Abstract
Background
Rhabdomyolysis is frequently associated with acute kidney injury (AKI). Due to the nephrotoxic properties of myoglobin, its rapid removal is relevant. If kidney replacement therapy (KRT) is necessary for AKI, a procedure with effective myoglobin elimination should be preferred. This pilot trial was designed to compare different KRT modes that enable myoglobin elimination.
Methods
In this prospective randomized single-center study, 15 patients with rhabdomyolysis and severe AKI requiring KRT were randomized 1:1:1 into three groups: continuous veno-venous hemofiltration (CVVH), continuous veno-venous hemodialysis (CVVHD) using a high cut-off dialyzer (CVVHD-HCO), or CVVHD using a high-flux dialyzer in combination with the adsorber CytoSorb (CVVHD-CS). Concentrations of serum myoglobin, urea, creatinine, β2-microglobulin, interleukin-6, and albumin were measured before and after the dialyzer 1, 6, 12, and 24 h after initiating KRT.
Results
There was no significant difference in the median myoglobin clearance between the KRT modes during the 24-h study period. Nevertheless, the CVVHD-CS group showed a significantly higher myoglobin elimination compared to the other modes in the first hours of treatment. However, as a greater decline in clearance performance was observed over time, no better performance was detected over the whole study period. Simulation of different device combinations showed the highest myoglobin clearance for CVVHD-HCO combined with CS with a 12-hourly adsorber exchange interval.
Conclusions
All tested modes showed an effective myoglobin elimination capacity. The time-dependent elimination performance could be further increased by combining KRT with more frequent adsorber exchange.
Trial registration
German Clinical Trials Registry (DRKS00023998); date of registration 03/03/2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-025-03945-3.
Keywords: Myoglobin clearance, Rhabdomyolysis, Acute kidney injury (AKI), High cut-off dialyzer, Adsorber, CytoSorb, Continuous veno-venous hemodialysis (CVVHD), Continuous veno-venous hemofitration (CVVH)
Background
The association between crush syndrome following trauma and acute kidney injury (AKI) was first described in 1941 with muscle necrosis identified as the cause [1]. In addition to trauma, muscle hypoxia and overexertion, infections, autoimmune diseases, metabolic and electrolyte disorders, poisoning, drugs, hypo- and hyperthermia as well as genetic defects can trigger rhabdomyolysis (RM) [2–4]. Damaged skeletal muscle cells release intracellular contents, including myoglobin. These in turn result in damage to the kidneys by several mechanisms: Firstly, the formation of free radicals increases the oxidative stress of the renal tubular epithelial cells. Secondly, vasoconstriction due to cytokine release from the damaged muscles and volume depletion after trauma lead to renal hypoperfusion. Thirdly, myoglobin forms casts that obstruct the renal tubules and collecting ducts [2, 4–6].
About 5–25% of AKI cases are associated with RM [2, 5]. Harmful serum myoglobin levels correlate with the severity of AKI, with high mortality rates reported in patients with RM [7–9]. Therefore, rapid removal of myoglobin from the circulation can be useful in terms of preventing kidney damage.
If kidney function is preserved, myoglobin can be eliminated with forced diuresis and urin alkalinization [3, 4]. However, high-volume fluid therapy also appears to be unfavorable [10]. In oligo-anuric AKI, serum myoglobin can only be eliminated with kidney replacement therapy (KRT). Since myoglobin has a molecular weight of 17.1 kDa, sufficient middle molecule clearance is required. Effective myoglobin clearance was demonstrated with continuous veno-venous hemofiltration (CVVH) [11]. Subsequent animal experiments showed that early CVVH can reduce renal damage in RM [12]. Accordingly, this mode has been suggested as the method of choice [2, 4].
Newer KRT modes that enable effective myoglobin elimination, such as continuous veno-venous hemodialysis (CVVHD) using a high cut-off (HCO) dialyzer [13–16] and CVVHD in combination with an adsorber, have been investigated during the last years [15–19].
Based on these observations regarding the impact of myoglobin on renal function and the various modes available for its clearance, this pilot study aimed to compare the myoglobin elimination performance of different KRT modes. For this purpose, CVVH, CVVHD-HCO and CVVHD-CS were compared in patients suffering from RM and requiring KRT for severe AKI.
Methods
Study design
This study is a prospective, randomized, single-blinded and single-center pilot trial. It was registered at the German trial registry (DRKS00023998) and approved by the local ethics committee (ethics committee at the medical faculty, University Leipzig, 361/20-ek) and adheres to CONSORT guidelines (Additional file 1).
We planned to enroll 15 critically ill adult medical patients managed in the medical intensive care unit (ICU) of the University Hospital Leipzig. A written informed consent was obtained from all patients or their legal guardians.
Allocation concealment and unrestricted randomization were guaranteed by using 15 sequentially numbered, opaque sealed envelopes to avoid the possibility to decipher the allocation arm, with five patients planned for each intervention arm. The 15 envelopes were then thoroughly mixed and marked with unique numbers, and then kept in a container [20]. For technical reasons, only patients were blinded to the treatment arm. Enrollment of participants, randomization and assignment to interventions were carried out by trained medical staff.
The study received funding from CytoSorbents Europe, 12587 Berlin, Germany.
Patients
Patients with severe AKI and indication for KRT (based on the recommendations of the Kidney Disease, Improving Global Outcomes [21]) as well as RM with serum myoglobin > 4000 µg/l were included. Exclusion criteria were refusal of study participation, participation in other trials, age < 18 years, pregnancy and lactation, futility of ICU treatment due to end-stage disease or expected death within the next 24 h, preexisting chronic kidney disease with glomerular filtration rate < 15 ml/min/1.73m2 (CKD G5), contraindications for systemic anticoagulation with unfractionated heparin.
All patients admitted to the ICU during the study period were screened for eligibility. The screening ended after the inclusion of 15 patients randomized to one of the following treatment groups:
CVVH with high-flux dialyzer
CVVHD with HCO dialyzer
CVVHD with high-flux dialyzer and additional adsorber CytoSorb®
CVVH with high-flux dialyzer was defined as the control mode, because this has been the traditional procedure of choice in the literature [2, 4].
Data collection
Demographic and clinical data were collected at the time of initiating KRT. These included age, gender, height, weight, body mass index (BMI), diuresis, presence of AKI stage 3, serum creatinine and estimated glomerular filtration rate according the CKD-EPI equation [22], serum urea, blood gas analysis, serum electrolytes, hemoglobin, platelet and leucocyte count, creatinine kinase, myoglobin, Acute Physiology And Chronic Health Evaluation (APACHE)-II score [23]), Sequential Organ Failure Assessment (SOFA) score [24], mean arterial blood pressure, need for mechanical ventilation, need for vasopressors, diagnosis of sepsis according to the sepsis-3 criteria [25], comorbidities, causes of rhabdomyolysis and indications for KRT.
Outcome and safety parameters were taken from electronic patient records or generated through telephone calls to patients, legal representatives or managing physicians.
Procedure
A 13 French double-lumen high-flow catheter (Achim Schulz-Lauterbach VMP, Iserloh, Germany) was inserted as a central venous access for KRT. All KRT modes were performed using MultiFiltrate® with bicarbonate-buffered replacement fluid (multiBic® K4 or K2) or dialysate (CiCa® dialysate K4 or K2) (both: Fresenius medical care, Bad Homburg, Germany). The following dialysers were used: In CVVH, postdilution with a high-flux dialyzer (Ultraflux AV1000S; Fresenius Medical Care, Bad Homburg, Germany), in CVVHD-HCO the high-cut-off dialyzer (Ultraflux EMiC2, Fresenius Medical Care, Bad Homburg, Germany), and in CVVHD-CS, the same high-flux dialyzer together with the adsorber CytoSorb® (CytoSorbents Europe GmbH, Berlin, Germany). The adsorber was integrated into the extracorporeal circuit before the dialyzer. The anticoagulation of the extracorporeal circuit was performed in CVVHD-HCO and CVVHD-CS with regional citrate anticoagulation (RCA) and in CVVH using systemic anticoagulation with unfractionated heparin. Heparin was started at a dose of 18 IU/kg/h (ideal body weight) and adjusted during the treatment period based on the activated partial thromboplastin time (aPTT) monitored twice daily. The target aPTT was set at 60 s. RCA in CVVHD-HCO and CVVHD-CS was monitored by measuring ionized postfilter calcium and systemic ionized calcium every 6 h. Citrate supply (citrate: 136 mmol/l) was started with a citrate flow of 4.0 mmol citrate/l blood to achieve an ionized postfilter calcium concentration of 0.25–0.34 mmol/l. A calcium chloride solution (calcium: 83 mmol/l) was added simultaneously to the extracorporeal circuit near the backflow to the patient to keep systemic ionized calcium stable between 1.12–1.20 mmol/l. The calcium flow was started with 1.7 mmol/l Ca2+/l dialysate.
