Abstract
Colorectal cancer (CRC) is widely recognized as the third most prevalent malignancy globally and the second leading cause of cancer-related mortality. Traditional treatment modalities for CRC, including surgery, chemotherapy, and radiotherapy, can be utilized either individually or in combination. However, these treatments frequently result in significant side effects due to their non-specificity and cytotoxicity affecting all cells. Moreover, a considerable number of patients face relapses following these treatments. Consequently, it is imperative to explore more efficacious treatment interventions for CRC patients. Immunotherapy, an emerging frontier in oncology, represents a novel therapeutic approach that leverages the body's immune system to target cancer cells. The principal advantage of immunotherapy is its capacity to selectively target cancer cells while minimizing damage to healthy cells. Its recent adoption as a neoadjuvant therapy presents significant potential to transform the treatment landscape for both primary resectable and metastatic CRC. This review endeavors to offer a comprehensive overview of current strategies in CRC immunotherapy, critically analyze existing literature, underscore anticipated outcomes from ongoing clinical trials, and deliberate on the challenges and impediments encountered within the field of immunotherapy.
1. Introduction
Cancer is a leading cause of mortality and represents a significant challenge to improving life expectancy worldwide. Colorectal cancer (CRC) is ranked as the third most frequently diagnosed malignancy globally and is the second most common cause of cancer-related deaths [1,2]. Colon cancer exhibits higher prevalence in North America, Europe, and Australia/New Zealand, whereas rectal cancer incidence is notably high in Eastern Asia. Conversely, the incidence rates of CRC are generally lower in most parts of Africa and South-Central Asia [3]. Notably, CRC incidence rates have demonstrated a consistent increase in regions undergoing rapid economic development, including Eastern Europe, Southeast Asia, South-Central Asia, and South America [4,5]. An emerging concern is the rising prevalence of CRC among individuals under the age of 50 [6].
CRC originates from the colon and rectum, beginning with the transformation of a normal colonic crypt into a benign intestinal polyp. Some adenomatous polyps can progress into advanced adenomas, which may eventually develop into malignant tumors capable of metastasis. However, the majority of polyps, including inflammatory and hyperplastic polyps, are not considered precancerous lesions [7]. Adenocarcinomas represent the most common type of CRC. The progression of CRC, termed “multi-step carcinogenesis”, involves a series of progressive changes [[8], [9], [10]]. Three key molecular pathways have been identified in CRC carcinogenesis: chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylation pathways (CIMP) [11]. Approximately 80 % of spontaneous CRC cases follow the CIN pathway, characterized by the classic adenoma-carcinoma sequence involving mutations in genes such as Kirsten rat sarcoma viral oncogene homolog (KRAS), adenomatous polyposis coli (APC), and tumor protein p53 (TP53). Mutations in APC are found in the majority (80–90 %) of both hereditary and sporadic CRC cases. These mutations disrupt the APC complex formation, leading to β-catenin translocation into the nucleus, hyperactivation of the Wnt signaling, and increased cell proliferation [9,12,13]. The development of cancer involves complex interactions among malignant cells, microenvironment components (such as stromal and vascular endothelial cells), and the immune system [14,15]. Consequently, the CRC microenvironment is characterized by intricate components and relationships, presenting significant challenges for therapeutic interventions.
Traditional approaches, such as surgical resection and chemotherapy, have long been considered the standard of care for CRC [16]. Despite efforts to raise public awareness for early CRC screening and advancements in cancer therapy, a considerable number of cases are diagnosed at advanced stages, contributing to the unfavorable prognosis of the disease [6,17]. For patients with unresectable lesions or those unable to undergo surgery, radiotherapy and chemotherapy remain key strategies for disease management. While radiotherapy can modulate the immune contexture of the tumor microenvironment and impact immunosurveillance, its efficacy in treating disseminated metastases is limited [16]. Recent studies have shown that chemotherapy in CRC patients, especially those with metastases, has improved their overall survival (OS), resulting in chemotherapy becoming the cornerstone of CRC treatment [[18], [19], [20]]. However, chemotherapy is associated with challenges such as systemic toxicity, variable response rates, and the development of resistance [21]. Despite the use of neoadjuvant or adjuvant chemotherapy or radiotherapy to reduce tumor burden, the prognosis for CRC, especially for patients with metastatic disease, remains unsatisfactory [6,22,23]. Therefore, the development of alternative and more effective treatments for CRC patients is imperative.
