Abstract
EB1 proteins are ubiquitous microtubule-associated proteins involved in microtubule search and capture, regulation of microtubule dynamics, cell polarity, and chromosome stability. We have cloned a complete cDNA of Dictyostelium EB1 (DdEB1), the largest known EB1 homolog (57 kDa). Immunofluorescence analysis and expression of a green fluorescent protein-DdEB1 fusion protein revealed that DdEB1 localizes along microtubules, at microtubule tips, centrosomes, and protruding pseudopods. During mitosis, it was found at the spindle, spindle poles, and kinetochores. DdEB1 is the first EB1-homolog that is also a genuine centrosomal component, because it was localized at isolated centrosomes that are free of microtubules. Furthermore, centrosomal DdEB1 distribution was unaffected by nocodazole treatment. DdEB1 colocalized with DdCP224, the XMAP215 homolog, at microtubule tips, the centrosome, and kinetochores. Furthermore, both proteins were part of the same cytosolic protein complex, suggesting that they may act together in their functions. DdEB1 deletion mutants expressed as green fluorescent protein or maltose-binding fusion proteins indicated that microtubule binding requires homo-oligomerization, which is mediated by a coiled-coil domain. A DdEB1 null mutant was viable but retarded in prometaphase progression due to a defect in spindle formation. Because spindle elongation was normal, DdEB1 seems to be required for the initiation of the outgrowth of spindle microtubules.
INTRODUCTION
In living cells, organization and dynamics of the microtubule cytoskeleton are highly regulated by proteins binding directly or indirectly to microtubules, in particular to their plus and minus ends. The minus ends are bound to the centrosome, the largest known protein complex in the cell, which regulates microtubule nucleation. The microtubule plus ends are the main sites for growth and shrinkage and involved in tethering of microtubules to cortical sites and kinetochores. Recent work has shown that plus ends are also associated with a protein complex, including CLIP170, dynein, dynactin, LIS-1, the adenomatous polyposis coli protein (APC), and EB1 (reviewed by Schroer, 2001). Among these, EB1 (for end binding) proteins are of special interest because they were found at the tips of growing astral microtubules (Mimori-Kiyosue et al., 2000) as well as at centrosomes and kinetochores (reviewed by Pellman, 2001; Tirnauer and Bierer, 2000). Homologs of human EB1 (Su et al., 1995) have been found and characterized in organisms as diverse as budding yeast (Bim1p; Schwartz et al., 1997), fission yeast (Mal3; Beinhauer et al., 1997), and Drosophila (dEB1; Lu et al., 2001). Originally, EB1 was identified as a binding partner of the tumor suppressor protein APC (Su et al., 1995). The EB1–APC interaction is disturbed in the majority of human colorectal tumors. Recent work suggests that the EB1–APC interaction may provide the physical link between growing microtubules and kinetochores. Loss of the APC–EB1 interaction could therefore cause chromosomal instability, which is usually observed in colorectal cancers (Fodde et al., 2001). APC and EB1 seem to have a comparable function at cortical microtubule-capture sites where both proteins are essential for the interaction of microtubule ends with adherens junctions. Cortical anchorage, in turn, is required for spindle orientation and symmetrical division of epithelial cells (Lu et al., 2001). This function is also reflected by budding yeast BIM1 and fission yeast mal3 deletion mutants that are viable but display spindle and nuclear positioning defects (Beinhauer et al., 1997; Schwartz et al., 1997; Tirnauer et al., 1999). Furthermore, Bim1p also affects microtubule dynamics. It increases the microtubule depolymerization rate but promotes net polymerization by increasing both the time spent growing and the rescue frequency, resulting in longer, more dynamic microtubules (Tirnauer et al., 1999). Thus, Bim1p is mainly a protein that promotes dynamic instability, similar to Xenopus XMAP215 (Gard and Kirschner, 1987; Vasquez et al., 1994; Tournebize et al., 2000). XMAP215 homologs have been studied in organisms as different as humans, Drosophila, Arabidopsis, yeast, and Dictyostelium (Ohkura et al., 2001). The effect of Bim1p and XMAP215 on microtubule dynamics suggests that members of these two protein families act together in this function.
Herein, we characterize DdEB1, a novel member of the EB1 protein family from Dictyostelium amoebae. Dictyostelium is a valuable model organism for the study of chemotaxis, signal transduction, development, cytoskeletal dynamics, and the centrosome (reviewed by Gräf et al., 2000a; Kessin, 2001). The Dictyostelium centrosome contains no centrioles but consists of a three-layered core structure that is surrounded by a matrix, called corona, which contains the microtubule nucleation sites. Unlike mammalian cells or budding yeast, centrosome duplication is not synchronized with the entry into S phase but is initiated in prophase and separation of the two centrosomal entities starts in prometaphase (Ueda et al., 1999). One of the well-investigated centrosomal components in Dictyostelium is the XMAP215 homolog DdCP224, a permanent centrosomal resident that is also localized at kinetochores and plays a role in centrosome duplication (Gräf et al., 2000b). In this work we show for the first time that EB1 and XMAP215 homologs colocalize at peripheral microtubule tips and interact in cytosolic complexes that can be coimmunoprecipitated. Furthermore, we provide strong evidence that the association of DdEB1 with microtubules requires homo-oligomerization and that DdEB1 is a genuine centrosomal component required for the formation of the mitotic spindle.
MATERIALS AND METHODS
Cloning of Complete DdEB1 cDNA
A size-fractionated Dictyostelium cDNA library containing cDNAs from 1–2 kb (Gräf et al., 2000b) was screened with a labeled DNA probe corresponding to the sequence from base position 641-1121 of the complete cDNA clone. The hybridization probe was generated and labeled with digoxigenin by polymerase chain reaction (PCR) by using clone JAX4a75d11 as a template (containing the DdEB1 genomic sequence from base position 641-1287; kindly provided by Dr. L. Eichinger from the Dictyostelium genome project). Seven clones contained the complete EB1 coding sequence (EMBL data library accession no. AJ426053).
