrect instruction during the inpatient period (from 0 error/patient in control to 0.02 error/patient in intervention), and incorrect dosing at discharge (from 0.11 error/patient in control to 0.17 error/patient in intervention). It is important to note that statistical significance was not reported for any of these endpoints [27].
Other studies demonstrating statistically significant reductions in commission errors with pharmacist intervention compared to usual care include Marotti et al. [25], Hale et al. [34], and Luo et al. [30]. These studies reported significant improvements (P < 0.001) in errors related to the drug [30, 34], dose [25, 30, 34], frequency [25, 34], duration [30], and route [30]. In contrast, Kwan et al. [35] reported conflicting findings, showing reductions between groups in the incidence of incorrect dose, incorrect frequency, and no indication, yet no effects were observed for drug interactions, inappropriate route, and therapeutic duplication.
Some studies reported findings related to particular classes of medication. For instance, Falconer et al. [40] reported increase in the number of stopped medications including antihypertensives (44.7% vs 85.4%; P < 0.001) and antidiabetics (65.9% vs. 66.7%; P = 0.43) after conducting medication optimization interventions by the pharmacist. Fitzpatrick et al. [28] claims improper venous thromboembolism prophylaxis, with 15% of nonsteroidal anti-inflammatory drugs (NSAID) prescriptions despite caution, contraindication or existing NSAID prescriptions, and a 6.3% QT-prolonging medication prescribed despite a borderline or prolonged QTc on preoperative ECG. Both Chen et al. [39] and Zhang et al. [37] explored errors related to PPI use. The former showed that the proportion of unindicated PPI use, utilization rate, average defined daily dose (DDD), drug costs, and PPI costs were significantly lower in the intervention group than in the control group (P < 0.05) [39]. Similarly, the latter reported that the rates of inappropriate PPI use before and after the intervention were 48.9 and 22.7 per 100 patient-days, respectively [37]. Both studies showed that most errors were related to therapy appropriateness, indication, dosage, routes, frequency, and duration, although exact numbers were not provided.
Van Prooyen et al. [36] investigated proper dosage formulation via pharmacist consultation after bariatric surgery compared to a historical control group: extended-release medication (28.7% vs 9.4%; P = 0.0005), capsules that could not be opened (28.7% vs 22.4%; P = 0.27), noncrushable tablets (4.2% vs 1.2%; P = 0.27), and enteric- or film-coated tablets (1.2% vs 3.5%; P = 0.34). Moreover, medications that were recommended to be discontinued (e.g., NSAIDs, loop diuretics) were prescribed less frequently in the intervention group, yet the difference was not statistically significant.
Description of pharmacist interventions
Yang et al. [42] included 630 pharmacist interventions, and the accepted interventions included changes in drug treatment regimens (n = 396), dose adjustments (n = 61), discontinuation of a drug (n = 121), and order entry errors (n = 34). Han et al. [41] showed that pharmacists made a median of 13 interventions per patient during clinic consultation, including instruction changes (n = 58), hold medications (n = 53), change medications (n = 45), discontinue medications (n = 41), dose changes and tapering (n = 10), monitoring (n = 7), and other interventions (n = 8). Similarly, Bansal et al. [38] showed that 234 (55.7%) of the interventions were perioperative drug management, while 186 (44.3%) were medicine optimization.
Severity of medication errors and acceptance rate
There is a considerable lack of reporting in relation to the severity of errors and acceptance rate (Table 3). Inconsistency in reporting has also been noted across the studies. The acceptance rate, for example, was reported by only 29% of articles, and it ranged widely from 77% [40] to 97.1% [42].
Only two studies reported on the severity of errors with a notable lack of standardized reporting system for medication errors severity. The first study reported the probability of the error to cause harm or discomfort, and the errors were categorized as probable, possible, and unlikely [35]. The second study showed that most errors were of moderate severity [27]. The lack of reporting and substantial heterogeneity challenged the ability to compare results.