The mismatch repair (MMR)/microstatellite instability (MSI) system plays a pivotal role in the classification and therapeutic strategies for CRC. Microsatellites, comprised of repetitive nucleotide units, can undergo instability due to insertions or deletions in tumor cells, leading to frameshift mutations [24]. The MMR system, responsible for recognizing and repairing DNA damage, plays a critical role in correcting errors during DNA replication, including insertions, deletions, and mismatched bases [25]. The MMR system is categorized into mismatch repair deficiency (dMMR) and mismatch repair proficiency (pMMR). dMMR, characterized by the absence of MMR proteins, results in the accumulation of genetic errors and high instability (MSI-H) in tumor DNA. In contrast, pMMR indicates normal MMR protein expression and can be further subdivided into low instability (MSI-L) and stable (MSS) categories [26]. The dMMR/MSI-H subtype accounts for approximately 15 % of CRC cases and 5 % of metastatic CRC (mCRC) cases [[27], [28], [29], [30]]. Due to the high mutation rate in dMMR/MSI-H tumors, they exhibit increased immunogenicity, activating the immune system against the tumor. Consequently, patients with dMMR/MSI-H show enhanced responsiveness to immune-based therapies, particularly immune checkpoint inhibitors (ICI) [31,32]. Hence, the exploration of novel and more effective immunotherapeutic strategies to address the diverse subtypes of CRC has become a primary focus of current research.
Immunotherapy has emerged as a promising and effective approach in cancer treatment by harnessing the patient's own immune system [33]. It has demonstrated remarkable success, particularly in melanoma and lung cancer [34], establishing it as a primary treatment modality for challenging solid tumors, including CRC [33]. In CRC immunotherapy, various strategies are being explored, including small molecule drugs, macromolecular drugs, cancer vaccines, adoptive cell transfer, and gene therapy [35]. Small molecule drugs target specific molecular markers on cancer cells, enhancing the immune response against them. Macromolecular drugs, such as immune checkpoint inhibitors, reinvigorate immune cells and unleash their antitumor potential. Cancer vaccines aim to stimulate the immune system through diverse approaches, such as peptide or protein-based vaccines, dendritic cell vaccines, or DNA/RNA-based vaccines. Adoptive cell transfer involves the infusion of expanded immune cells, such as T cells or natural killer cells, to enhance the immune response against CRC. Gene therapy manipulates gene expression in either cancer cells or immune cells to augment antitumor activity. This comprehensive review provides an in-depth summary of CRC immunotherapy, encompassing various strategies and their underlying mechanisms. By evaluating both successes and limitations (Table 1), this review offers valuable insights into future research directions and the development of effective and personalized immunotherapeutic approaches for CRC treatment.
Table 1.
Overview of immunotherapies, their advantages and limitations.
| Types of immunotherapies | Advantages | Limitations |
|---|---|---|
| Small molecule drugs |
|
|
| Macromolecular drugs |
|
|
| Adoptive Cell Therapy |
|
|
| Cancer Vaccines | Shared advantages:
|
Shared limitations:
|
| Gene Therapy |
|
|
2. Small molecule drugs
Small molecule drugs have exhibited significant efficacy in targeting both extracellular and intracellular components, thereby modulating immune tolerance and suppression through molecular pathway alterations. This approach can elicit a robust antitumor response. By specifically manipulating key pathways and cells implicated in immune modulation, small molecules possess substantial potential to enhance the efficacy of cancer immunotherapy. The approval of imatinib, a small molecule tyrosine kinase inhibitor, in 2001 represented a seminal milestone in the development of small molecule drugs, heralding an exciting new chapter in the field of research.