Vector Construction, Protein Expression, and Antibodies
All green fluorescent protein (GFP)- and maltose-binding protein (MBP)-fusion vectors were generated by PCR with linker primers with restriction enzyme recognition sequences (base positions in parentheses refer to the complete cDNA sequence). The pMALc2 (NEB, Schwalbach, Germany) constructs for expression in Escherichia coli were MBP-DdEB1 (17–1537; BamHI/HindIII), MBP-DdEB1Δ129N (404–1537; BamHI/HindIII), and MBP-DdEB1Δ281C (17–860; BamHI/HindIII). All fragments were cloned using BamHI/HindIII sites. For MBP-DdEB1 expression in Dictyostelium, the complete MBP-DdEB1 sequence amplified by PCR with KpnI/NsiI linker primers and the respective E. coli expression vector as a template was cloned into p1ABsr8 (Gräf et al., 2000b). MBP-fusion proteins were purified by amylose chromatography with E. coli or Dictyostelium extracts (Gräf, 2001). Cell lysis and column washing were performed in lysis buffer containing 150 mM KCl, 2 mM MgCl2, and 50 mM HEPES/K, pH 7.4. The buffer was supplemented with 10 mM maltose for elution. Polyclonal antibodies were raised against highly purified, bacterially expressed MBP-DdEB1 (Dr. J. Pineda, Antikörperservice, Berlin, Germany).
The pA6PsgGFPXN constructs for expression of GFP fusion proteins in Dictyostelium were GFP-DdEB1 (17–1537), GFP-DdEB1Δ129N (404–1537), GFP-DdEB1Δ328C (17–1055), and GFP-DdEB1Δ281C (17–860). All fragments were cloned using SalI/NsiI sites. The N-terminal GFP fusion vector pA6PsgGFPXN was constructed by replacement of the actin15 promoter/polylinker/GFP cassette of the C-terminal GFP fusion vector p1ABsr8 (Gräf et al., 2000b) by an actin6 promoter/GFP/polylinker cassette.
For expression of the GFP-α-tubulin in Dictyostelium, the complete coding sequence of Dictyostelium-α-tubulin (EMBL data library accession no. L13999) was amplified by reverse transcription-PCR with SalI/BamHI-linker primers and Dictyostelium mRNA as a template. The fragment was cloned into pDiscGFPSSEB2 (Daunderer and Gräf, 2002).
Size Determination of Native MBP-DdEB1 Fusion Proteins
Purified bacterially expressed MBP-DdEB1 and its truncation mutants were analyzed by native gradient gel electrophoresis with 4–20% acrylamide precast gradient gels (Ready Gel; Bio-Rad, Munich, Germany).
Size exclusion chromatography by using a Superdex 200HR10/30 column (Amersham Biosciences, Freiburg, Germany) was performed in lysis buffer containing 1 mM maltose at a flow rate of 0.3 ml/min. The sample volume was 0.2 ml and the fraction volume was 0.75 ml each.
DdEB1/DdCP224 Coprecipitation Assays
Dictyostelium cytosolic extracts were prepared from MBP-DdEB1 or AX2 (wild-type) cells in lysis buffer containing 10% sucrose as described recently (Gräf, 2001). For coprecipitation of DdCP224, MBP-DdEB1–containing extracts were incubated for 20 min with 1/10 volume of amylose beads on a rotator. After washing with 10 volumes of lysis buffer the bound protein was eluted with 0.5× urea sample buffer (9 M urea, 10% SDS, and 5% 2-mercaptoethanol). Cell extracts from AX2 cells were used as negative control. Coprecipitation of DdEB1 was performed accordingly, but using extracts from wild-type cells and N-hydroxyl-succinimido–activated Sepharose 4B beads (Amersham Biosciences) covalently coupled to the anti-DdCP224 antibody 4/148 (Gräf et al., 1999). In this case anti-γ-tubulin–Sepharose beads coupled to rabbit antibodies with no affinity to DdCP224 or DdEB1 were used as a control for unspecific binding.
Construction of DdEB1Δ Mutants
The DdEB1 sequence from base position 85–1537 was amplified by PCR with KpnI/BamHI linker-primers and cloned into pSPORT1 (Invitrogen, Karlsruhe, Germany). After the HindIII site of pSPORT1 had been destroyed, the entire pSPORT1 sequence and the N- and C-terminal parts of DdEB1 were amplified by inverted PCR with XbaI/HindIII-linker primers, so that the sequence from base position 767–835 was deleted. The resulting PCR product was ligated with the Blasticidin resistance cassette obtained after XbaI/HindIII digestion of pUCBsrΔBam (Adachi et al., 1994). Before transformation, the DdEB1 knockout plasmid was cut with XbaI and HindIII, which increased homologous recombination efficiency. After transformation into Dictyostelium cells (strain AX2) the desired null mutants were screened for the absence of DdEB1 staining by immunofluorescence microscopy with the anti-EB1 antibody.
Light Microscopy
Indirect immunofluorescence microscopy and confocal light microscopy were performed as described previously (Gräf et al., 1998) using secondary anti-rabbit, anti-rat, and anti-mouse antibodies coupled to Alexa 488, Alexa 568 (Molecular Probes, Leiden, The Netherlands) or Cy3 (Jackson Laboratories, Bar Harbor, ME) dyes. DNA was stained either with 4,6-diamidino-2-phenylindole (wide field microscopy) or TOPRO3 (Molecular Probes; for confocal microscopy). Confocal microscopic image stacks were processed with the Huygens 2.2 deconvolution software (Bitplane AG, Zürich, Switzerland) by using a computed theoretical point spread function and the Maximum Likelihood Estimation algorithm. Z-projections of image stacks were made with the ImageJ program (National Institutes of Health, Bethesda, MD). Live cell imaging was performed under agar overlay (Fukui et al., 1987) at the PerkinElmer Ultraview real-time confocal system equipped with a 12-bit charge-coupled device camera, piezo stepper, and a 100×/1.3 lens.
Other Methods
Cultivation of Dictyostelium cells, transformation of Dictyostelium, and selection of clones was performed as described previously (Gräf et al., 2000b). Centrosome isolation, SDS gel electrophoresis, and Western blotting were carried out according to Gräf et al. (1998).
RESULTS
DdEB1 Is the Largest Member of the EB1 Family
A partial DNA sequence of a putative Dictyostelium homolog of human EB1 (DdEB1) was identified in the Dictyostelium genome project. This sequence was used as a probe for the isolation of a cDNA containing the complete coding sequence for DdEB1. EB1 proteins usually have a size of 35–38 kDa. With a calculated molecular mass of 57 kDa and a length of 506 amino acids, DdEB1 is the largest known member of the EB1 family. Among all family members it is most closely related to human and mouse EB1 (43% amino acid identity). Its remarkable size is due to several sequence insertions and a C-terminal extension (Figure 1).