Discussion
Statement of principal findings
All included studies incorporated multicomponent interventions primarily focused on medication reconciliation, medicine-related recommendations, education delivered to other healthcare professionals, and patient counselling. Reporting of intervention development processes was unclear and lacking. Large inconsistencies have been observed across studies in error identification methods, definitions, and categorization of identified errors. This variation prevented a thorough and structured investigation into the impact of pharmacists on the sub-categories of errors; hence, we classified them into two broad categories of omission and commission errors. Pharmacist interventions in the surgical setting were associated with a reduction in the overall medication error rate before admission, during hospitalization, and upon discharge. Similarly, pharmacist interventions generally tended to reduce the prevalence of the sub-categories of medication errors, though there are some inconsistencies. Medicine optimization during the perioperative period was the main areas of intervention for pharmacists in this review.
Context of these findings
Our findings showed that pharmacist interventions could potentially reduce the occurrence of overall medication errors in perioperative settings. This is consistent with previous research that investigated the impact of pharmacists on medication errors across a wide range of settings [43–46]. For example, a meta-analysis that focused on emergency departments reported that pharmacist activities significantly reduced medication errors by a mean of 0.33 per patient (95% CI − 0.42 to − 0.23) and the proportion of patients with at least one error by 73% (RR 0.27, 95% CI 0.19 to 0.40, I2 = 85.3%) [45].
Nonetheless, findings from our review revealed inconsistencies in relation to the impact of pharmacist interventions on the subcategories of medication errors. This was particularly evident in studies that explored commission errors. It is noteworthy that some of the included studies only investigated the subcategories of errors without reporting on the overall incidence; hence, the impact of the pharmacist on the overall error occurrence was not assessed. Additionally, high inconsistency has been noted in the number of error subcategories (e.g., wrong drug or wrong dose) used across studies, and there appears to be no standard approach for the categorization of these errors. It is likely that the variation in the number and type of error subcategories included may influence the overall reported medication error rate (e.g., a greater number of errors subcategories is likely to result in a greater incidence of overall medication error) [47].
While dosage adjustments remain the predominant trigger for pharmacist interventions in various settings [44, 48–50], findings from the current review highlight that most interventions within the surgical context were medication optimization. This could be attributed to the need for adjusting some of the patients’ chronic medications around the time of the surgery to improve safety in surgery. Perioperative medication management continues to grow as pressing health concern, particularly with the progressively aging and sick population. Recent statistics show that over half of the general surgical patients take medications unrelated to surgery [51]. Therefore, a unique role for pharmacists emerges in this specific setting as they can provide evidence-based recommendations regarding when to continue, when to withhold, and when to restart home medications. Additionally, the pharmacist could also advise on alternative medications to control the chronic conditions throughout the spectrum of surgical care. The American Society of Health-System Pharmacists (ASHP), in their 2019 guidelines on perioperative pharmacy services, emphasized the need for a pharmacist to review orders and provide pharmacotherapeutic recommendations during the preoperative and post-anesthesia periods [12].
Identification and classification of medication errors
The included studies greatly varied in their error detection methods, definitions, and categorization of these errors. The majority of studies lacked reporting of medication errors using established classification systems. These systems could include classifications of severity, such as the NCCMERP classification system, or classification based on the medication management process (prescribing, transcribing, dispensing, administering, and monitoring) [52]. Research studying pharmacist intervention needs to collect and report data on medication errors in a more specific manner, which will enhance our ability to understand the role of pharmacist, as their role is likely to vary within the different steps of the drug utilization process [52, 53]. Once the pharmacist’s role is better understood, interventions could be better planned and studied based on these findings.
Characteristics of pharmacist intervention
All studies encompassed services within the realm of clinical pharmacy practice, such as admission reconciliation, medication review, communication with prescribers for medication optimization, monitoring, and patient education. All the referenced studies employed a comprehensive approach to clinical pharmacy services as the pharmacist intervention, except for one article. Only two of the included studies introduced a novel, structured intervention services [35, 38]. Multifaceted pharmacist interventions enable proactive engagement at different care stages. Existing research has substantiated that transitions of care, such as discharge or transfer, rank among the primary contributors to avoidable medication errors [54], and the number of transitions within the perioperative setting far outweighs that of other care domains. Patients experience many transitions of care and shifts of locations and healthcare providers within a short period [55]; therefore, pharmacist interventions must be dynamic and diverse to effectively address these complexities.