The total turnover rate (TTR) (dialysate or replacement fluid) was calculated at 25 ml/kg ideal body weight or, in obese patients, adjusted body weight/h [26]. We applied the Hamwi equation to calculate the ideal body weight (for males: 48 kg for the first 152 cm + 1.1 kg for each additional cm; for females 45 kg for the first 152 cm + 0.9 kg for each additional cm). The adjusted body weight was used for calculation of dialysate or replacement fluid flow if the quotient of actual body weight divided by ideal weight was more than 1.3: for males: (actual body weight-ideal body weight) * 0.38 + ideal body weight; for females: (actual body weight-ideal body weight) * 0.32 + ideal body weight) [27, 28].
In patients with CVVHD, the blood flow (Qb) was set to be three times higher than the dialysate flow. CVVH was adjusted to filter a maximum of 20% of the plasma water.
Ultrafiltration was set at zero ten minutes before collecting blood samples to avoid additional effect of hemoconcentration at the dialyzer. Blood samples were taken before (pre) and after (post) the dialyzer in patients with CVVH and CVVHD-HCO. In patients with CVVHD-CS, blood samples were collected before the adsorber (pre), between the adsorber and dialyzer (int) and after (post) the dialyzer.
Dialyzer and adsorber were exchanged according to the specifications of the manufacturer regarding the maximum lifespan (maximum dialyzer lifespan limited to 72 h and adsorber lifespan to 24 h). After the study-related interventions, the decision to continue or discontinue KRT was left to the discretion of the treating physician.
Endpoints
The primary endpoint was defined as the difference of marginal means in myoglobin clearance from an ANOVA model with repeated measurements using CVVH as reference category. We used estimated marginal means taking into account the unbalanced dataset resulting from the small sample size and repeated measurements at different time points. Secondary endpoints of the study were: 1. myoglobin clearance after 1 h, 6 h, 12 h, and 24 h; 2. clearance of other molecules with different molecular weights (urea, creatinine, β2-microglobulin, interleukin-6 (IL-6), and albumin); 3. lifetime of the extracorporeal circuits. Adverse events (AEs) and serious adverse events (SAEs) were systematically recorded up to 72 h after the start of the study intervention. Furthermore, ICU length of stay, ICU mortality, hospital mortality as well as mortality at day 28 and day 90 were presented.
Calculations
The calculations have been adapted as already described [13, 14] and are listed in detail under Additional file 2.
Laboratory analyses
All biochemical analyses were performed at the Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, Leipzig University Hospital. Serum samples were analyzed shortly after blood withdrawal on a Cobas 8000 automated laboratory analyzer (Roche Diagnostics, Mannheim, Germany) in accordance with the manufacturer's protocol. The exact methods used in the field can be found under Additional file 3.
Laboratory samples with serum myoglobin concentrations above the measurement limit of 30,000 µg/l were diluted 1:20 as well as 1:50 and measured again. The concentration accurately measured with the lowest dilution was used for further analysis.
Statistical analyses
This study was designed as a pilot trial, as a sample size calculation was not possible due to lack of appropriate published data. Therefore, the aims of the study were primarily exploratory.
Categorical variables were displayed as frequencies with percentages and tested with the Chi-square test (two-sided) or Fisher exact test as appropriate. Because of the small number of cases in the three groups and generally unreliable tests for normal distribution for such small sample sizes, continuous variables were presented as median with 25th and 75th quantiles in square brackets. Group comparisons were performed with the Kruskal–Wallis test. Since a combination of adsorber and high-flux dialyzer was used in CVVHD-CS, we compared in detail the substance-specific clearances between adsorber and dialyzer (Clint) as well as after both (Clpost) using Wilcoxon signed-rank test. By adding up the medians of myoglobin clearances, models for the combination of different modes and more frequent adsorber exchange were developed.
Marginal means and their differences were displayed with 95% confidence interval and calculated by a mixed linear model for comparisons of clearance between the different KRT modalities using the CVVH as the reference mode. Dialyzer performance in one group over time has been analyzed by a general linear model with repeated measurements. The Kaplan–Meier estimate was applied to calculate and depict the survival function of the dialyzer lifetime. All analyses were performed using IBM SPSS, version 29 (Minneapolis, USA). The significance level was set at p < 0.05 for two-sided tests.
Results
Patients
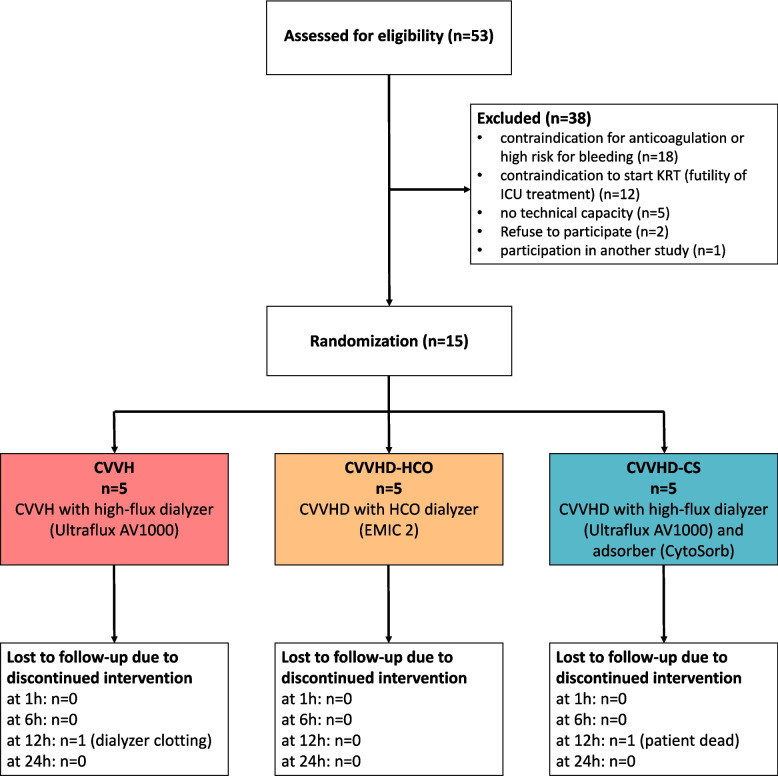
A total of 53 patients were screened between April 2021 and December 2023. The main reasons for exclusion were contraindication for heparin anticoagulation related to bleeding (n = 18) and futility of intensive care treatment because of moribund patients (n = 12). Finally, 15 patients could be included in the study (Fig. 1). The baseline clinical characteristics, comorbidities, causes of RM and the indications for KRT did not differ between the three groups (Table 1). The initial laboratory parameters at the time of diagnosis were also similar (Table 2).
Fig. 1.
Recruitment flowchart
AV1000S type of high-flux dialyzer, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, EMiC2 type of high cut-off dialyzer, HCO high cut-off
Table 1.