Figure 1.
Alignment of the DdEB1 and human EB1 amino acid sequences. Top trace (Dd), DdEB1; bottom trace (Hs), human EB1. Identical amino acids are shaded in black, similar ones in gray. The coiled-coil domain predicted with the Coilscan program (Lupas et al., 1991) is underlined and italicized in both sequences.
DdEB1 Is a Genuine Centrosomal Component
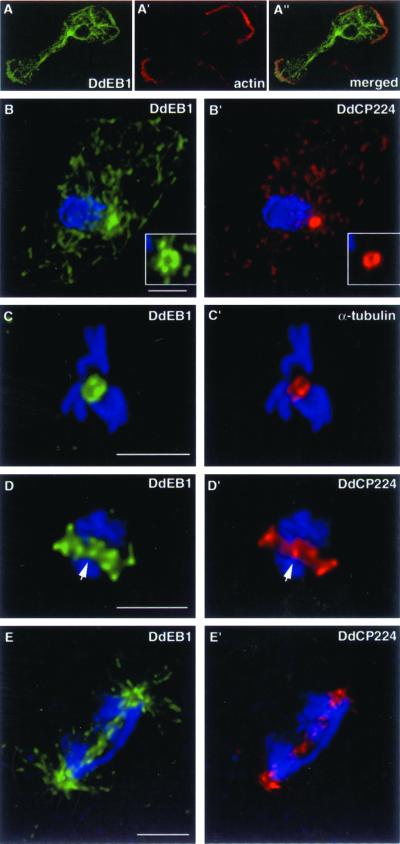
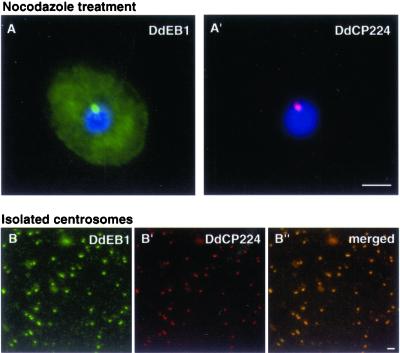
Polyclonal antibodies were raised against a bacterially expressed maltose-binding DdEB1 fusion protein (MBP-DdEB1), and used for subcellular localization of DdEB1 by immunofluorescence microscopy (Figure 2; the specificity of the antibodies for DdEB1 is shown in Figure 7A). DdEB1 was most prominent at microtubule tips and the centrosome, but it was also present along astral microtubules and weakly at the edges of protruding pseudopods where it colocalized with actin (Figure 2, A and B). During mitosis, it was found at the spindle, spindle poles (Figure 2, C–E), and in the kinetochore region (Figure 2D). These localizations were confirmed in mutants expressing DdEB1 fused to the GFP (Figure 4A). Confocal immunofluorescence microscopy revealed a ring-like appearance of the centrosome when wild-type cells were stained with anti-DdEB1 and anti-DdCP224 antibodies, respectively (Figure 2B). The Dictyostelium centrosome does not contain centrioles but consists of a three-layered core structure surrounded by a matrix called corona, which contains the microtubule nucleation sites (Daunderer et al., 1999). When stained with corona-specific antibodies, the corona appears as a ring in confocal sections (Gräf et al., 1998) (Figure 2, B and B′). Colocalization of DdEB1 with DdCP224, a permanent centrosomal resident and known component of the corona (Gräf et al., 2000b), suggested that DdEB1 could be an integral centrosomal component and part of the corona as well. Indeed, centrosomal localization of DdEB1 did not require an intact microtubule network because centrosomal DdEB1 localization was unaffected by treating the cells with 30 μM nocodazole for 2 h (Figure 3A). Furthermore, DdEB1 was present at isolated centrosomes, which are devoid of microtubules (Gräf et al., 1998) (Figure 3B).
Figure 2.
Cell cycle-dependent localization of DdEB1. DdEB1 (A) colocalizes with actin labeled with anti-Dictyostelium actin (Simpson et al., 1984) (A′) at protruding pseudopods. The merged image is displayed in A". The cell cycle stages shown are interphase (B), prophase (C), metaphase (D), and anaphase/telophase transition (E). Counterstaining was performed with the anti-DdCP224 monoclonal antibody 4/148 (B′, D′, and E′) (Gräf et al., 1999) or with the monoclonal anti-α-tubulin antibody YL1/2 (C′) (Chemicon, Hofheim, Germany). The arrows in D and D′ point to the kinetochore region, which is characterized by DdCP224 labeling. DNA (blue) was stained with TOPRO3. All images are maximum intensity projections of image stacks obtained by confocal microscopy and deconvolution, except A to A", and the insets in B and B′ show single confocal planes. The insets demonstrate the ring-like appearance of the interphase centrosome. Bar, 2 μm.
Figure 7.
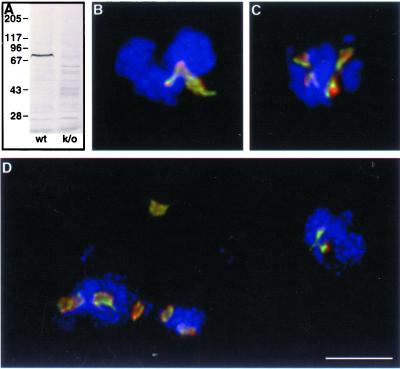
and Movie 2. Aberrant mitotic figures in DdEB1Δ mutants. The absence of DdEB1 in the knockout mutants (k/o) is shown in the Western blot (A), where cytosolic extracts of wild-type (wt) and DdEB1Δ mutants were probed with the anti-DdEB1 antibodies. The DdEB1Δ cell extract was prepared >2 mo after transformation. (B–D) Merged images of DdEB1Δ/GFP-α-tubulin mutants ∼2 wk after transformation of the DdEB1 knockout construct. The nuclei of a single cell are shown in each image. GFP fluorescence is shown in green, anti-DdCP224 staining for centrosomes in red, and DNA staining by TOPRO3 in blue. The images are maximum intensity projections of image stacks obtained by confocal microscopy and deconvolution. Bar, 2 μm. Movie 2 shows an animation of the deconvoluted confocal sections of the projection shown in D.
Figure 4.