Many of the included articles employed pharmacist interventions with limited contact frequencies, usually limited to one or two contact points within the process. This limitation of contact could considerably underestimate the pharmacist’s role as many errors will be missed, and no intervention will be undertaken in an attempt to reduce them. In this review, only six of the included articles [27, 28, 34, 35, 38, 41] reported pharmacist interventions in the preadmission period; this is important to note because in the setting of surgery, mainly elective surgery, there is a dire need for preadmission medication adjustment [56]. George et al. revealed that pharmacist involvement in preadmission care resulted in an increased number of interventions compared to restricting the pharmacist services to the admission period [57].
Increasing efforts have been made to include pharmacists in the preadmission, admission, and discharge processes. However, there is a growing body of evidence that shows a great portion of medication errors occurs within the operation itself; in an observational study on 227 operations, in which 3671 medication administrations were observed, 193 (5.3%; 95% CI, 4.5 to 6.0) included a medication error, of which 79.3% were preventable, 64.7% were serious, and 2% were life-threatening [7]. Nevertheless, in the context of this review, no articles documented interventions examining the involvement of pharmacists in intraoperative settings, representing a substantial gap given that this phase constitutes a pivotal part of the surgical process. Intraoperative settings lack several checkpoints for medication validation and error prevention that are typically present in ward settings. The inherent nature of the intraoperative environment results in a consistent bypass of validated systems known for their efficacy in reducing medication errors [58, 59].
Educational services were prevalent interventions within this review, but there was a noticeable inclination toward directing educational efforts more toward healthcare providers than patients. This is, however, understandable, as significant medication errors could occur within the prescribing and administration processes [60]. A meta-analysis conducted by Jaam et al. reported that pharmacist-led educational endeavors targeted and delivered to healthcare providers result in a significant reduction in medication errors (OR 0.38, 95% CI 0.22 to 0.65) (P = 0.0004).
Only two studies in this review examined the utilization of pharmacy prescribing services, specifically independent pharmacist prescribers (IPPs) [27, 34]. A comprehensive cross-sectional study demonstrated that IPPs exhibited an error rate of 0.7% (95% CI 0.0 to 1.0%) in contrast to physicians, who displayed a substantially higher error rate of 9.8% (95% CI 9.0 to 11.0%) [61].
Furthermore, an observation within the reviewed studies was the lack of reporting on the pharmacist-to-patient ratio. The deficiency in pharmacist staffing is particularly evident in surgical settings, where pharmacists are often responsible for a higher patient load than their counterparts in medical or intensive care unit (ICU) settings. This understaffing could potentially underestimate the positive role of pharmacy intervention. The issue of understaffing remains prevalent in various countries, emphasizing the urgency of addressing and rectifying this concern within the realm of pharmacy practice [15].
Development of pharmacist intervention
A considerable number of the encompassed studies adopted a pragmatic methodology in implementing pharmacist interventions, with the majority relying on international, national, or institutional guidelines as the basis for their interventions. Notably, there is a significant shortfall in the execution and documentation of the development and adaptation processes employed for pharmacist interventions within the prevailing settings. This deficiency extends to elucidating the rationale behind selecting each element comprising the intervention and the scientific expectations regarding its impact on outcomes [62]. Enhancing comprehension in these aspects could contribute to heightened participant engagement in the studies and augment the generalizability and reproducibility of the research findings [63, 64]. The omission of reporting the theoretical foundations of the interventions included constrains our ability to provide a comprehensive analysis of their impact. Consequently, the effectiveness of theory-driven interventions in this domain remains uncertain. While the theory may not necessarily result in a favorable impact on outcomes supporting the intervention, it aids in pinpointing, from a vast array, the intervention components that could prove effective, which would further support the development of further interventions in future research [65].