2.1. Targeted tumor drugs
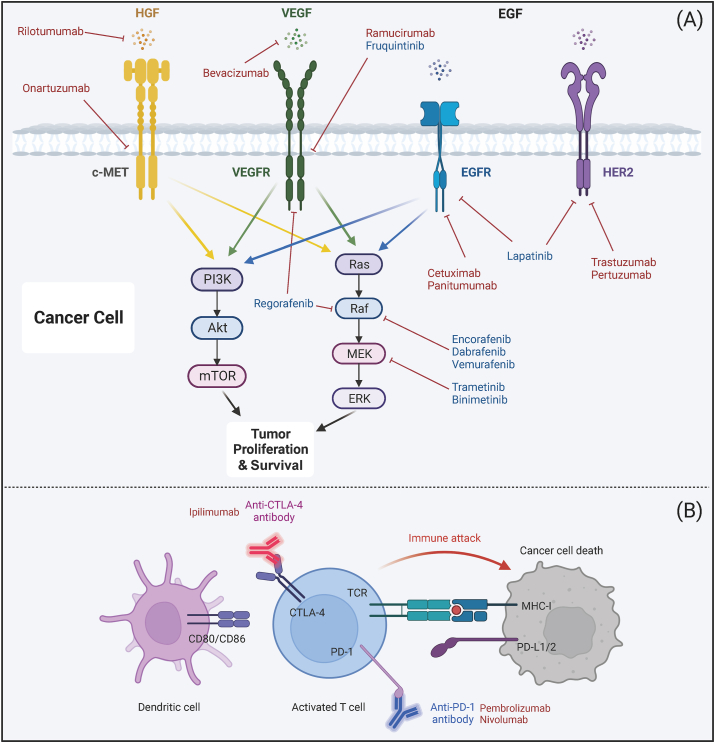
Specific small molecule drugs that target key pathways and receptors have demonstrated potential in the treatment of CRC (Fig. 1A). The epidermal growth factor receptor (EGFR), a member of the ErbB/HER family, activates intracellular signaling pathways such as RAS/RAF/MEK/ERK, PI3K/AKT, and JAK/STAT3, which are involved in regulating cellular growth, survival, and migration. In certain subgroups of CRC patients, EGFR and vascular endothelial growth factor receptor (VEGFR) inhibitors have successfully slowed disease progression, as observed in metastatic CRC (mCRC) [36] and rat sarcoma viral oncogene (RAS) wild-type mCRC patients [37]. Dysregulation of EGFR and human epidermal growth factor receptor (HER) expression is a common feature in various malignancies, rendering them potential therapeutic targets [38]. HER2 is particularly recognized as a significant therapeutic target in CRC. Recently, the FDA approved the use of pertuzumab and trastuzumab for treating adult patients with advanced RAS wild-type HER2-positive CRC [39]. The dual-targeting of HER2 and EGFR may offer increased efficacy compared to single-agent HER2-targeted therapy [40]. Angiogenesis-related factors, such as VEGF, fibroblast growth factors (FGFs), transforming growth factors (TGFs), platelet-derived growth factor (PDGF), and angiopoietins, play critical roles in tumor progression and resent potential targets for therapeutic intervention [41]. The hepatocyte growth factor (HGF), acting as an epithelial transition factor (MET) ligand, significantly contributes to tumor growth, survival, metastasis, and drug resistance [42]. Inhibition of the HGF-MET interaction could offer potential prognostic value in cancer [43].
Fig. 1.
Mechanisms of tumor target inhibitors and immunomodulatory mAbs. (A) Clinical utilization of targeted tumor inhibitors. This section illustrates targeted tumor inhibitors currently in clinical use. The inhibition of signaling pathways associated with cancer cell survival and growth leads to the death of cancer cells. Small molecule inhibitors are indicated in blue, while mAbs are indicated in red. (B) mAbs targeting immunomodulatory T cells. This section describes mAbs that target T cells, specifically anti-CTLA-4 and anti-PD-1. These mAbs counteract T cell suppression, thereby facilitating the release from immunosuppression and the subsequent elimination of cancer cells. Abbreviations: mAbs, monoclonal antibodies; HGF, hepatocyte growth factor; c-MET, mesenchymal–epithelial transition factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; EGFR, epidermal growth factor receptor; EGF, epidermal growth factor; HER2, human epidermal growth factor 2; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; PD-1, programmed death-1; PD-L1, programmed death ligand 1; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B, also known as PKB; mTOR, mammalian target of rapamycin; MEK, mitogen-activated protein kinase; ERK, extracellular signal regulated kinase. Created with BioRender.com.