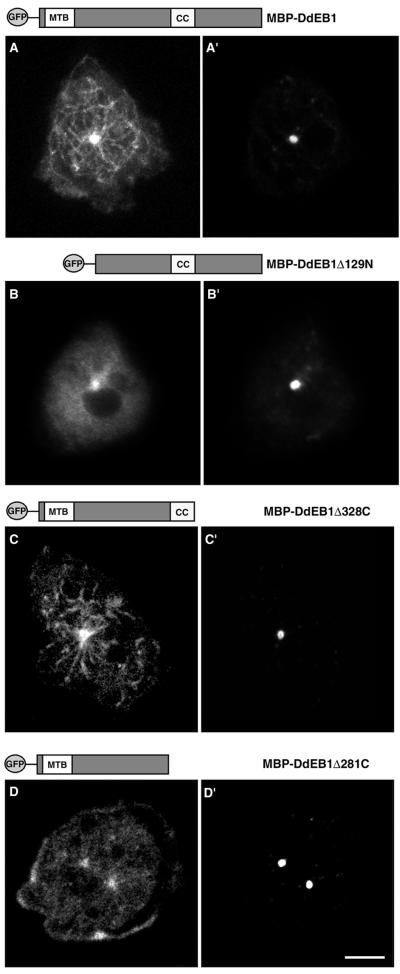
Microtubule binding of DdEB1 requires the presence of the coiled-coil domain. DdEB1 and its deletion mutants were expressed as GFP-fusion proteins. (A–D) GFP fluorescence; a scheme of the respective mutant is shown at the top of each panel. (A′–D′) Centrosomes were counterstained with the anti-DdCP224 monoclonal antibody 4/148. The cell shown in D is dinucleated. Such cells are not unusual in axenically grown Dictyostelium cultures and occur at the same frequency in the mutant as in wild-type cells. MTB, microtubule-binding domain; CC, coiled-coil. Bar, 2 μm.
Figure 3.
Centrosomal localization of DdEB1 does not require microtubules. (A) Treatment of wild-type cells with 30 μM nocodazole for 2 h did not reduce centrosomal labeling with DdEB1 antibodies. (B) DdEB1 is also present at isolated centrosomes. The centrosomes were counterstained with the anti-DdCP224 monoclonal antibody 4/148 (A′ and B′). DNA was stained with 4,6-diamidino-2-phenylindole (blue). Bar, 2 μm.
Microtubule Binding Requires the Coiled-Coil Domain
The structural determinants for microtubule binding of DdEB1 were analyzed with GFP-tagged deletion mutants expressed in Dictyostelium. Recently, Juwana et al. (1999) suggested a microtubule-binding domain residing within the highly conserved N-terminal half of human EB1, which was narrowed down to amino acids 79–134 by sequence comparison between several EB1 homologs. This microtubule-binding domain was confirmed in Dictyostelium because the N-terminal DdEB1 deletion mutant (DdEB1Δ129N), where the first 128 amino acids were missing, did not localize to microtubules or microtubule tips anymore, whereas it was still present at the centrosome (Figure 4B). In contrast, C-terminal deletions downstream from the coiled-coil domain predicted for all known EB1 family members (DdEB1Δ328C) had no effect on any of the DdEB1 localizations (Figure 4C). However, when the coiled-coil region was deleted as well (DdEB1Δ281C) the GFP fusion was neither present along microtubules nor at their tips, whereas it was still present at the centrosome and at the cell edges (Figure 4D). This suggested that the coiled-coil domain also contributes to the association of DdEB1 with microtubules. Because coiled-coil domains mediate intermolecular interactions, our findings indicate that microtubule binding requires the association of DdEB1 with a second DdEB1 molecule or a different binding partner. The latter possibility is rather unlikely, because EB1 proteins are known to bind directly to microtubules (Schwartz et al., 1997; Juwana et al., 1999). Thus, the requirement of the coiled-coil domain for homo-oligomerization was tested with DdEB1 deletion mutants that were expressed as MBP fusion proteins in E. coli and corresponded to the GFP mutants described above. Native gradient gel electrophoresis revealed that highly purified full-length MBP-DdEB1 (∼100 kDa) and the N-terminally truncated MBP-DdEB1Δ129N (∼85-kDa) mutant behaved like homotetramers on these gels, with apparent molecular masses of ∼400 and ∼340 kDa, respectively (Figure 5A). In contrast, the C-terminally truncated MBP-DdEB1Δ281C mutant (∼73 kDa), where the C-terminal domain including the coiled-coil was deleted, exhibited the electrophoretic mobility of a monomer (Figure 5A). These results were confirmed by size exclusion chromatography where MBP-DdEB1 fractionated with a molecular mass of ∼500 kDa (Figure 5B). As a control for the native state of the bacterially expressed proteins, DdEB1 was expressed as an MBP fusion protein in Dictyostelium (Gräf, 2001). The fusion protein had the same localization pattern as endogenous DdEB1 and the same elution behavior in size exclusion chromatography as the bacterially expressed protein (our unpublished data).
Figure 5.
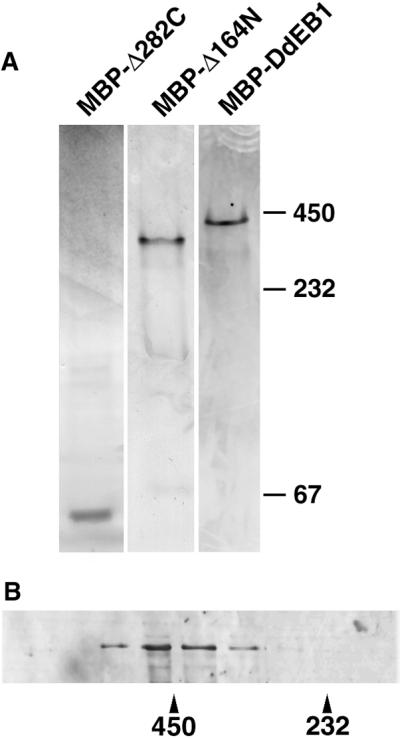
Coiled-coil domain is required for homo-oligomerization. (A) Sizing of MBP-DdEB1 and its deletion mutants by native polyacrylamide gradient gel electrophoresis. (B) Sizing of MBP-DdEB1 by size exclusion chromatography. Numbers refer to the molecular masses in kilodaltons.