Strengths and limitations
To our best understanding, this systematic review represents the first attempt to evaluate the influence of pharmacist intervention on medication errors in the perioperative setting. The study protocol was preregistered on PROSPERO [66]. Data extraction was performed by a team of four researchers utilizing the DEPICT-2 tool, ensuring a consistent and unbiased approach [22]. Adhering to PRISMA guidelines, the systematic review’s reporting was meticulously executed.
Several limitations are associated with the current review. Firstly, the search was restricted to English and Arabic, potentially excluding relevant literature in other languages. Secondly, we acknowledge the heterogeneity of results, considering the diverse range of pharmacist interventions and outcomes under investigation. Thirdly, a notable limitation is the small sample size in many studies, suggesting insufficient statistical power to demonstrate the impact of pharmacist intervention. Fourth, the short follow-up periods in most studies, often limited to the admission period, pose a challenge. Prior research indicates that up to half of discharged patients experience medication errors when followed after discharge, particularly since, in many cases, patients do not have contact with healthcare providers during that timeframe [67]. Fifth, the generalizability of our findings is constrained due to the predominant inclusion of studies from the USA and China.
Future directions
The review findings suggest that pharmacist-led interventions exhibit promise in reducing medication errors within perioperative settings. However, a research gap exists in developing and implementing interventions tailored to this setting, considering its unique characteristics. Researchers are urged to explore medication errors to identify specific gaps and areas conducive to pharmacist intervention. The study underscores the absence of theory-driven interventions in perioperative settings, advocating for robust randomized studies using theoretical frameworks. Future research is encouraged to provide detailed descriptions of interventions, encompassing structures, processes, and outcomes, to ensure reproducibility, with the endorsement of the DEPICT-2 tool for this purpose. Additionally, there is a call for further investigation into the impact of pharmacist prescribing in clinical pharmacy practice due to its promising advantages, such as expedited access to medications and reduced physician workload.
Conclusion
While there is some evidence of a positive impact of the pharmacist-led interventions on medication errors in perioperative settings, this evidence is generally of low quality and insufficient volume. Heterogeneity in study design, definitions, and case detection is common; hence, high-quality research that applies more stringent controls and uses clearer definitions is warranted.
Supplementary Information
Supplementary Material 1: Supplement S1 – PRISMA checklist. Supplement S2 – Search strategy.
Authors’ contributions
LN contributed to the study design, systematic review planning, data extraction, interpretation of data, and data analysis and led the article screening, article selection, and manuscript writing. SK contributed to the study design, data extraction and interpretation of data, article screening, article selection, and revision of the manuscript. BA and MA were involved in the article selection, data extraction, manuscript writing, and revision of the manuscript. The authors attest that all authors meet authorship criteria, and that no authors who meet the criteria have been omitted. All authors have read and approved the final manuscript.
Funding
None.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors provided consent for the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lisby M, Nielsen LP, Brock B, et al. How are medication errors defined? A systematic literature review of definitions and characteristics. Int J Qual Health Care. 2010;22(6):507–18. [DOI] [PubMed] [Google Scholar]
- 2.About medication errors: National Coordinating Council for Medication Error Reporting and Prevention. Available from: https://www.nccmerp.org/about-medication-errors.