Abnormal activation of tyrosine kinases, resulting from gene mutation, translocation, or overexpression, contributes to the tumorigenesis and progression of CRC. Although some tyrosine kinase inhibitors (TKIs) have demonstrated effectiveness in CRC patients, only a select few have received regulatory approval. Regorafenib, a multi-target TKI, has been shown to improve survival outcomes in patients with refractory mCRC [44]. Famitinib, which targets the c-KIT receptor, and fruquintinib, which inhibits VEGFR-1, 2, and 3, have also exhibited positive effects [45]. Cabozantinib, a broad-spectrum kinase inhibitor, has displayed promising antitumor activity in CRC models, whereas nintedanib has shown inconclusive benefits [45]. Further research is warranted to investigate the efficacy of newer agents, such as tepotinib, foretinib, glesatinib, and sitravatinib, in the treatment of CRC.
Small molecule drugs that target specific pathways and receptors offer promising therapeutic avenues for the treatment of CRC. However, the intricacy of signaling pathways and the propensity of tumor cells to develop resistance through diverse mechanisms pose significant challenges. To surmount these obstacles, the employment of combination therapies and the pursuit of further research into the mechanisms and antitumor effects of small molecule drugs are essential. By delving into the complex signaling networks that underlie CRC and elucidating the mechanisms of drug resistance, it becomes feasible to devise more targeted and efficacious treatment strategies aimed at enhancing patient outcomes.
3. Macromolecular drugs
Macromolecular drugs, particularly monoclonal antibodies (mAbs), have significantly influenced the field of oncology. Over the years, there has been a marked increase in the development and approval of mAbs for clinical applications, rendering targeted therapy with mAbs a pivotal strategy in cancer treatment (Fig. 1A). Innovative formats, such as bispecific antibodies and antibody-drug conjugates, exhibit substantial potential for the next generation of tumor-targeting therapies.
3.1. Monoclonal antibodies (mAbs)
3.1.1. Targeting tumor mAbs
Unlike polyclonal antibodies, which bind to multiple epitopes, monoclonal antibodies exhibit monovalent affinity, enabling their specific interaction with antigenic epitopes. mAbs can recognize and specifically bind to tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs) present on the surface of cancer cells (Fig. 1). Cetuximab and panitumumab, monoclonal antibodies targeting EGFR, have demonstrated significant benefits in the treatment of mCRC [46,47]. Cetuximab has shown a positive impact on progression-free survival (PFS) in irinotecan-resistant patients, while panitumumab has been used as a first-line treatment, improving PFS and overall survival (OS) [48]. Both antibodies are recommended for CRC patients with specific mutations that typically confer lower response to anti-EGFR therapies. Another antibody, bevacizumab, which targets VEGF-A, has been approved for use in mCRC and enhances PFS and OS, despite associated adverse effects [49]. Ramucirumab, targeting VEGFR-2, when combined with FOLFIRI (Fluorouracil + Irinotecan + Leucovorin), has also shown improved OS. However, further evaluation is needed for its combination efficacy with other medications [50,51]. In contrast, inhibitors of HGF and MET have not demonstrated significant benefits in mCRC patients [52]. The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) presents a potential therapeutic opportunity, and selective DR5-binding monoclonal antibodies, such as conatumumab, have shown promise in preclinical tests and phase I trials [53]. Additionally, eftozanermin alfa, a TRAIL-R agonist, exhibits anticancer activity but requires further verification [54].