DdEB1 Interacts with DdCP224
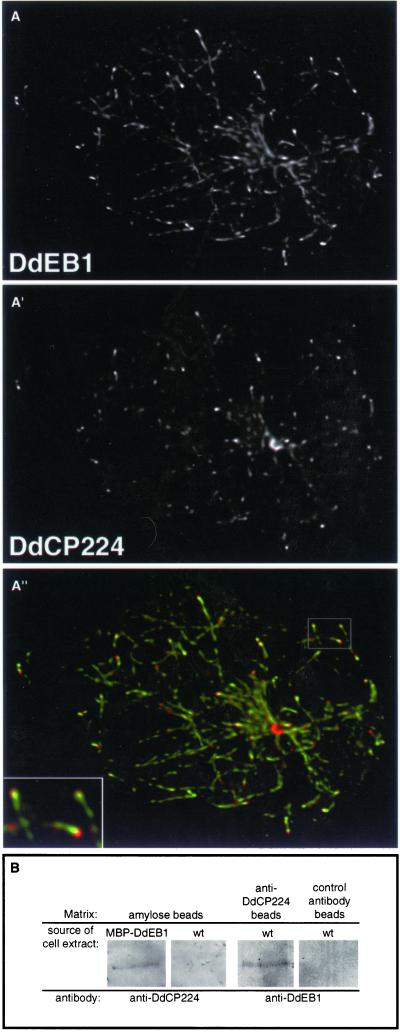
So far, EB1 and XMAP215 homologs were not colocalized at microtubule tips, although both proteins are believed to regulate microtubule dynamics at the microtubule plus ends (see INTRODUCTION). Herein, we show that DdCP224 and DdEB1 clearly colocalized at the microtubule tips in immunofluorescence preparations of Dictyostelium cells stained with anti-DdEB1 antibodies and a monoclonal anti-DdCP224 antibody (Figure 6A and Movie 1). Moreover, using cytosolic extracts from the Dictyostelium mutant expressing MBP-DdEB1, we could specifically coprecipitate DdCP224 with MBP-DdEB1 bound to amylose beads. Vice versa, using wild-type cell cytosolic extracts, DdEB1 was coprecipitated with DdCP224 that was immobilized on beads coated with the anti-DdCP224 monoclonal antibody (Figure 6B). Because we have already shown that most of the cytosolic DdCP224 behaves as a monomer in density gradient centrifugation experiments (Gräf et al., 2000b), only a small fraction of DdCP224 and DdEB1 was expected to be part of the same cytosolic protein complex. Consequently, we could coprecipitate only small amounts of DdCP224 with DdEB1 and vice versa. However, the mock precipitations with control beads proved that the direct or indirect interaction of the cytosolic forms of DdCP224 and DdEB1 was specific.
Figure 6.
and Movie 1. DdEB1 interacts and colocalizes with DdCP224. (A–A") Colocalization of DdEB1 and DdCP224 at microtubule tips. Wild-type (AX2) Dictyostelium cells were stained with anti-DdEB1 antibodies (A) and the monoclonal anti-DdCP224 antibody 4/148 (A′). The inset in the merged image (A") is a magnification of the area indicated in the upper right part of the image. The images are maximum intensity projections of image stacks obtained by confocal microscopy and deconvolution. Movie 1 shows an animation of the deconvoluted confocal sections of the projection shown in A". (B) Coprecipitation of DdEB1 and DdCP224 from cytosolic Dictyostelium extracts from MBP-DdEB1 mutants or wild-type cells. Coprecipitates were loaded onto 12.5% acrylamide gels and blotted after electrophoresis. The matrix used for precipitation is given on the top and the antibodies used for Western blot staining are indicated on the bottom.
Prometaphase Progression Is Retarded in DdEB1Δ Mutants
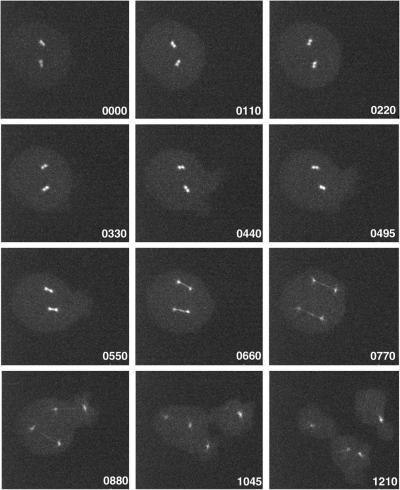
To investigate the cellular functions of DdEB1, we have created a DdEB1 null mutant (DdEB1Δ) by homologous recombination. The DdEB1 gene disruption was very efficient because the homologous integration occurred in >80% of all transformants. DdEB1Δ mutants were easily recognized by the lack of DdEB1 staining in immunofluorescence microscopy (our unpublished data) and immunoblots of cytosolic extracts (Figure 7A). DdEB1Δ mutants were viable and showed normal development of fruiting bodies, but frequently displayed disordered mitotic figures. Many mitotic cells were in a prophase- or prometaphase-like stage characterized by condensed but unsegregated chromosomes (Figure 7). Usually, mitotic spindles were deformed (Figure 7B) or lacking (Figure 7D and Movie 2). Often nuclei were associated with more than one duplicated centrosome (Figure 7, C and D, and Movie 2), and the mutants showed signs of aneuploidy such as additional, small cytosolic DNA masses (Figure 7D and Movie 2). With continuous cultivation, the proportion of cells with unusual mitotic figures decreased and there were more and more cells significantly retarded in prometaphase progression. This was observed in three independent transformations. After approximately 2 mo the DdEB1Δ phenotype was stable, and there was still no DdEB1 detectable in these cells (Figure 7A). The percentage of prometaphase cells among all mitotic DdEB1Δ cells was increased by a factor of three compared with untransformed cells (Table 1). As expected, the generation time of DdEB1Δ mutants was increased from ∼8 to ∼12 h. These results were confirmed by live cell analysis of DdEB1Δ cells expressing GFP-α-tubulin. The expression of GFP-α-tubulin in wild-type cells does not affect mitotic progression (Neujahr et al., 1998; Kimble et al., 2000). However, in GFP-α-tubulin/DdEB1Δ mutants, prometaphase often lasted longer than 8 min compared with the usual ∼2 min (Ueda et al., 1999), before the spindle poles started to separate and a spindle was formed (Figure 8 and Movie 3). However, spindle elongation was unaffected in DdEB1Δ mutants, because once a spindle was formed, its elongation proceeded at normal speed.