- 3.Mekonnen AB, Alhawassi TM, McLachlan AJ, et al. Adverse drug events and medication errors in African hospitals: a systematic review. Drugs Real World Outcomes. 2018;5(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tariq RA, Vashisht R, Sinha A, et al. Medication dispensing errors and prevention. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed]
- 5.Wittich CM, Burkle CM, Lanier WL. Medication errors: an overview for clinicians. Mayo Clin Proc. 2014;89(8):1116–25. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker CF, Miklich MA, Patel RS, et al. Medication safety principles and practice in CKD. Clin J Am Soc Nephrol. 2018;13(11):1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanji KC, Patel A, Shaikh S, et al. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenwasser R, Winterstein AG, Rosenberg AF, et al. Perioperative medication errors in otolaryngology. Laryngoscope. 2010;120(6):1214–9. [DOI] [PubMed] [Google Scholar]
- 9.van Waes JA, de Graaff JC, Egberts AC, et al. Medication discontinuity errors in the perioperative period. Acta Anaesthesiol Scand. 2010;54(10):1185–91. [DOI] [PubMed] [Google Scholar]
- 10.Stipp MM, Deng H, Kong K, et al. Medication safety in the perioperative setting: a comparison of methods for detecting medication errors and adverse medication events. Medicine (Baltimore). 2022;101(44): e31432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahr JA, Abernathy JH 3rd, Lazarra EH, et al. Medication safety in the operating room: literature and expert-based recommendations. Br J Anaesth. 2017;118(1):32–43. [DOI] [PubMed] [Google Scholar]
- 12.Bickham P, Golembiewski J, Meyer T, et al. ASHP guidelines on perioperative pharmacy services. Am J Health Syst Pharm. 2019;76(12):903–820. [DOI] [PubMed] [Google Scholar]
- 13.The definition of clinical pharmacy. Pharmacotherapy. 2008;28(6):816–7. [DOI] [PubMed] [Google Scholar]
- 14.Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wireko AA, Ohenewaa Tenkorang P, Tope Adebusoye F, et al. The importance of pharmacists in modern day surgery - editorial. Int J Surg. 2023;109(2):88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naseralallah L, Koraysh S, Alasmar M, et al. Effect of pharmacist care on clinical outcomes and therapy optimization in perioperative settings: a systematic review. Am J Health Syst Pharm. 2024;82(1):44–73. [DOI] [PMC free article] [PubMed]
- 17.Naseralallah L, Koraysh S, Aboujabal B, et al. Effectiveness of pharmacist-led antimicrobial stewardship programs in perioperative settings: a systematic review and meta-analysis. Res Social Adm Pharm. 2024;20(11):1023–37. [DOI] [PubMed] [Google Scholar]
- 18.Naseralallah L, Koraysh S, Aboujabal B, et al. Interventions and impact of pharmacist-delivered services in perioperative setting on clinically important outcomes: a systematic review and meta-analysis. Ther Adv Drug Saf. 2024;15:20420986241260170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefera GM, Zeleke AZ, Jima YM, et al. Drug therapy problems and the role of clinical pharmacist in surgery ward: prospective observational and interventional study. Drug Healthc Patient Saf. 2020;12:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotta I, Salgado TM, Felix DC, et al. Ensuring consistent reporting of clinical pharmacy services to enhance reproducibility in practice: an improved version of DEPICT. J Eval Clin Pract. 2015;21(4):584–90. [DOI] [PubMed] [Google Scholar]
- 23.Crowe M, Sheppard L. A general critical appraisal tool: an evaluation of construct validity. Int J Nurs Stud. 2011;48(12):1505–16. [DOI] [PubMed] [Google Scholar]
- 24.Crowe M, Sheppard L, Campbell A. Reliability analysis for a proposed critical appraisal tool demonstrated value for diverse research designs. J Clin Epidemiol. 2012;65(4):375–83. [DOI] [PubMed] [Google Scholar]
- 25.Marotti SB, Kerridge RK, Grimer MD. A randomised controlled trial of pharmacist medication histories and supplementary prescribing on medication errors in postoperative medications. Anaesth Intensive Care. 2011;39(6):1064–70. [DOI] [PubMed] [Google Scholar]