3.1.2. Immunomodulatory mAbs
Immunomodulatory monoclonal antibodies (mAbs) have emerged as potent cancer therapeutics due to their ability to inhibit tumor growth and augment the immune system's recognition of cancer cells. Immune Checkpoint Inhibitors (ICIs) represent a significant class of mAbs that target proteins involved in immune checkpoint pathways, predominantly expressed on T cells or various immune cell subsets. Prominent targets include Programmed Death receptor 1 (PD-1) and Cytotoxic T-Lymphocyte Antigen 4 (CTLA4), as depicted in Fig. 1B. In CRC, the efficacy of ICIs is substantially contingent upon the immune infiltration status of the tumors. Patients with tumors exhibiting dMMR or MSI-H, which are characterized by heightened lymphocyte infiltration, have been observed to have improved superior survival outcomes. Illustratively, a study documented a notably high 12-month overall survival rate of 73 % among such patients administered nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor) [55]. In contrast, CRC tumors with MSS and pMMR typically display a diminished presence of immune cells and neoantigens, resulting in a subdued immune response. The FDA has granted approval for the anti-PD-1 antibodies pembolizumab and nivolumab for the treatment of MSI-H CRC [56,57].
While CTLA-4 inhibitors like Ipilimumab can counteract CTLA-4-mediated immunosuppression and promote T-cell activation, their clinical response in mCRC remains limited [58]. PD-1/PD-L1 inhibitors, such as pembrolizumab and nivolumab, restore effector T-cell function by disrupting the inhibitory signaling of PD-1 and its ligands. Although these inhibitors have demonstrated efficacy across various solid tumors [59], they confer more substantial benefits in CRC patients with dMMR or MSI-H, who exhibit significantly higher PFS rates compared to pMMR/MSS patients [30]. However, the therapeutic efficacy of both ICI monotherapy and combination therapy in mCRC, particularly in pMMR/MSS patients, still falls short of optimal outcomes [60,61]. Ongoing clinical trials are exploring dual immune checkpoint therapy for MSS patients, such as a combination of PD-L1 inhibitor and CTLA-4 inhibitor, but have not yet achieved improved treatment outcomes [62]. Ongoing investigations are focused on identifying new immune checkpoint targets, including T cell immunoglobulin domain and mucin domain-3 (TIM3), T cell immunoreceptor with Ig and ITIM domains (TIGIT), Lymphocyte Activation Gene-3 (LAG-3), and CD47, to expand the therapeutic options for CRC. These investigations hold promise for further enhancing treatment strategies for CRC patients.
3.2. Antibody complex
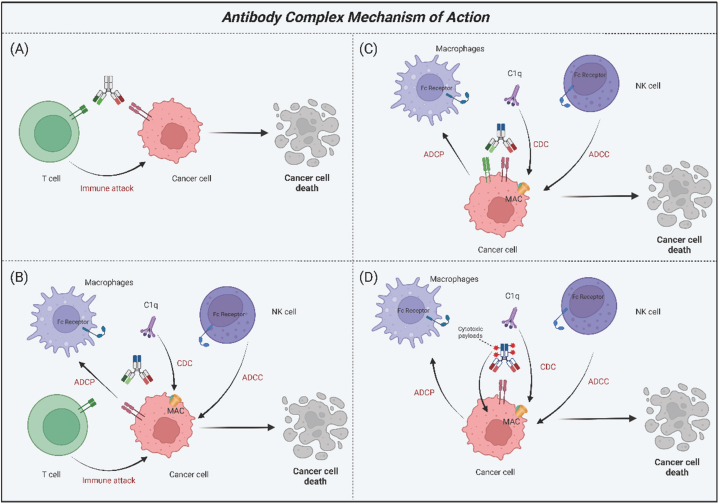
Monoclonal antibodies are characterized by their specificity to a single target, thereby conferring a singular biological effect. To mitigate unintended effects, it is essential in cancer therapy to concurrently disrupt multiple signaling pathways. Consequently, bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs) offer enhanced benefits for the treatment of cancer (Fig. 2).
Fig. 2.