Patient characteristics of study population
| Parameter | All n = 15 | CVVH n = 5 | CVVHD-HCO n = 5 | CVVHD-CS n = 5 | p |
|---|---|---|---|---|---|
| General characteristics: | |||||
| Age (years) | 68 [62; 77] | 62 [47; 72] | 74 [66; 77] | 75 [61; 81] | 0.223 |
| Sex (male) n, (%) | 12 (80.0) | 5 (100.0) | 4 (80.0) | 3 (60.0) | 0.287 |
| High (cm) | 175 [169; 180] | 180 [175; 182] | 175 [167; 180] | 172 [160; 178] | 0.200 |
| Weight (kg) | 94 [82; 108] | 102 [91; 150] | 94 [80; 104] | 82 [72; 96] | 0.129 |
| BMI (kg/m2) | 31.6 [26.8; 34.5] | 34,5 [28.4; 45.3] | 32.2 [27.0; 33.0] | 27.7 [25.2; 33.4] | 0.289 |
| Mechanical ventilation n, (%) | 9 (60) | 4 (80) | 2 (40) | 3 (60) | 0.435 |
| Sepsis n, (%) | 7 (47) | 2 (40) | 3 (60) | 2 (40) | 0.765 |
| Vasopressors n, (%) | 10 (67) | 4 (80) | 3 (60) | 3 (60) | 0.741 |
| AKI 3 n, (%) | 13 (87) | 4 (80) | 4 (80) | 5 (100) | 0.562 |
| SOFA | 13 [11; 15] | 12 [10; 17] | 12 [10; 14] | 14 [12; 16] | 0.586 |
| APACHE II | 31 [28; 39] | 29 [28; 39] | 30 [29; 35] | 42 [30; 44] | 0.277 |
| MAP (mmHg) | 65 [61; 72] | 65 [59; 76] | 65 [61; 74] | 62 [59; 68] | 0.618 |
| 24-h diuresis (ml)a | 180 [68; 433] | 215 [43; 508] | 100 [45; 455] | 260 [45; 570] | 0.997 |
| Comorbidities: | |||||
| • Chronic heart failure (NYHA IV) n, (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| • Pre-existing immunosuppression n, (%) | 1 (7) | 1 (20) | 0 (0) | 0 (0) | 0.343 |
| • Liver cirrhosis n, (%) | 1 (7) | 1 (20) | 0 (0) | 0 (0) | 0.343 |
| • Active malignancy n, (%) | 2 (13) | 1 (20) | 0 (0) | 1 (20) | 0.562 |
| • Chronic pulmonary disease n, (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| • eGFR < 45 ml/min/1.73m2 n, (%) | 2 (13) | 1 (20) | 1 (20) | 0 (0) | 0.562 |
| Causes of rhabdomyolysis: | |||||
| • Muscle hypoxia n, (%) | 6 (40) | 1 (20) | 2 (40) | 3 (60) | |
| • Drugs and toxins n, (%) | 1 (7) | 1 (20) | 0 (0) | 0 (0) | |
| • Shock and post CPR n, (%) | 8 (53) | 3 (60) | 3(60) | 2 (40) | 0.517 |
| Indications for KRT: | |||||
| • Acidosis (pH < 7,25) n, (%) | 6 (40) | 2 (40) | 3 (60) | 1 (20) | 0.435 |
| • High potassium level (> 6 mmol/l) n, (%) | 2 (13) | 2 (40) | 0 (0) | 0 (0) | 0.099 |
| • Pulmonary edema n, (%) | 6 (40) | 2 (40) | 2 (40) | 2 (40) | 1.000 |
| • Uremia (urea > 25 mmol/l) n, (%) | 6 (40) | 2 (40) | 3 (60) | 1 (20) | 0.435 |
| • Oliguria/Anuria n, (%) | 11 (73) | 3 (60) | 5 (100) | 3 (60) | 0.256 |
Data presented as n (%) or median [25th, 75th quantile]
AKI acute kidney injury, APACHE II Acute Physiology And Chronic Health Evaluation II, BMI body mass index, CPR cardiopulmonary resuscitation, CytoSorb®, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, GFR glomerular filtration rate, HCO high cut-off, HR heart rate, KRT kidney replacement therapy, MAP mean arterial pressure, NYHA New York Heart Association, SOFA sequential organ failure assessment
a24-h-diuresis before initiation of KRT
Table 2.
Laboratory parameters at diagnosis
| Parameter | All n = 15 | CVVH n = 5 | CVVHD-HCO n = 5 | CVVHD-CS n = 5 | p |
|---|---|---|---|---|---|
| Myoglobin (µg/l) | 9791 [6522; 27931] | 17560 [8157; 23816] | 7692 [6434; 19938] | 7773 [4821; 28966] | 0.689 |
| CK (µkat/l) | 133 [44; 367] | 212 [132; 344] | 68 [47; 254] | 37 [20; 367] | 0.497 |
| Urea (mmol/l) | 17.0 [12.0; 28.0] | 23.0 [11.5; 32.5] | 19.0 [10.5; 27.5] | 16.0 [12.0; 22.5] | 0.733 |
| Creatinine (µmol/l) | 296 [179; 362] | 296 [228; 351] | 362 [132; 441] | 275 [166; 659] | 0.925 |
| Phosphate (mmol/l) | 1.98 [0.96; 2.68] | 2.29 [1.11; 3.62] | 1.15 [0.91; 2.44] | 1.82 [1.03; 3.28] | 0.659 |
| Calcium (mmol/l) | 1.95 [1.73; 2.2] | 1.9 [1.77; 1.97] | 2.07 [1.82; 2.33] | 1.79 [1.70; 2.23] | 0.589 |
| Magnesium (mmol/l) | 1.05 [0.95; 1.15] | 1.15 [1.06; 1,27] | 0.99 [0.79; 1.1] | 1.04 [0.94; 1.13] | 0.121 |
| Sodium (mmol/l) | 139.0 [132.0; 142.0] | 137.0 [131.5; 143.0] | 139.0 [138.5; 141.5] | 141.0 [132.0; 144.5] | 0.488 |
| Potassium (mmol/l) | 4.7 [4.6; 5.3] | 5.2 [4.7; 5.6] | 4.6 [4.1; 5.2] | 4.7 [4.35; 5.1] | 0.247 |
| pH | 7.32 [7.31; 7.41] | 7.32 [7.27; 7.34] | 7.39 [7.25; 7.42] | 7.32 [7.27; 7.42] | 0.481 |
| Bicarbonate (mmol/l) | 19.7 [16.7; 27.7] | 16.7 [15.3; 30.4] | 22.0 [17.75; 31.0] | 19.7 [16.5; 26.8] | 0.651 |
| Base excess (mmol(l) | -4.1 [-9.7; 3.3] | -8.8 [-10.05; 3.85] | -3.3 [-7.15; 6.3] | -4.1 [-8.85; 1.8] | 0.651 |
| Lactate (mmol/l) | 2.2 [1.5; 2.5] | 2.4 [2.25; 2.85] | 2.1 [1.0; 2.35] | 1.6 [1.25; 3.6] | 0.295 |
| Hemoglobin (mmol/l) | 6.0 [4.4; 6.9] | 6.9 [5.65; 7.85] | 5.2 [4.2; 8.1] | 4.5 [4.3; 6.4] | 0.195 |
| Leukocytes (109/l) | 16.7 [8.1; 18.8] | 7.8 [6.3; 21.2] | 17.1 [13.5; 18.65] | 16.7 [9.95; 28.7] | 0.468 |
| Platelets (109/l) | 183.0 [100.0; 252.0] | 162.0 [55.0; 194.0] | 187.0 [162.5; 284.0] | 201.0 [77.0; 270.5] | 0.310 |
Data presented as median [25th, 75th quantile]
CK creatin kinase, CS CytoSorb®, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, HCO high cut-off
According to the technical specification, the blood flow in CVVH was significantly higher to avoid hemoconcentration in the dialyzer compared with that of the two CVVHD—based procedures. The TTR was also higher with CVVH, but the difference was not significant (Additional file 4).
Myoglobin clearance with CVVHD-HCO and CVVHD-CS compared to CVVH
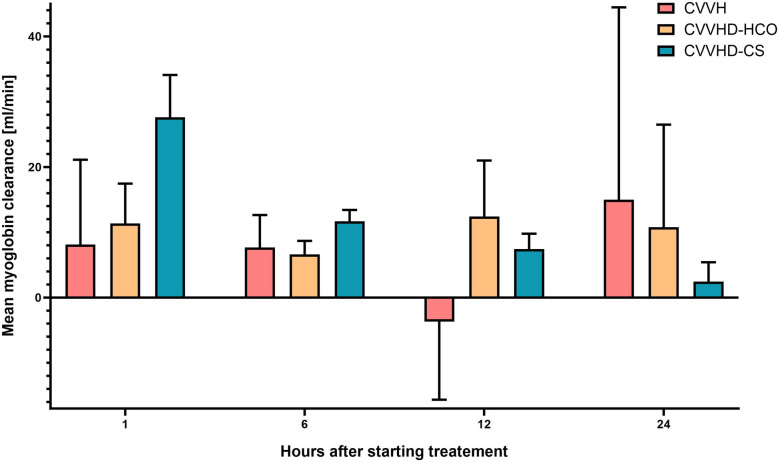
The myoglobin elimination capacity was significantly higher after 12 h with CVVHD-HCO than with CVVH (p = 0.004). However, the differences in marginal means at the other time points were not significantly different.
A significantly better myoglobin elimination was observed with CVVHD-CS after 1 h (p = 0.005) and 12 h (p = 0.019) compared to CVVH, while the difference in the marginal means was not significant after 6 h (p = 0.051). This difference became negative after 24 h (-12.57 (CI: -29.90–4.76)), indicating a decreasing elimination capacity with CVVHD-CS (Table 3, Fig. 2).
Table 3.