- 26.SUREPILL. Effect of a ward-based pharmacy team on preventable adverse drug events in surgical patients (SUREPILL study). Br J Surg. 2015;102(10):1204–12. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen AD, Lam A, Banakh I, et al. Improved medication management with introduction of a perioperative and prescribing pharmacist service. J Pharm Pract. 2020;33(3):299–305. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick K, Addie K, Shaw M, et al. Implementing an innovative, patient-centered approach to day case arthroplasty: improving patient outcomes through remote preoperative pharmacist consultations. Eur J Hosp Pharm. 2024;31(4):321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Léguillon R, Varin R, Pressat-Laffouilhère T, et al. Clinical pharmacist intervention reduces potentially inappropriate prescriptions in a geriatric perioperative care unit dedicated to hip fracture. Gerontology. 2023;69(4):386–95. [DOI] [PubMed] [Google Scholar]
- 30.Luo H, Fan Q, Xiao S, et al. Impact of clinical pharmacist interventions on inappropriate prophylactic acid suppressant use in hepatobiliary surgical patients undergoing elective operations. PLoS ONE. 2017;12(10): e0186302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly N-A, Hickey A, Burns A, et al. Systematic review and meta-analysis of the impact of carer stress on subsequent institutionalisation of community-dwelling older people. PLoS ONE. 2015;10(6): e0128213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Awaisi A, Koummich S, Koraysh S, et al. Patient safety education in entry to practice pharmacy programs: a systematic review. J Patient Saf. 2022;18(2):e373–86. [DOI] [PubMed] [Google Scholar]
- 33.AbuRuz S, Jaber D, Basheti I, et al. Impact of pharmacist interventions on drug-related problems in general surgery patients: a randomised controlled trial. Eur J Hosp Pharm. 2021;28(Suppl 2):e72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3(7): e003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034–40. [DOI] [PubMed] [Google Scholar]
- 36.Van Prooyen AM, Hicks JL, Lin E, et al. Evaluation of an inpatient pharmacy consult on discharge medications in bariatric surgery patients. J Pharm Pract. 2023;36(2):203–12. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Yang H, Kong J, et al. Impact of interventions targeting the inappropriate use of proton-pump inhibitors by clinical pharmacists in a hepatobiliary surgery department. J Clin Pharm Ther. 2021;46(1):149–57. [DOI] [PubMed] [Google Scholar]
- 38.Bansal N, Tai WT, Chen LC. Implementation of an innovative surgical pharmacy service to improve patient outcomes-twelve-month outcomes of the Enhanced Surgical Medicines Optimization Service. J Clin Pharm Ther. 2019;44(6):904–11. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Wang Q, Zhang Y. Clinical intervention increases rational use of proton pump inhibitors in the general surgery department. Front Pharmacol. 2022;13: 864081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falconer EA, Harris DA, Van Prooyen A, et al. Pharmacy-led initiative for improving peri-operative medication reconciliation among bariatric surgical patients: what is the role? Surg Endosc. 2022;36(2):1593–600. [DOI] [PubMed] [Google Scholar]
- 41.Han A, Nguyen NY, Hung N, et al. Efficacy of a bariatric surgery clinic-based pharmacist. Obes Surg. 2022;32(8):2618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Li L, Hu X, et al. Impact of pharmacist-led post-transplant medication management for kidney transplant recipients: a retrospective pre- and post-intervention study. J Clin Pharm Ther. 2019;44(4):603–10. [DOI] [PubMed] [Google Scholar]
- 43.Jaam M, Naseralallah LM, Hussain TA, et al. Pharmacist-led educational interventions provided to healthcare providers to reduce medication errors: a systematic review and meta-analysis. PLoS ONE. 2021;16(6): e0253588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naseralallah LM, Hussain TA, Jaam M, et al. Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: a systematic review and meta-analysis. Int J Clin Pharm. 2020;42(4):979–94. [DOI] [PubMed] [Google Scholar]
- 45.Atey TM, Peterson GM, Salahudeen MS, et al. Impact of pharmacist interventions provided in the emergency department on quality use of medicines: a systematic review and meta-analysis. Emerg Med J. 2023;40(2):120–7. [DOI] [PubMed] [Google Scholar]
- 46.Manias E, Kusljic S, Wu A. Interventions to reduce medication errors in adult medical and surgical settings: a systematic review. Ther Adv Drug Saf. 2020;11:2042098620968309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gates PJ, Baysari MT, Gazarian M, et al. Prevalence of medication errors among paediatric inpatients: systematic review and meta-analysis. Drug Saf. 2019;42(11):1329–42. [DOI] [PubMed] [Google Scholar]