Mechanisms of action of antibody complex. This figure illustrates the mechanisms of four distinct types of antibody complexes. (A) T cell recruitment. This class of antibodies facilitates the recruitment of T lymphocytes to target and attack cancer cells. (B) Enhanced immune responses. Beyond T cell recruitment, antibodies in this class possess Fc regions that mediate ADCC, ADCP, and CDC effects. (C) Dual cancer cell antigen targeting. Antibodies of this class are designed to target two distinct antigens on cancer cell, potentially enhancing their therapeutic efficacy. (D) Antibody-drug conjugates. These antibodies are capable of killing cancer cells by delivering a cytotoxic payload while also stimulating immune cells. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CDC, complement-dependent cytotoxicity; MAC, membrane attack complex, ADC, antibody-drug conjugates. Created with BioRender.com.
3.2.1. Bispecific antibodies (BsAbs)
Bispecific antibodies (BsAbs) represent a promising therapeutic approach for cancer treatment. These molecules possess the unique capability to recognize two distinct epitopes or antigens, thereby activating immune cells to target and eradicate tumor cells more effectively (Fig. 2A and B). Moreover, BsAbs have the capacity to concurrently inhibit signaling pathways associated with these disparate antigens (Fig. 2C). A notable subtype of BsAb is the bispecific T-cell engagers (BiTEs), which are recombinant proteins composed of two single-stranded variable fragments (scFv), devoid of the crystallizable fragment (Fc) region. Each scFv within a BiTE binds to either a T cell-specific molecule or a tumor-associated antigen, thereby facilitating robust T cell activation and enhancing the physical interaction between T cells and tumor cells.
Catumaxomab, a trifunctional antibody, targets three different cell types: tumor cells, T cells, and accessory immune cells, including macrophages, dendritic cells (DCs), and natural killer (NK) cells. It represents the first anti-EpCAM antibody to be approved for the treatment of malignant ascites in patients with EpCAM-positive tumors. However, caution should be exercised regarding its potential side effects [63]. Vanucizumab and duligotuzumab are other notable dual inhibitors, but their efficacy appears to be confined to specific subsets of CRC patients [64]. Bintrafusp alfa, a novel bifunctional fusion protein, inhibits immunosuppression and diminishes TGF levels within the tumor microenvironment [65]. Early clinical trials have demonstrated promising anticancer activity in patients who were previously considered untreatable [66].
3.2.2. Antibody-drug conjugate (ADC)
ADCs represent a novel class of anti-cancer treatments that combine the selectivity of monoclonal antibodies with the cytotoxic capabilities of chemotherapy agents. Upon binding to a specific antigen on cancer cells, ADCs are internalized into early endosomes, which mature into late endosomes and subsequently fuse with lysosomes (Fig. 2D). Within this compartment, the cytotoxic payload is released, triggering apoptosis in the cancer cell. As our understanding of ADCs advances alongside the development of new therapeutics, these conjugates are poised to offer significant potential in targeted cancer therapies. A notable example is cetuximab sarotalocan, an anti-EGFR ADC that demonstrates promising applications for the treatment of EGFR-expressing solid tumors, including CRC [67].
Immunotherapy utilizing mAbs has achieved significant advancements in the treatment of CRC. These mAbs demonstrate high selectivity due to their capacity to target specific antigens, positioning them as a vital therapeutic strategy for CRC. Combination therapies, which entail the simultaneous application of multiple therapeutic agents, have exhibited potential in augmenting the anticancer efficacy of macromolecular drug therapies. Despite the notable progress in macromolecular drug therapy for CRC, ongoing research and refinement are essential to maximize their effectiveness. Approaches such as personalized medicine, combination therapies, the discovery of novel targets, and the enhancement of immunotherapy response rates possess considerable potential to ameliorate treatment outcomes and yield superior clinical results for patients with CRC. Sustained endeavors in these domains will foster the evolution of CRC treatment, ultimately benefiting patients.