Differences of marginal means of myoglobin clearances in CVVHD-HCO and CVVHD-CS (ml/min)a
| Time after starting KRT | n | CVVHD-HCO n = 5 | CI | p |
| 1 h | 15 | 3.39 | -8.67–15.45 | 0.545 |
| 6 h | 15 | -0.96 | -5.46–3.54 | 0.644 |
| 12 h | 14 | 17.42 | 6.99–27.84 | 0.004 |
| 24 h | 13 | -4.24 | -22.51–14.04 | 0.617 |
| Time after starting KRT | n | CVVHD-CS n = 5 | CI | p |
| 1 h | 15 | 18.37 | 6.923- 29.80 | 0.005 |
| 6 h | 15 | 4.24 | -0.03- 8,51 | 0.051 |
| 12 h | 14 | 12.42 | 2.53- 22.31 | 0.019 |
| 24 h | 13 | -12.57 | -29.90- 4.76 | 0.137 |
Data presented as marginal means and 95% confidence interval of marginal means
CI 95% confidence interval, CS CytoSorb®, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, HCO high cut-off, KRT kidney replacement therapy
aThe clearance in reference group (CVVH) was set equal to zero
Fig. 2.
Mean myoglobin clearance
Graphical data position as a bar chart with error bars. CI confidence interval, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, HCO high cut-off
Substance specific clearances of other molecules
Elimination of small molecules was better with CVVH at the start of treatment (for urea at 1 h and 6 h; for creatinine at 1 h). The β2-microglobulin clearance was higher with CVVHD-CS one hour after initiation of the procedure (p = 0.007), although this kinetics was not maintained during further course. Similarly, the IL-6 clearance was higher after one (p = 0.015) and 6 h (p = 0.036) with CVVHD-CS, but also decreased over time. There was no relevant elimination of albumin in all investigated modes at all time points.
The calculated median clearance rates over the whole observation period did not significantly differ between the KRT modes for all investigated molecules (Table 4).
Table 4.
Substance specific clearances (ml/min)
| Time after starting KRT | n | CVVH n = 5 | CVVHD-HCO n = 5 | CVVHD-CS n = 5 | p |
|---|---|---|---|---|---|
| Myoglobin | |||||
| 1 h | 15 | 4.82 [0.33; 17.61] | 10.62 [7.78; 15.32] | 28.16 [23.39; 31.57] | 0.014 |
| 6 h | 15 | 8.56 [4.53; 10.38] | 6.11 [5.33; 8.16] | 11.33 [10.56; 13.00] | 0.009 |
| 12 h | 14 | -0.50 [-13.64; 4.72] | 12.54 [7.30; 17.42] | 6.93 [5.78; 9.31] | 0.031 |
| 24 h | 13 | 10.14 [0.53; 34.32] | 6.00 [5.59; 20.70] | 1.94 [0.38; 4.71] | 0.089 |
| Clmedian | 13 | 2.56 [-3.57; 15.25] | 11.19 [8.32; 12.27] | 9.60 [8.19; 10.35] | 0.391 |
| Urea | |||||
| 1 h | 15 | 40.00 [34.28; 47.81] | 27.37 [22.67; 39.45] | 25.19 [19.46; 30.25] | 0.031 |
| 6 h | 15 | 40.92 [35.67; 47.39] | 25.83 [22.02; 37.15] | 27.65 [20.47; 31.78] | 0.025 |
| 12 h | 14 | 40.00 [29.82; 46.28] | 33.93 [23.86; 36.82] | 30.72 [22.87; 36.82] | 0.273 |
| 24 h | 13 | 40.45 [34.73; 50.85] | 25.36 [23.45; 41.06] | 29.80 [25.13; 33.00] | 0.096 |
| Clmedian | 13 | 39.76 [32.11; 48.85] | 32.12 [23.85; 37.92] | 29.06 [22.78; 33.68] | 0.136 |
| Creatinine | |||||
| 1 h | 15 | 41.14 [35.06; 51.21] | 31.42 [26.61; 41.72] | 25.56 [20.98; 31.51] | 0.022 |
| 6 h | 15 | 43.01 [36.09; 48.85] | 29.61 [28.00; 39.45] | 28.25 [22.95; 35.66] | 0.054 |
| 12 h | 14 | 40.82 [29.27; 47.54] | 36.48 [27.27; 40.20] | 31.24 [25.26; 37.54] | 0.343 |
| 24 h | 13 | 39.97 [34.81; 52.02] | 28.93 [27.62; 46.01] | 31.43 [26.82; 36.16] | 0.131 |
| Clmedian | 13 | 41.12 [31.47; 50.11] | 33.90 [28.05; 41.36] | 30.52 [24.70; 35.75] | 0.301 |
| β2-microglobulin | |||||
| 1 h | 15 | 35.15 [25.12; 36.98] | 26.41 [23.98; 33.73] | 44.16 [41.89; 55.86] | 0.007 |
| 6 h | 15 | 28.71 [15.14; 33.07] | 21.73 [18.87; 35.26] | 30.83 [25.18; 32.94] | 0.756 |
| 12 h | 14 | 26.42 [2.72; 30.04] | 21.83 [18.05; 32.00] | 24.51 [22.97; 30.70] | 0.741 |
| 24 h | 13 | 32.39 [22.64; 45.80] | 20.97 [19.14; 41.57] | 18.98 [16.57; 22.02] | 0.082 |
| Clmedian | 13 | 28.94 [12.64; 31.29] | 24.55 [19.08; 34.97] | 27.40 [24.62; 30.63] | 0.826 |
| Interleukin-6 | |||||
| 1 h | 15 | 10.09 [4.60; 18.45] | 3.26 [2.07; 6.68] | 19.18 [16.24; 22.70] | 0.015 |
| 6 h | 15 | 8.33 [4.65; 11.27] | 1.71 [-0.61; 4.54] | 8.06 [5.47; 9.88] | 0.036 |
| 12 h | 14 | 4.17 [2.21; 8.15] | 1.55 [0.62; 2.38] | 3.26 [3.01; 7.11] | 0.056 |
| 24 h | 13 | 10.84 [5.12; 34.38] | 0.27 [-1.33; 16.71] | 1.81 [0.14; 3.10] | 0.067 |
| Clmedian | 13 | 7.00 [5.57; 15.84] | 1.53 [0.51; 6.30] | 5.88 [5.68; 6.97] | 0.205 |
| Albumin | |||||
| 1 h | 15 | 1.34 [-13.23; 15.15] | -1.44 [-3.44; 0.93] | 1.08 [0.14; 4.80] | 0.310 |
| 6 h | 15 | 0.86 [-3.14; 8.53] | -3.03 [-7.18; -1.35] | -2.08 [-3.44; 3.31] | 0.230 |
| 12 h | 14 | -4.13 [-18.93; 3.95] | -2.60 [-7.18; 1.24] | -0.82 [-1.93; 0.07] | 0.195 |
| 24 h | 13 | 5.06 [-5.94; 32.31] | -4.28 [-5.11; 11.53] | 0.32 [-1.27; 1.40] | 0.446 |
| Clmedian | 13 | 1.25 [-12.04; 6.35] | -2.05 [-6.12; 1.20] | -0.48 [-1.74; 1.49] | 0.552 |
Data presented as median [25th, 75th quantile]
Clmedian calculated median clearance between 1 and 24 h, CS CytoSorb®, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, HCO high cut-off, KRT kidney replacement therapy
Time dependent elimination of molecules with CVVHD-CS
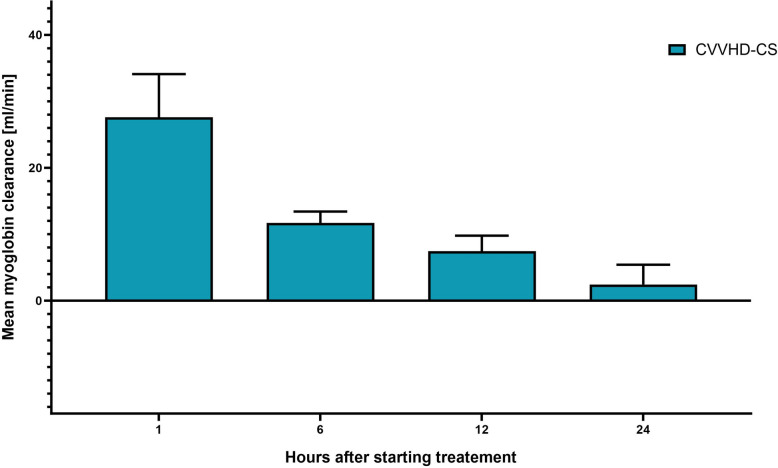
While myoglobin elimination using CVVHD-CS was the highest at the beginning, this clearance sharply decreased over time (Fig. 3). This effect was significant using a general linear model for this group (p = 0.023), while this could not be proved for the other modes (CVVH: p = 0.125, CVVHD-HCO: p = 0.644). This observation was confirmed by the decreasing substance-specific clearances of other molecules with middle molecule weight such as β2-microglobulin (p < 0.001) and IL-6 (p = 0.029). In contrast, the elimination performance for small molecules was stable during the observation period (urea (p = 0.206) and creatinine (p = 0.155)). There were no significant changes regarding albumin clearance during the study time points (p = 0.090) (Table 5).