Table 1.
Percentage of prometaphase cells is increased in DdEB1Δ mutants
| Prophase | Prometaphase | Metaphase | Anaphase | Telophase | |
|---|---|---|---|---|---|
| DdEB1Δ (n = 109) | 3 | 37 | 27 | 7 | 27 |
| Wild-type (n = 120) | 3 | 13 | 43 | 4 | 38 |
Percentage of cells in the individual mitotic stages among all mitotic cells in a logarithmically growing culture of wild-type or DdEB1Δ cells, respectively, after 2 mo of cell culture. The distribution was almost identical when DdEB1Δ/GFP-α-tubulin mutants were compared with GFP-α-tubulin cells (our unpublished data).
Figure 8.
and Movie 3. DdEB1Δ mutants are retarded in prometaphase progression. Live cell observation of a dinucleated DdEB1Δ/GFP-α-tubulin cell by four-dimensional confocal microscopy. The time is indicated in seconds. Prometaphase lasts for >8 min before a spindle is formed and starts to elongate. The time-lapse movie is an animation of brightest point projections of z-stacks consisting of three confocal slices with a distance of 0.5 μm each. Z-stacks were recorded using 2 × 2 binning, a time delay of 2.5 s between each stack and an exposure 500 ms/frame.
DISCUSSION
Similarly to the other members of the EB1 protein family, DdEB1 is a microtubule-associated protein concentrated at the microtubule tips. However, DdEB1 is exceptional because it is almost twice as long as all other EB1 homologs and also a genuine centrosomal component. The amino acid sequence alignment with human EB1 reveals that the first 122 amino acids, including the putative microtubule-binding domain (Juwana et al., 1999), are highly conserved. The remaining DdEB1 sequence is less conserved but contains a coiled-coil domain, which was predicted for all EB1 proteins. The sequence insertions of DdEB1 could be important for the striking microtubule-independent centrosomal association of DdEB1, which was not observed in case of the smaller human EB1 protein (Morrison et al., 1998). Although deletion of the N-terminal microtubule-binding domain as well as truncation of the C-terminal domain, including the coiled-coil, diminished microtubule binding of DdEB1, the mutant proteins still localized to the centrosome. Thus, centrosomal binding of DdEB1 seems to be independent of homo-oligomerization and the centrosomal targeting domain is likely to reside within the sequence shared by the N- and C-terminal deletion mutants (amino acids 129–281). Our DdEB1 deletion mutants demonstrated that the N-terminal microtubule-binding domain is not the only determinant for the association of DdEB1 with microtubules. The two green fluorescent deletion mutants GFP-DdEB1Δ328C and GFP-DdEB1Δ281C, which differ only in the presence of the predicted coiled-coil domain, revealed that the C-terminal part downstream from the coiled-coil domain is not required for any of the DdEB1 localizations, whereas the coiled-coil domain is essential for microtubule binding. Our studies with recombinant MBP-DdEB1 suggested that the coiled-coil domain promotes formation of a DdEB1 homo-oligomer (presumably a tetramer). This oligomerization seems to be a prerequisite for microtubule binding in vivo. The intermolecular interaction between the single DdEB1 chains may be similar to other homotetrameric coiled-coil proteins such as the bcr protein, the cartilage matrix protein, or the viral NSP4 protein (McWhirter et al., 1993; Taylor et al., 1996; Beck et al., 1997). The presence of the coiled-coil domain in all EB1 proteins indicates that homo-oligomerization may generally be required for binding of EB1 to microtubules.
Despite its capacity to bind directly to microtubules (Juwana et al., 1999), it is possible that other binding partners such as XMAP215 family members mediate the presence of EB1 at microtubule tips, kinetochores, and the centrosome (Ohkura et al., 2001). The XMAP215 homolog in Dictyostelium, DdCP224, was originally identified as a centrosomal component (Gräf et al., 2000b). Herein, we could show for the first time colocalization of members of the XMAP215 and EB1 families of microtubule-associated proteins at microtubule tips, the centrosome, and the kinetochore region. This may indicate that both proteins act together within the same protein complex. Indeed, yeast EB1 (Bim1p) was found as an interactor of Stu2p (XMAP215 in yeast), in a two-hybrid screen (Chen et al., 1998), but so far this interaction could not be verified by biochemical means and it cannot be excluded that it is indirect. Although most of the cytosolic fraction of DdCP224 seems to be monomeric (Gräf et al., 2000b), we could show coprecipitation of a fraction of cytosolic DdCP224 with DdEB1. The absence of a prevailing cytosolic DdEB1/DdCP224 complex does not rule out a direct interaction of both proteins at microtubule tips or at the centrosome because both proteins could assemble to these localizations subsequently. We also cannot exclude the involvement of further binding partners in an indirect DdEB1–DdCP224 interaction. So far, both proteins failed to interact in a yeast two-hybrid assay (our unpublished data). Moreover, confocal microscopy images revealed that the localizations of the two proteins do not overlap exactly. DdCP224 is localized a bit farther distal from DdEB1 at both ends of microtubules, i.e., closer to the cell cortex at the microtubule tips and closer to the centrosomal core at the centrosome. These data suggest that DdCP224 and DdEB1 are linked together by at least one additional binding partner. Potential candidates are the dynein and dynactin subunits, which coimmunoprecipitated with EB1 (Berrueta et al., 1999). These interactions were confirmed in yeast, where mutations in BIM1 were synthetically lethal with deletions in certain dynein (DHC1) and dynactin (ACT5) genes (Muhua et al., 1998), and in Dictyostelium, where the dynein IC (Ma et al., 1999) also coprecipitated with MBP-DdEB1 (our unpublished data). DdEB1 interactions with dynein/dynactin could also explain its colocalization with actin at protruding pseudopods because dynactin subunits have been shown to interact with the cortical actin cytoskeleton (Garces et al., 1999; Goode et al., 2000).