- 48.Al Rahbi HA, Al-Sabri RM, Chitme HR. Interventions by pharmacists in out-patient pharmaceutical care. Saudi Pharm J. 2014;22(2):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed A, Tanveer M, Shrestha S, et al. Interventions and impact of pharmacist-delivered services for people infected with COVID-19: a systematic review. Healthcare (Basel). 2022;10(9):1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez M, Masse M, Deldicque A, et al. Analysis of clinical pharmacist interventions in the COVID-19 units of a French University Hospital. Eur J Hosp Pharm. 2022;29(e1):e30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castanheira L, Fresco P, Macedo AF. Guidelines for the management of chronic medication in the perioperative period: systematic review and formal consensus. J Clin Pharm Ther. 2011;36(4):446–67. [DOI] [PubMed] [Google Scholar]
- 52.Brixey J, Johnson TR, Zhang J. Evaluating a medical error taxonomy. Proceedings. AMIA Symp. 2002:71–75. [PMC free article] [PubMed]
- 53.Koffuor GA, Anto BP, Abaitey AK. Error-provoking conditions in the medication use process: the case of a government hospital in Ghana. J Patient Saf. 2012;8(1):22–5. [DOI] [PubMed] [Google Scholar]
- 54.De Oliveira GS, Jr., Castro-Alves LJ, Kendall MC, et al. Effectiveness of pharmacist intervention to reduce medication errors and health-care resources utilization after transitions of care: a meta-analysis of randomized controlled trials. J Patient Saf. 2021;17(5):375–80. [DOI] [PubMed] [Google Scholar]
- 55.Bougeard AM, Watkins B. Transitions of care in the perioperative period - a review. Clin Med (Lond). 2019;19(6):446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whinney C. Perioperative medication management: general principles and practical applications. Cleve Clin J Med. 2009;76(Suppl 4):S126–32. [DOI] [PubMed] [Google Scholar]
- 57.George LJ, Senturk-Raif R, Hodgkinson MR, et al. Impact of a surgical preadmission clinic pharmacist on the quality of medication management from preadmission to discharge: a randomised controlled study. J Pharm Pract Res. 2011;41(3):212–6. [Google Scholar]
- 58.Workie MM, Chekol WB, Fentie DY, et al. Drug safety management in the operation room of referral hospital: cross-sectional study. Int J Surgery Open. 2020;26:97–100. [Google Scholar]
- 59.Suzuki R, Imai T, Sakai T, et al. Medication errors in the operating room: an analysis of contributing factors and related drugs in case reports from a Japanese Medication Error Database. J Patient Saf. 2022;18(2):e496–502. [DOI] [PubMed] [Google Scholar]
- 60.Institute of Medicine Committee on Quality of Health Care in America. In: Kohn LT, Corrigan JM, Donaldson MS, editors. To err is human: building a safer health system. Washington (DC): National Academies Press (US); 2000. [PubMed]
- 61.Turner E, Kennedy M-C, Barrowcliffe A. An investigation into prescribing errors made by independent pharmacist prescribers and medical prescribers at a large acute NHS hospital trust: a cross-sectional study. Eur J Hosp Pharm. 2021;28(3):149–53. [Google Scholar]
- 62.French SD, Green SE, O’Connor DA, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci. 2012;7(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolanowski AM, Litaker MS, Baumann MA. Theory-based intervention for dementia behaviors: a within-person analysis over time. Appl Nurs Res. 2002;15(2):87–96. [DOI] [PubMed] [Google Scholar]
- 64.Duncan EM, Bennett T, Gillies K. Assessing effective interventions to improve trial retention: do they contain behaviour change techniques? Trials. 2020;21(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Silva MJ, Breuer E, Lee L, et al. Theory of change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials. 2014;15:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiavo JH. PROSPERO: an International Register of Systematic Review Protocols. Med Ref Serv Q. 2019;38(2):171–80. [DOI] [PubMed] [Google Scholar]
- 67.Mixon AS, Myers AP, Leak CL, et al. Characteristics associated with postdischarge medication errors. Mayo Clin Proc. 2014;89(8):1042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplement S1 – PRISMA checklist. Supplement S2 – Search strategy.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.