Fig. 3.
Myoglobin clearance in CVVHD-adsorber over time
Graphical data position as a bar chart with error bars. CI confidence interval, CVVHD continuous veno-venous hemodialysis
Table 5.
Substance-specific clearances in CVVHD-CS over time
| Parameter, time after starting KRT | n | mean | CI | P* |
|---|---|---|---|---|
| Myoglobin | ||||
| 1 h | 5 | 27.62 | 21.13–34.10 | |
| 6 h | 5 | 11.69 | 9.96–13.42 | 0.002 |
| 12 h | 5 | 7.42 | 5.07–9.78 | 0.001 |
| 24 h | 5 | 2.43 | -0.56–5.41 | < 0.001 |
| Urea | ||||
| 1 h | 5 | 24.92 | 17,63–32.21 | |
| 6 h | 5 | 26.43 | 17.68–35.19 | 0.496 |
| 12 h | 5 | 30.02 | 20.33–39.72 | 0.077 |
| 24 h | 5 | 29.21 | 22.99–35.44 | 0.098 |
| Creatinine | ||||
| 1 h | 5 | 26.11 | 19.34–32.87 | |
| 6 h | 5 | 29.09 | 19.72–38.47 | 0.365 |
| 12 h | 5 | 31.37 | 22.36–40.37 | 0.135 |
| 24 h | 5 | 31.48 | 24.33–38.64 | 0.090 |
| β2-microglobulin | ||||
| 1 h | 5 | 47,9333 | 38.78–57.08 | |
| 6 h | 5 | 29,4148 | 24.18–34.65 | 0.005 |
| 12 h | 5 | 26,3672 | 20.05–32.69 | 0.008 |
| 24 h | 5 | 19,2308 | 15.73–22.73 | 0.001 |
| Interleukin-6 | ||||
| 1 h | 5 | 19.41 | 14.63–24.20 | |
| 6 h | 5 | 7.75 | 4.83–10.68 | 0.006 |
| 12 h | 5 | 4.70 | 1.95–7.46 | 0.002 |
| 24 h | 5 | 1.66 | -0.31–3.62 | 0.001 |
| Albumin | ||||
| 1 h | 5 | 2.19 | -1.65–6.03 | |
| 6 h | 5 | -0.47 | -5.71–4.78 | 0.043 |
| 12 h | 5 | -0.91 | -2.37–0.56 | 0.048 |
| 24 h | 5 | 0.12 | -1.78–2.02 | 0.118 |
Data presented as means and 95% confidence interval of means
CI 95% confidence interval, CS CytoSorb®, CVVHD continuous veno-venous hemodialysis, KRT kidney replacement therapy
*general linear model with repeated measurement (reference category: 1 h value)
Specific clearance performance of the adsorber and the high-flux dialyzer with CVVHD-CS
The calculated Clint for the middle molecular weight substances such as myoglobin and IL-6 immediately after the adsorber was higher compared to the final Clpost after 6, 12 and 24 h. In contrast, the Clint of urea and creatinine was nearly zero. These substances were only removed through the high-flux dialyzer. For β2-microglobulin, hemoadsorption and hemodialysis have an additive effect with an increasing clearance along the extracorporeal circuit. While the elimination of albumin in the CVVHD-CS group was negligible, the calculated clearance rate for albumin was higher at Clint after 24 h (Table 6).
Table 6.
Substance specific clearances between (Clint) and after (Clpost) CS and dialyzer (ml/min)
| Time after starting KRT | n | Clint | Clpost | P* |
|---|---|---|---|---|
| Myoglobin | ||||
| 1 h | 5 | 29.33 [24.20; 32.47] | 28.16 [23.39; 31.57] | 0.138 |
| 6 h | 5 | 14.37 [12.79; 15.75] | 11.33 [10.56; 13.00] | 0.043 |
| 12 h | 5 | 10.52 [9.27; 11.54] | 6.93 [5.78; 9.31] | 0.043 |
| 24 h | 5 | 5.30 [4.22; 6.56] | 1.94 [0.38; 4.71] | 0.043 |
| Urea | ||||
| 1 h | 5 | 1.77 [0.97; 2.48] | 25.19 [19.46; 30.25] | 0.043 |
| 6 h | 5 | 1.51 [0.99; 2.71] | 27.65 [20.47; 31.78] | 0.043 |
| 12 h | 5 | 4.05 [0.72; 4.49] | 30.72 [22.87; 36.82] | 0.043 |
| 24 h | 5 | 0.76 [-0.43; 3.56] | 29.80 [25.13; 33.00] | 0.043 |
| Creatinine | ||||
| 1 h | 5 | 0.56 [-0.38; 1.18] | 25.56 [20.98; 31.51] | 0.043 |
| 6 h | 5 | 2.39 [1.41; 3.09] | 28.25 [22.95; 35.66] | 0.043 |
| 12 h | 5 | 2.17 [1.63; 3.12] | 31.24 [25.26; 37.54] | 0.043 |
| 24 h | 5 | 1.57 [0.00; 3.80] | 31.43 [26.82; 36.16] | 0.043 |
| β2-microglobulin | ||||
| 1 h | 5 | 37.87 [36.77; 46.86] | 44.16 [41.89; 55.86] | 0.043 |
| 6 h | 5 | 19.10 [17.24; 23.10] | 30.83 [25.18; 32.94] | 0.043 |
| 12 h | 5 | 14.09 [12.56; 20.07] | 24.51 [22.97; 30.70] | 0.043 |
| 24 h | 5 | 9.67 [7.41; 10.43] | 18.98 [16.57; 22.02] | 0.043 |
| Interleukin-6 | ||||
| 1 h | 5 | 21.86 [17.40; 23.95] | 19.18 [16.24; 22.70] | 0.068 |
| 6 h | 5 | 11.50 [8.57; 13.63] | 8.06 [5.47; 9.88] | 0.043 |
| 12 h | 5 | 6.18 [5.96; 8.81] | 3.26 [3.01; 7.11] | 0.043 |
| 24 h | 5 | 4.83 [3.54; 6.10] | 1.81 [0.14; 3.10] | 0.043 |
| Albumin | ||||
| 1 h | 5 | 4.45 [1.07; 5.51] | 1.08 [0.14; 4.80] | 0.500 |
| 6 h | 5 | 0.91 [-1.24; 2.81] | -2.08 [-3.44; 3.31] | 0.500 |
| 12 h | 5 | 0.89 [-3.22; 2.67] | -0.82 [-1.93; 0.07] | 0.500 |
| 24 h | 5 | 3.26 [1.23; 4.60] | 0.32 [-1.27; 1.40] | 0.043 |
Data presented as median [25th, 75th quantile]
CS CytoSorb®, KRT renal replacement therapy
*Wilcoxon signed-rank test
Safety endpoints and lifetime of extracorporeal circuit
Adverse events and severe adverse events did not differ significantly between the groups. Two patients with CVVHD-HCO died during the intervention due to a refractory septic shock. Bleeding complications and clotting of extracorporeal circuit were observed only in the CVVH group (Table 7). There was no difference regarding the ICU length of stay between the groups. A total of 11 patients died, with no difference between the groups regarding overall mortality rates. The cause of death was sepsis in six patients, acute heart failure in three, hypoxic ischemic encephalopathy and acute on chronic liver failure in one each.
Table 7.