The phenotype of DdEB1Δ mutants indicated that DdEB1 fulfills its main function in mitosis because the lack of DdEB1 caused a significant retardation of prometaphase progression. In Dictyostelium, prometaphase is characterized by the formation of the mitotic spindle whose elongation separates the two spindle poles (Ueda et al., 1999). The defect in prometaphase progression of DdEB1Δ mutants strongly reminded of Dictyostelium cells incubated with microtubule-depolymerizing drugs. This treatment causes a block of spindle formation and prometaphase progression, whereas centrosome duplication, which takes place in prophase, is unaffected (Welker and Williams, 1980; Kitanishi et al., 1984). Due to the lack of a spindle checkpoint in Dictyostelium (Welker and Williams, 1980; Ma et al., 1999), the cells proceed to the next cell cycle, and thus giant cells with multiple nuclei or huge, aberrantly shaped nuclei are produced (Kitanishi et al., 1984; Kitanishi-Yumura et al., 1985). Because centrosome duplication is independent of microtubules, the nuclei in such cells are often associated with more than one duplicated, but unseparated centrosome as in DdEB1Δ mutants. The DdEB1Δ phenotype was most severe in cells viewed as early as possible after transformation and selection for transformants (i.e., after ∼2 wk). In a considerable fraction of these cells, spindle formation seemed to be blocked completely. It is likely that a strong selective pressure against these severe aberrations caused a partial compensation of these defects upon prolonged cultivation of these mutants, presumably by up- or down-regulation of other proteins that are involved in the same pathway. After 2 mo the DdEB1Δ phenotype was stable. Aberrant mitotic figures were only rarely encountered, and spindle formation was not blocked anymore but still significantly retarded. However, once spindle elongation has started, the kinetics of elongation was normal in these cells. Taken together, DdEB1 assists in formation but not elongation of the mitotic spindle. Thus, DdEB1Δ mutants were similar to yeast bim1Δ mutants where formation of the preanaphase bipolar spindle was delayed, whereas spindle elongation was unaffected (Schwartz et al., 1997; Muhua et al., 1998). bim1Δ mutants were also characterized by shorter, less dynamic microtubules (Tirnauer et al., 1999). In contrast, the interphase microtubule cytoskeleton seemed normal in DdEB1Δ/GFP-α-tubulin mutants. Compared with GFP-α-tubulin cells (Neujahr et al., 1998; Kimble et al., 2000), there were no obvious differences in length, distribution, and dynamics of astral microtubules in interphase. In Dictyostelium, the astral microtubules have a relatively constant length (Kimble et al., 2000) and show only little plus end dynamics. They are lost in prophase and most, if not all interphase microtubules of the later daughter cells start to grow out in metaphase (Roos et al., 1984; Ueda et al., 1999), i.e., after the prometaphase block in microtubule outgrowth of DdEB1Δ mutants has been overcome. Consequently, obvious defects of the interphase microtubule cytoskeleton of DdEB1Δ mutants were not expected, because DdEB1 does not seem to play a major role in microtubule elongation. Although DdEB1 was localized to microtubule tips, the formation of a normal astral microtubule network in DdEB1Δ mutants argues against an essential role of DdEB1 in capturing of microtubule plus ends at cortical sites. This is supported by the observation that DdEB1Δ mutants undergo normal development of fruiting bodies, indicating that the absence of DdEB1 does not affect amoeboid cell motility and chemotaxis. Both processes are thought to require an intact microtubule cytoskeleton where most of the microtubule tips are tethered to the cell cortex (Ueda et al., 1997). The strong centrosomal presence of DdEB1 could reflect an additional centrosomal function that remains to be identified. However, DdEB1Δ mutants exhibited no centrosomal defects that cannot be explained by the defect in spindle formation alone. Thus, the centrosome could simply serve as a source of DdEB1 for its distribution to the outgrowing plus ends. Because GFP-α-tubulin was always present at prometaphase spindle poles (Figure 8 and Movie 3), DdEB1 acts downstream from the recruitment of the first α/β-tubulin dimers to the nascent spindle poles, which are thought to be initiated by γ-tubulin complexes. Taken together, our data suggest that the main function of DdEB1 is the initiation of microtubule growth and not microtubule elongation.
Supplementary Material
ACKNOWLEDGMENTS
We deeply acknowledge Thi-Hieu Ho for expert technical assistance and Manfred Schliwa for critical comments and the opportunity to work together. We are also grateful to Rainer Pepperkok and everyone at the Advanced Light Microscopy Facility at the EMBL in Heidelberg for the use of the real-time confocal microscope. Furthermore, we thank Andrea Hestermann, Alexandra Lepier, Jan Faix, and Manfred Schliwa for critically reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 413) and the Friedrich-Baur-Stiftung.
Footnotes
Online version of this article contains video material for some figures. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0054. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0054.
REFERENCES
- Adachi H, Hasebe T, Yoshinaga K, Ohta T, Sutoh K. Isolation of Dictyostelium discoideum cytokinesis mutants by restriction enzyme-mediated integration of the blasticidin S resistance marker. Biochem Biophys Res Commun. 1994;205:1808–1814. doi: 10.1006/bbrc.1994.2880. [DOI] [PubMed] [Google Scholar]
- Beck K, Gambee JE, Kamawal A, Bachinger HP. A single amino acid can switch the oligomerization state of the α-helical coiled-coil domain of cartilage matrix protein. EMBO J. 1997;16:3767–3777. doi: 10.1093/emboj/16.13.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinhauer JD, Hagan IM, Hegemann JH, Fleig U. Mal3, the fission yeast homologue of the human APC-interacting protein EB-1 is required for microtubule integrity and the maintenance of cell form. J Cell Biol. 1997;139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- Chen XP, Yin H, Huffaker TC. The yeast spindle pole body component Spc72p interacts with Stu2p and is required for proper microtubule assembly. J Cell Biol. 1998;141:1169–1179. doi: 10.1083/jcb.141.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunderer C, Gräf R. Molecular analysis of the cytosolic Dictyostelium γ-tubulin complex. Eur J Cell Biol. 2002;81:175–184. doi: 10.1078/0171-9335-00241. [DOI] [PubMed] [Google Scholar]
- Daunderer C, Schliwa M, Gräf R. Dictyostelium discoideum: a promising centrosome model system. Biol Cell. 1999;91:313–320. doi: 10.1016/s0248-4900(99)80092-6. [DOI] [PubMed] [Google Scholar]