4. Adoptive cell therapy (ACT)
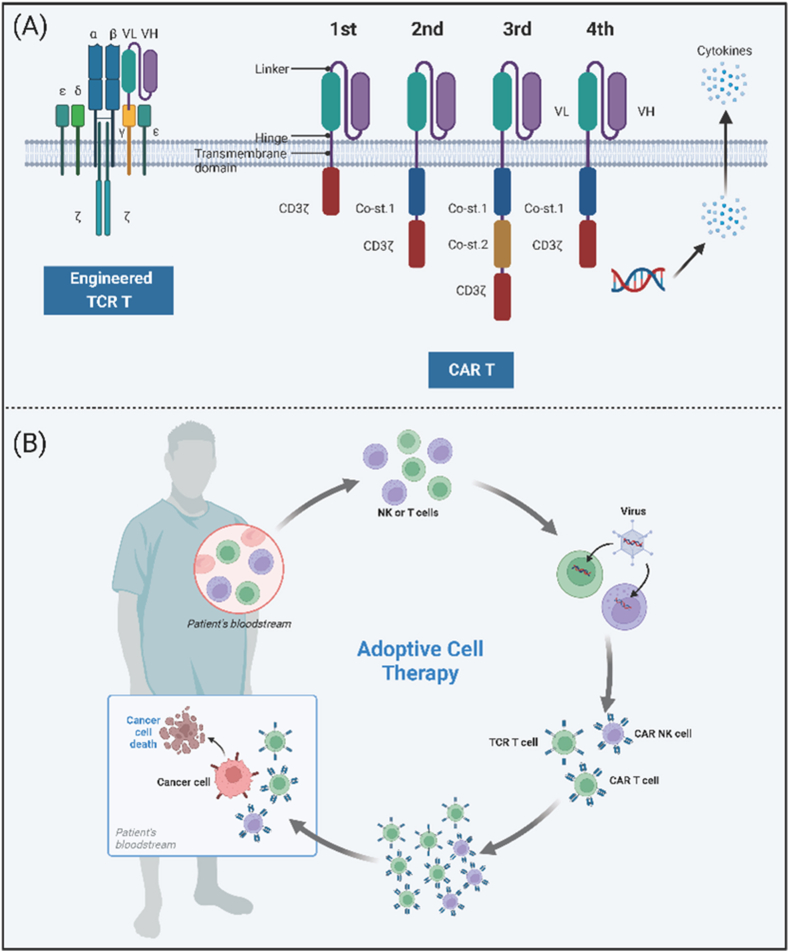
Adoptive immunotherapy presents a novel therapeutic approach for cancer treatment, harnessing immune cells such as T cells, DCs, NK cells, and cytokine-induced killer (CIK) cells. These cells, sourced from either the patient or a donor, are employed to stimulate an antitumor response in cancer patients (Fig. 3B). Among these strategies, T cell-based therapies have demonstrated the most significant promise, encompassing the utilization of tumor-infiltrating lymphocytes (TILs) and genetically modified T cells equipped with receptors intended to target the tumor, such as T cell receptors (TCRs) and chimeric antigen receptors (CARs). Although the FDA has granted approval for adoptive cell therapy (ACT) for a variety of hematologic malignancies, the majority of solid tumors remain in the clinical trial phase. This review is intended to provide a comprehensive discussion of the predominant cellular therapeutic approaches that are specifically tailored for the treatment of CRC.
Fig. 3.
Structure and therapeutic overview of adoptive cell therapy. (A) Architectural components. This section details the structure of T cells engineered with T cell receptors (TCR T) and each generation of CAR T cells. (B) Adoptive cell therapy procedure. 1) Isolation: The patient's T cells or NK cells are isolated from their blood; 2) Gene transfer: A viral vector is utilized to introduce the CAR-encoding gene into the T cells or NK cells; 3) Expression: The modified T cells or NK cells express the CAR on their cell surfaces; 4) Expansion: The CAR T cells, TCR T cells, or CAR NK cells are expanded in number; 5) Reinfusion: The engineered cells are reintroduced into the patient's bloodstream; 6) Targeting and elimination: The CAR T cells, TCR T cells, or CAR NK cells identify cancer cells expressing the target antigens and eliminate them. Abbreviations: TCR T, T cell receptor engineered T; CAR T, chimeric antigen receptor T; NK, natural killer. Created with BioRender.com.