Safety endpoints
| Parameter | All n = 15 | CVVH n = 5 | CVVHD-HCO n = 5 | CVVHD-CS n = 5 | p |
|---|---|---|---|---|---|
| ICU stay (d) | 10 [4; 31] | 12 [5; 51] | 10 [1; 29] | 8 [6; 21] | 0.689 |
| ICU mortality n, (%) | 9/15 (60) | 3/5 (60) | 3/5 (60) | 3/5 (60) | 1.000 |
| hospital mortality n, (%) | 11/15 (73) | 4/5 (80) | 4/5 (80) | 3/5 (60) | 0.711 |
| 28d mortality n, (%) | 8/15 (53) | 2/5 (40) | 3/5 (60) | 3/5 (60) | 0.765 |
| 90d mortality n, (%) | 11/15 (73) | 4/5 (80) | 4/5 (80) | 3/5 (60) | 0.711 |
| SAE | |||||
| Treatment associated life-threatening complications n, (%) | 0/15 (0) | ||||
| Dead of any cause during intervention n, (%) | 2/15 (13) | 0/5 (0) | 2/5 (40)a | 0/5 (0) | 0.099 |
| AE | |||||
| Hypocalcaemia n, (%) | 1/15 (7) | 0/5 (0) | 0/5 (0) | 1/5 (20) | 0.343 |
| Metabolic alkalosis n, (%) | 0/15 (0) | ||||
| Citrate accumulation n, (%) | 1/15 (7) | 0/5 (0) | 1/5 (20) | 0/5 (0) | 0.343 |
| Bleeding with transfusion n, (%) | 0/15 (0) | ||||
| Bleeding without transfusion n, (%) | 2/15 (13) | 2/5 (40) | 0/5 (0) | 0/5 (0) | 0.099 |
| Clotting of extracorporeal circuit n, (%) | 2/15 (13) | 2/5 (40)b | 0/5 (0) | 0/5 (0) | 0.099 |
| HIT n, (%) | 0/15 (0) | ||||
| Dysfunction of dialysis catheter n, (%) | 2/15 (13) | 1/5 (20) | 1/5 (20) | 0/5 (0) | 0.562 |
Data presented as n (%) or median [25th, 75th quantile]
AE adverse event, CS CytoSorb®, CVVH continuous veno-venous hemofiltration, CVVHD continuous veno-venous hemodialysis, HCO high cut-off, HIT heparin-induced thrombocytopenia, ICU intensive care unit, SAE sever adverse event
adead 10 h and 29 h after initiation CVVHD-HCO
bclotting of extracorporeal circuit 14 h and 25 h after initiation of CVVH
The median filter lifespans for the different KRT modes were 60 h [0; 135] in CVVH, 61 h [0; 132] in CVVHD-HCO and 67 h [42; 92] in CVVHD-CS group. These differences were not significant (p = 0.863).
Myoglobin clearance with simulated alternative KRT settings
The potential performance of alternative KRT settings were estimated. First, a model combining CVVHD-HCO with the adsorber gave a median myoglobin clearance of 20.79 ml/min. Second, a more frequent adsorber exchange (every 12 h) in a CVVHD with a standard high-flux dialyzer resulted in a median myoglobin clearance of 14.91 ml/min. Finally, both tested models were merged. CVVHD-HCO combined with the adsorber with a 12-hourly adsorber exchange could presumably generate a myoglobin clearance of 26.10 ml/min.
Discussion
In this prospective randomized controlled pilot trial on critically ill patients with RM and severe AKI requiring KRT, myoglobin elimination using CVVHD with a high-flux dialyzer in combination with an adsorber was found to be the most effective mode during the first hours of treatment. This finding is supported by other studies that also showed a high elimination capacity for myoglobin in the early stages of treatment by combining CVVHD with CS [16–18]. The high myoglobin elimination rates within the first hour reported by those studies suggests that our findings on the performance of the CVVHD combined with the adsorber may have been underestimated, because we did not take into account the effects of the first treatment hour.
Nevertheless, there were no significant differences between the three studied modes regarding the average myoglobin clearance during the first 24 h of treatment. This can be explained by the rapid decline in myoglobin clearance in CVVHD-CS during the longer observation period. A retrospective comparative study in patients with severe RM, notably with a short treatment period of only 6–12 h, has also shown no differences in the myoglobin reduction rate between intermittent hemodiafiltration with high cut-off dialyzer, intermittent hemodialysis with medium cut-off dialyzer and CVVHD-CS [15].
CVVH has been defined as the control mode based on previous reports that proposed this as the standard procedure for myoglobin elimination in AKI [2, 4]. However, despite similar TTRs, we have observed considerably lower myoglobin clearance rates with this mode compared to that reported by Amyot using a 0.9m2 polyacrylnitril (AN69) dialyzer [11]. The myoglobin clearance using CVVHD-HCO was similar to that in our previous study [14].
Small molecules such as urea and creatinine were best eliminated with CVVH, most probably due to the significantly higher blood flow and TTR [29]. The higher blood flow is required in order to avoid a critical hemoconcentration along the dialyzer in the postdilution mode [30].
Similar to the elimination kinetics for myoglobin, the best initial clearance for the other middle molecular weight substances β2-microglobulin and IL-6 was found in CVVHD-CS. In due course and when averaged over time, no differences in clearance could be observed between the various modes. The high IL-6 clearance, a pro-inflammatory cytokine, might be an interesting adjunctive therapeutic approach for patients with sepsis. Although case series and retrospective studies on patients with septic shock demonstrated improved survival using hemoperfusion with CS [31, 32], no survival benefit has so far been documented in prospective studies [33].
β2-microglobulin, on the other hand, is a surrogate parameter for medium molecular weight uremic toxins. However, the clinical effects of reducing serum concentrations through extracorporeal measures are still uncertain [13, 34].
Finally we did not observe any relevant loss of albumin with the three modes studied. This was not surprising since the cutoff for the dialyzers applied is 30 kDa. Previous elimination studies using CVVHD-HCO with the EMiC2 filter also showed no albumin loss [13, 14]. There is currently a lack of clinical data regarding a potential albumin adsorption with the Cytosorb adsorber. Since the cut-off for this adsorber with 55 kDa [17] is not far lower than the molecular mass of albumin (66.5 kDa), minimal albumin loss could be assumed.
Increasing saturation of the adsorber during CVVHD-CS leads to a loss of elimination ability despite the extensive adsorption surface of 45,000 m2 [17]. In contrast, the elimination performance of a dialyzer is largely maintained by continuous fresh dialysis or substitution solution and is only slightly affected by membrane fouling over time [35, 36]. Other recently published reports have also shown a rapid decline in myoglobin and IL-6 elimination performance using the combination of continuous KRT and CS [16, 18, 37]. This study showed that myoglobin (17.1 kDa) and IL-6 (26.0 kDa) are only removed with the adsorber in CVVHD-CS. For the smaller β2-microglobulin (11.8 kDa), elimination obviously occurs through both the adsorption and the dialysis. Over time, the elimination decreased until the adsorber was saturated, while the elimination with dialysis remained almost constant. Surprisingly, Clint was slightly higher than Clpost for myoglobin and IL-6. Filtration-backfiltration processes may explain this phenomenon [38]. Despite the negligible clearance, this phenomenon could also be observed for albumin. Creatinine and urea were hardly removed by the adsorber in this study. Although the adsorber binds a wide variety of hydrophobic substances up to a molecular weight of 55 kDa [17, 39], it does not seem to play a role in the elimination of small hydrophilic molecules. These are almost completely eliminated by the dialysis part of the system [16].
We did not observe any significant differences between the groups regarding ICU-length of stay, hospital-, 28- and 90-day mortality. However, these findings should be carefully interpreted due to the small sample sizes. The very high 90-day mortality of 73% is in the range of other studies on critically ill patients with AKI, RM and need for KRT [8, 14, 40]. The SOFA score and the need for KRT were recently identified as independent risk factors for ICU mortality in patients with RM [8].
In the group with CVVH, two patients experienced bleeding problems and the extracorporeal circuit was clotted before the scheduled end of treatment in two patients. This observation is in line with the findings of a recent multicenter study [41]. To avoid these problems of systemic anticoagulation, performing CVVH with RCA could be a possible alternative. However, due to the required higher blood flow, there would be a significant increase in citrate intake with the risk of citrate accumulation. A high disease severity, such as observed in this study, is often associated with citrate accumulation [42].
Using the calculated clearance performances of the different KRT modes, our study showed the modelling of alternative KRT settings with the aim of further increasing myoglobin elimination. The knowledge gained from the additional determination of Clint in CVVHD-CS was a prerequisite for the corresponding calculations. Higher effectiveness was recently demonstrated for the combination of CVVHD-HCO and CS [16]. Additionally, based on the observed saturation kinetics of the adsorber, a model with an adsorber exchange every 12 h would increase the median myoglobin clearance. A recently published consensus paper also recommends an adsorber exchange every 12 h until the serum myoglobin concentration falls below 10,000 ug/l [19]. Finally, the model combination of CVVHD-HCO and CS with a 12-hourly adsorber exchange resulted in a sustained high median myoglobin clearance throughout the entire assumed treatment period. The effectiveness of this concept has been demonstrated in a case report [43].