- Fodde R, et al. Mutations in the APC tumor suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Yumura S, Yumura TK. Agar-overlay immunofluorescence: high resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987;28:347–356. doi: 10.1016/s0091-679x(08)61655-6. [DOI] [PubMed] [Google Scholar]
- Garces JA, Clark IB, Meyer DI, Vallee RB. Interaction of the p62 subunit of dynactin with Arp1 and the cortical actin cytoskeleton. Curr Biol. 1999;9:1497–1500. doi: 10.1016/s0960-9822(00)80122-0. [DOI] [PubMed] [Google Scholar]
- Gard DL, Kirschner MW. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol. 1987;105:2203–2215. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Gräf R. Maltose-binding protein as a fusion tag for the localization and purification of cloned proteins in Dictyostelium. Anal Biochem. 2001;289:297–300. doi: 10.1006/abio.2000.4961. [DOI] [PubMed] [Google Scholar]
- Gräf R, Brusis N, Daunderer C, Euteneuer U, Hestermann A, Schliwa M, Ueda M. Comparative structural, molecular and functional aspects of the Dictyostelium discoideum centrosome. Curr Top Dev Biol. 2000a;49:161–185. doi: 10.1016/s0070-2153(99)49008-8. [DOI] [PubMed] [Google Scholar]
- Gräf R, Daunderer C, Schliwa M. Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol Cell. 1999;91:471–477. doi: 10.1111/j.1768-322x.1999.tb01102.x. [DOI] [PubMed] [Google Scholar]
- Gräf R, Daunderer C, Schliwa M. Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J Cell Sci. 2000b;113:1747–1758. doi: 10.1242/jcs.113.10.1747. [DOI] [PubMed] [Google Scholar]
- Gräf R, Euteneuer U, Ueda M, Schliwa M. Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur J Cell Biol. 1998;76:167–175. doi: 10.1016/S0171-9335(98)80031-9. [DOI] [PubMed] [Google Scholar]
- Juwana JP, et al. EB/RP gene family encodes tubulin binding proteins. Int J Cancer. 1999;81:275–284. doi: 10.1002/(sici)1097-0215(19990412)81:2<275::aid-ijc18>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kessin RH. Dictyostelium: Evolution, Cell Biology, and the Development of Multicellularity. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Kimble M, Kuzmiak C, McGovern KN, de Hostos EL. Microtubule organization and the effects of GFP-tubulin expression in Dictyostelium discoideum. Cell Motil Cytoskeleton. 2000;47:48–62. doi: 10.1002/1097-0169(200009)47:1<48::AID-CM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kitanishi T, Shibaoka H, Fukui Y. Disruption of microtubules and retardation of development of Dictyostelium with ethyl N-phenylcarbamate and thiabendazole. Protoplasma. 1984;120:185–196. [Google Scholar]
- Kitanishi-Yumura T, Blose SH, Fukui Y. Role of the MT-MTOC complex in determination of the cellular locomotory unit in Dictyostelium. Protoplasma. 1985;127:133–146. [Google Scholar]
- Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- Lupas A, VanDyke M, Stock J. Predicting coiled-coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Ma S, Trivinos Lagos L, Gräf R, Chisholm RL. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J Cell Biol. 1999;147:1261–1274. doi: 10.1083/jcb.147.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter JR, Galasso DL, Wang JY. A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins. Mol Cell Biol. 1993;13:7587–7595. doi: 10.1128/mcb.13.12.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumor suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Muhua L, Adames NR, Murphy MD, Shields CR, Cooper JA. A cytokinesis checkpoint requiring the yeast homologue of an APC-binding protein. Nature. 1998;393:487–491. doi: 10.1038/31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr R, Albrecht R, Kohler J, Matzner M, Schwartz JM, Westphal M, Gerisch G. Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J Cell Sci. 1998;111:1227–1240. doi: 10.1242/jcs.111.9.1227. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Garcia MA, Toda T. Dis1/TOG universal microtubule adaptors - one MAP for all? J Cell Sci. 2001;114:3805–3812. doi: 10.1242/jcs.114.21.3805. [DOI] [PubMed] [Google Scholar]
- Pellman D. Cancer. A CINtillating new job for the APC tumor suppressor. Science. 2001;291:2555–2556. doi: 10.1126/science.1057337. [DOI] [PubMed] [Google Scholar]
- Roos UP, De Brabander M, De Mey J. Indirect immunofluorescence of microtubules in Dictyostelium discoideum. A study with polyclonal and monoclonal antibodies to tubulins. Exp Cell Res. 1984;151:183–193. doi: 10.1016/0014-4827(84)90367-7. [DOI] [PubMed] [Google Scholar]
- Schroer TA. Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr Opin Cell Biol. 2001;13:92–96. doi: 10.1016/s0955-0674(00)00179-4. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Richards K, Botstein D. BIM1 encodes a microtubule-binding protein in yeast. Mol Biol Cell. 1997;8:2677–2691. doi: 10.1091/mbc.8.12.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PA, Spudich JA, Parham P. Monoclonal antibodies prepared against Dictyostelium actin: characterization and interactions with actin. J Cell Biol. 1984;99:287–295. doi: 10.1083/jcb.99.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Taylor JA, O'Brien JA, Yeager M. The cytoplasmic tail of NSP4, the endoplasmic reticulum-localized non-structural glycoprotein of rotavirus, contains distinct virus binding and coiled-coil domains. EMBO J. 1996;15:4469–4476. [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, O'Toole E, Berrueta L, Bierer BE, Pellman D. Yeast Bim1p promotes the G1-specific dynamics of microtubules. J Cell Biol. 1999;145:993–1007. doi: 10.1083/jcb.145.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize R, et al. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat Cell Biol. 2000;2:13–19. doi: 10.1038/71330. [DOI] [PubMed] [Google Scholar]
- Ueda M, Gräf R, MacWilliams HK, Schliwa M, Euteneuer U. Centrosome positioning and directionality of cell movements. Proc Natl Acad Sci USA. 1997;94:9674–9678. doi: 10.1073/pnas.94.18.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Schliwa M, Euteneuer U. Unusual centrosome cycle in Dictyostelium: correlation of dynamic behavior and structural changes. Mol Biol Cell. 1999;10:151–160. doi: 10.1091/mbc.10.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RJ, Gard DL, Cassimeris L. XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J Cell Biol. 1994;127:985–993. doi: 10.1083/jcb.127.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker DL, Williams KL. Mitotic arrest and chromosome doubling using thiabendazole, cambendazole, nocodazole, and ben late in the slime mold Dictyostelium discoideum. J Gen Microbiol. 1980;116:397–407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.