Strengths
In this study, various options for myoglobin elimination were investigated for the first time in a prospective randomized design in critically ill patients with RM and AKI requiring KRT. Our trial provides precise data on the performance of the different modes studied and may help in decision making regarding the appropriate procedure.
Limitations
Our study has several limitations. Firstly, because of the small sample size, it is possible that relevant differences may have been missed (type 2 error). Secondly, there is a risk of a type 1 error due to multiple testing. Thirdly, the substance-specific elimination performance in CVVH may have been overestimated due to the higher blood flow and TTR. Fourth, the small sample size does not allow any valid statement on clinical outcome. Fifth, we did not take into account the effects of the first treatment hour. Thus, the high myoglobin elimination rate in CVVHD-CS within the first hour reported by previous studies [16, 18] suggests that the performance of the CVVHD-CS may have been underestimated. Finally, possible differences in baseline myoglobin concentration may have affected clearance performance over time due to the saturation kinetics of the adsorber. Nevertheless, this study allows the characterization of the elimination capacity of the examined KRT modes over time in RM.
Conclusions
CVVHD-CS provides by far the highest myoglobin clearance in the first hours of treatment. However, due to the rapid decline of the elimination capacity over the period of 24 h, no difference in the averaged clearance over time could be observed between CVVHD-CS, CVVHD-HCO and CVVH. In CVVH with heparin-based anticoagulation, the risk of bleeding or clotting was more frequent. Therefore, the old standard CVVH for the treatment of AKI in RM must be critically questioned in view of the newer available alternatives.
Theoretically, a further increase in myoglobin clearance could be achieved with the combination of CVVHD-CS-HCO with a 12-hourly exchange of the adsorber. However, large clinical trials are necessary to evaluate whether faster elimination of myoglobin could influence clinical outcome parameters.
Supplementary Information
Acknowledgements
We thank all patients and the ICU staff for their support.
Abbreviations
- AE
Adverse events
- AKI
Acute kidney injury
- APACHE
II Acute Physiology And Chronic Health Evaluation II
- aPTT
Activated partial thromboplastin time
- Cpost
Substance specific concentration after the dialyzer
- Cpre
Substance specific concentration before the dialyzer
- CKD
Chronic kidney disease
- Cl1h, 6h, 12h, 24h
Plasma clearance at different time points
- Clcorr
Corrected plasma clearance
- Clint
Plasma clearance between adsorber and dialyzer
- Clmedian
Median plasma clearance
- Clp
Substance-specific plasma clearance
- Clpost
Plasma clearance after adsorber and dialyzer
- Cpost,corr
Corrected plasma concentration
- Cltotal
Total plasma clearance
- CS
CytoSorb®
- CVVH
Continuous veno-venous hemofiltration
- CVVHD
Continuous veno-venous hemodialysis
- CVVHD-CS
Continuous veno-venous hemodialysis with the additional adsorber CytoSorb®
- CVVHD-HCO
Continuous veno-venous hemodialysis using a high cut-off dialyzer
- ECLIA
Electrochemiluminescence immunoassay
- FP
Filtration portion
- HCO
High cut-off
- hct
Hematocrit level
- ICU
Intensive care unit
- IL-6
Interleukin-6
- KRT
Kidney replacement therapy
- Qb
Blood flow
- Qppre
Plasma flow in the extracorporeal circuit
- Qppost
Postfilter plasma flow
- RCA
Regional citrate anticoagulation
- RM
Rhabdomyolysis
- SAE
Severe adverse events
- SOFA
Sequential Organ Failure Assessment
- TTR
Total turnover rate
Authors’ contributions
LW and JF contributed to the conception and design of the study, LW and AB wrote the first draft of the manuscript, MM and LW caried out the statistical evaluations, LW und JF designed the tables and illustrations, AB und LW coordinated the implementation of the clinical trial, AW supported and advised on laboratory diagnostics, SP and CS advised in all phases of scientific work, All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
Open Access funding enabled and organized by Projekt DEAL. LW received funding from CytoSorbents Europe GmbH (Müggelseedamm 131, Berlin, Germany).
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the local Institutional Review Board at the Medical Faculty of the University of Leipzig (361/20-ek). Informed consent to participate was obtained from all of the participants in the study. Patients under 18 years of age were excluded. All experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
Lorenz Weidhase was involved in scientific projects funded by Fresenius Medical Care Deutschland GmbH (Else-Kröner-Straße 1, D-61352 Bad Homburg, Germany). Christina Scharf got speaker honoraria by CytoSorbents Europe GmbH. The remaining authors have disclosed that they do not have any conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lorenz Weidhase and Antonia Borrmann contributed equally to this work.
References
- 1.Bywaters EG, Beall D. Crush injuries with impairment of renal function. Br Med J. 1941;1(4185):427–32. 10.1136/bmj.1.4185.427. PMID:20783577;PMCID:PMC2161734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62–72. 10.1056/NEJMra0801327. Erratum.In:NEnglJMed.2011May19;364(20):1982 PMID: 19571284. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058–65. 10.1378/chest.12-2016. PMID: 24008958. [DOI] [PubMed] [Google Scholar]
- 4.Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(3):224. 10.1186/cc13897. PMID:25043142;PMCID:PMC4056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis – an overview for clinicians. Crit Care. 2005;9(2):158–69. 10.1186/cc2978. Epub 2004 Oct 20. PMID: 15774072; PMCID: PMC1175909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Moreno JA. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res. 2015;40(5):520–32. 10.1159/000368528. Epub 2015 Oct 20 PMID: 26512883. [DOI] [PubMed] [Google Scholar]
- 7.El-Abdellati E, Eyselbergs M, Sirimsi H, Hoof VV, Wouters K, Verbrugghe W, Jorens PG. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care. 2013;3(1):8. 10.1186/2110-5820-3-8. PMID: 23497406; PMCID: PMC3614462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Fallois J, Scharm R, Lindner TH, Scharf C, Petros S, Weidhase L. Kidney replacement and conservative therapies in rhabdomyolysis: a retrospective analysis. BMC Nephrol. 2024;25(1):96. 10.1186/s12882-024-03536-8. PMID:38486159;PMCID:PMC10938657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasaoka S, Todani M, Kaneko T, Kawamura Y, Oda Y, Tsuruta R, Maekawa T. Peak value of blood myoglobin predicts acute renal failure induced by rhabdomyolysis. J Crit Care. 2010;25(4):601–4. 10.1016/j.jcrc.2010.04.002. PMID: 20537502. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Kim S, Ohn JH, Kim NH, Lee J, Kim ES, Lim Y, Cho JH, Park HS, Ryu J, Kim SW. Role of bicarbonate and volume therapy in the prevention of acute kidney injury in rhabdomyolysis: a retrospective propensity score-matched cohort study. Kidney Res Clin Pract. 2022;41(3):310–21. 10.23876/j.krcp.21.093. Epub 2021 Dec 2. PMID: 34974654; PMCID: PMC9184844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amyot SL, Leblanc M, Thibeault Y, Geadah D, Cardinal J. Myoglobin clearance and removal during continuous venovenous hemofiltration. Intensive Care Med. 1999;25(10):1169–72. 10.1007/s001340051031. PMID: 10551978. [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Chen Z, Wu W, Qiu H, Bo H, Zhang L, Fu P. Renal protective effects of early continuous venovenous hemofiltration in rhabdomyolysis: improved renal mitochondrial dysfunction and inhibited apoptosis. Artif Organs. 2013;37(4):390–400. 10.1111/j.1525-1594.2012.01574.x. Epub 2013 Feb 27 PMID: 23441644. [DOI] [PubMed] [Google Scholar]
- 13.Weidhase L, Haussig E, Haussig S, Kaiser T, de Fallois J, Petros S. Middle molecule clearance with high cut-off dialyzer versus high-flux dialyzer using continuous veno-venous hemodialysis with regional citrate anticoagulation: a prospective randomized controlled trial. PLoS ONE. 2019;14(4):e0215823. 10.1371/journal.pone.0215823. PMID:31026303;PMCID:PMC6485708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidhase L, de Fallois J, Haußig E, Kaiser T, Mende M, Petros S. Myoglobin clearance with continuous veno-venous hemodialysis using high cutoff dialyzer versus continuous veno-venous hemodiafiltration using high-flux dialyzer: a prospective randomized controlled trial. Crit Care. 2020;24(1):644. 10.1186/s13054-020-03366-8. PMID:33176824;PMCID:PMC7659077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerman A, Andonova M, Persic V, Gubensek J. Extracorporeal removal of myoglobin in patients with rhabdomyolysis and acute kidney injury: comparison of high and medium cut-off membrane and an adsorber cartridge. Blood Purif. 2022;51(11):907–11. 10.1159/000521923. Epub 2022 Mar 25. PMID: 35340002; PMCID: PMC9808672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrecht F, Schunk S, Fuchs M, Volk T, Geisel J, Fliser D, Meiser A. Rapid and effective elimination of myoglobin with CytoSorb® hemoadsorber in patients with severe rhabdomyolysis. Blood Purif. 2024;53(2):88–95. 10.1159/000534479. Epub 2023 Nov 2 PMID: 37918366. [DOI] [PubMed] [Google Scholar]
- 17.Scharf C, Liebchen U, Paal M, Irlbeck M, Zoller M, Schroeder I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit Care. 2021;25(1):41. 10.1186/s13054-021-03468-x. PMID:33509234;PMCID:PMC7844984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf H, Gräfe C, Bruegel M, Zoller M, Maciuga N, Frank S, Weidhase L, Paal M, Scharf C. Myoglobin adsorption and saturation kinetics of the cytokine adsorber Cytosorb® in patients with severe rhabdomyolysis: a prospective trial. Ann Intensive Care. 2024;14(1):96. 10.1186/s13613-024-01334-x. PMID:38907120;PMCID:PMC11192705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forni L, Aucella F, Bottari G, Büttner S, Cantaluppi V, Fries D, Kielstein J, Kindgen-Milles D, Krenn C, Kribben A, Meiser A, Mitzner S, Ostermann M, Premuzic V, Rolfes C, Scharf C, Schunk S, Molnar Z, Zarbock A. Hemoadsorption therapy for myoglobin removal in rhabdomyolysis: consensus of the hemoadsorption in rhabdomyolysis task force. BMC Nephrol. 2024;25(1):247. 10.1186/s12882-024-03679-8. PMID:39085790;PMCID:PMC11293130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20(2):187–91. 10.1016/j.jcrc.2005.04.005. discussion 191-3 PMID: 16139163. [DOI] [PubMed] [Google Scholar]
- 21.KDIGO. Clinical practice guideline for acute kidney injury (AKI). Kidney Int Suppl. 2012;2:4. 10.1038/kisup.2012.4. [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006. Erratum. In: Ann Intern Med. 2011 Sep 20;155(6):408. PMID: 19414839; PMCID: PMC2763564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29 PMID: 3928249. [PubMed] [Google Scholar]
- 24.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. 10.1007/BF01709751. PMID: 8844239. [DOI] [PubMed] [Google Scholar]
- 25.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. 10.1001/jama.2016.0287. PMID:26903338;PMCID:PMC4968574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fayad AI, Buamscha DG, Ciapponi A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst Rev. 2016;10(10):CD010613. 10.1002/14651858.CD010613.pub2. PMID: 27699760; PMCID: PMC6457961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glynn CC, Greene GW, Winkler MF, Albina JE. Predictive versus measured energy expenditure using limits-of-agreement analysis in hospitalized, obese patients. JPEN J Parenter Enteral Nutr. 1999;23(3):147–54. 10.1177/0148607199023003147. PMID: 10338222. [DOI] [PubMed] [Google Scholar]
- 28.Krenitsky J. Adjusted body weight, pro: evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr Clin Pract. 2005;20(4):468–73. 10.1177/0115426505020004468. PMID: 16207686. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzin A, Garzotto F, Alghisi A, Neri M, Galeano D, Aresu S, Pani A, Vidal E, Ricci Z, Murer L, Goldstein SL, Ronco C. CVVHD treatment with CARPEDIEM: small solute clearance at different blood and dialysate flows with three different surface area filter configurations. Pediatr Nephrol. 2016;31(10):1659–65. 10.1007/s00467-016-3397-2. Epub 2016 Apr 30 PMID: 27139897. [DOI] [PubMed] [Google Scholar]
- 30.Bellomo R, Ronco C. Continuous haemofiltration in the intensive care unit. Crit Care. 2000;4(6):339–45. 10.1186/cc718. Epub 2000 Oct 20. PMID: 11123877; PMCID: PMC137261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21(1):74. 10.1186/s13054-017-1662-9. PMID:28343448;PMCID:PMC5366999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz P, Schwier E, Eickmeyer C, Henzler D, Köhler T. High-dose CytoSorb hemoadsorption is associated with improved survival in patients with septic shock: a retrospective cohort study. J Crit Care. 2021;64:184–92. 10.1016/j.jcrc.2021.04.011. Epub 2021 Apr 20 PMID: 33962219. [DOI] [PubMed] [Google Scholar]
- 33.Becker S, Lang H, Vollmer Barbosa C, Tian Z, Melk A, Schmidt BMW. Efficacy of CytoSorb®: a systematic review and meta-analysis. Crit Care. 2023;27(1):215. 10.1186/s13054-023-04492-9. PMID:3037259160;PMCID:PMC10230475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke HD, Vanholder R, Diouf M, Choukroun G, Massy ZA, European Uremic Toxin Work Group (EUTox). Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–303. 10.1038/ki.2012.301. Epub 2012 Aug 15. PMID: 22895515. [DOI] [PubMed] [Google Scholar]
- 35.Villa G, Zaragoza JJ, Sharma A, Neri M, De Gaudio AR, Ronco C. Cytokine removal with high cut-off membrane: review of literature. Blood Purif. 2014;38(3–4):167–73. 10.1159/000369155. Epub 2014 Dec 3 PMID: 25471681. [DOI] [PubMed] [Google Scholar]
- 36.Whiting L, Bianchi N, Faouzi M, et al. Kinetics of small and middle molecule clearance during continuous hemodialysis. Sci Rep. 2023;13:12905. 10.1038/s41598-023-40075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graf H, Gräfe C, Bruegel M, Happich FL, Wustrow V, Wegener A, Wilfert W, Zoller M, Liebchen U, Paal M, Scharf C. Extracorporeal elimination of pro- and anti-inflammatory modulators by the cytokine adsorber CytoSorb® in patients with hyperinflammation: a prospective study. Infect Dis Ther. 2024;13(9):2089–101. 10.1007/s40121-024-01028-8. Epub 2024 Aug 18. PMID: 39154299; PMCID: PMC11343926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poorkhalil A, Mouzakis F, Kashefi A, Mottaghy K. The course of hematocrit value along the length of a dialyzer’s fiber: Hemoconcentration modeling and validation methods. Int J Artif Organs. 2019;42(9):482–9. 10.1177/0391398819847214. Epub 2019 May 23 PMID: 31122110. [DOI] [PubMed] [Google Scholar]
- 39.Poli EC, Rimmelé T, Schneider AG. Hemoadsorption with CytoSorb®. Intensive Care Med. 2019;45(2):236–9. 10.1007/s00134-018-5464-6. Epub 2018 Nov 16 PMID: 30446798. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Kang X, Shi Y, Bai ZH, Lv JH, Sun JL, Pei HH. SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren Fail. 2020;42(1):638–45. 10.1080/0886022X.2020.1788581. PMID:32660294;PMCID:PMC7470067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarbock A, Küllmar M, Kindgen-Milles D, Wempe C, Gerss J, Brandenburger T, Dimski T, Tyczynski B, Jahn M, Mülling N, Mehrländer M, Rosenberger P, Marx G, Simon TP, Jaschinski U, Deetjen P, Putensen C, Schewe JC, Kluge S, Jarczak D, Slowinski T, Bodenstein M, Meybohm P, Wirtz S, Moerer O, Kortgen A, Simon P, Bagshaw SM, Kellum JA, Meersch M, RICH Investigators and the Sepnet Trial Group. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA. 2020;324(16):1629–39. 10.1001/jama.2020.18618. PMID: 33095849; PMCID: PMC7585036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khadzhynov D, Schelter C, Lieker I, Mika A, Staeck O, Neumayer HH, Peters H, Slowinski T. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J Crit Care. 2014;29(2):265–71. 10.1016/j.jcrc.2013.10.015. Epub 2013 Nov 11 PMID: 24360392. [DOI] [PubMed] [Google Scholar]
- 43.Dilken O, Ince C, van der Hoven B, Thijsse S, Ormskerk P, de Geus HRH. Successful reduction of creatine kinase and myoglobin levels in severe rhabdomyolysis using extracorporeal blood purification (CytoSorb®). Blood Purif. 2020;49(6):743–7. 10.1159/000505899. Epub 2020 Feb 28 PMID: 32114569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.