4.1. Cytokine-induced killer (CIK)
Cytokine-Induced Killer (CIK) therapy represents an autologous form of adoptive immunotherapy, which entails the amplification of heterogeneous immune effector cells derived from peripheral blood mononuclear cells (PBMCs). CIK cells exhibit potent antitumor activity comparable to that of T lymphocytes, along with non-MHC-restricted tumoricidal properties akin to NK cells. Notably, CIK cells demonstrate robust recognition capabilities against tumor cells, functioning akin to “cellular missiles” that can precisely target tumor cells without affecting normal cells. They are especially efficacious following surgery or chemoradiotherapy, with the ability to eradicate residual micrometastases, prevent the dissemination and recurrence of cancer cells, and bolster overall immune function. Cell therapy products encompass conventional T cells (CD3+CD56−), NK-like T cells (CD3+CD56+), and NK cells (CD3−CD56+) [68]. CIK cells have demonstrated higher proliferation rates and more potent antitumor activity compared to other cell types, such as lymphokine-activated killer (LAK) cells and TILs, positioning them as a promising therapeutic option for solid tumors [68]. Research reports indicate that the proportion of the CD3+CD56+ subset increases in CRC patients following CIK cell therapy, and this increase correlates with improved OS and PFS in patients with mCRC [69,70]. A recent meta-analysis has revealed that patients who received additional CIK cell therapy achieved favorable outcomes without heightened toxicity compared to standard treatment, underscoring the necessity for further research into CIK therapy for CRC [71]. Nonetheless, optimizing cancer cell recognition in CIK therapy necessitates additional investigation [72]. Specific subsets of CIK cells, including CEA-specific CAR-CIK cells and DC-CIK cells derived from the co-culture of DCs and CIK cells, are under exploration to augment therapeutic efficacy and avert tumor recurrence [73]. While promising, further clinical studies are warranted to ascertain the effectiveness of DC-CIK therapy [74]. Addressing limitations of CIK therapy, such as constrained migration capacity, may be accomplished through strategies like the use of CEA-specific CAR-CIK cells or the modification of chemokine receptor expression in CIK cells. Recently, a novel study discovered that functional CIK cells can be cultivated from patients with liver metastatic disease from CRC, advocating for continued investigation into the therapeutic application of autologous CIK cells in the management of CRC liver metastases [75]. Elucidating the signaling pathways implicated in CIK therapy can offer valuable insights for refining this treatment modality.
4.2. Chimeric antigen receptor T (CAR-T)
CAR-T cells have demonstrated significant efficacy in immune-targeted treatments for hematological cancers, which has led to FDA approval for these applications. Recent studies indicate promising progress in CAR-T cell therapy for CRC [76]. CARs consist of four critical components: the extracellular region (typically the Fab or scFv of a monoclonal antibody for antigen specificity), hinge structure, transmembrane domain, and intracellular signaling domain. CAR-T cell immunotherapy involves genetically modifying isolated peripheral blood T lymphocytes from patients to express CARs, enabling them to target specific antigens independently of MHC and antigen-presenting cells (APCs), proliferate through T lymphocyte expansion, stimulate cytotoxic T lymphocyte effector functions, and selectively kill cancer cells [77]. First-generation CARs had limited antitumor activity due to the absence of costimulatory signaling. Second-generation CARs integrated a costimulatory domain, such as 4-1BB or CD28, to enhance CAR function. Fourth-generation CARs further improved upon this by secreting cytokines such as IL-2 and IL-12 [78] (Fig. 3A). A novel development in CAR-T cells involves focused ultrasound and heat-inducible genes, enabling localized temperature control and enhanced CAR-T targeting [79]. While CAR-T cell therapy has achieved success in B-cell leukemia and lymphoma, advancements in solid tumors lag behind. The most studied targets for CRC in CAR-T cell therapy are CEA and NKG2DL, followed by EGFR and HER2 [80]. The first clinical trial of CAR-T cells targeting CRC began in 2014, evaluating the safety and efficacy of second-generation CEA-CAR T cells in CRC patients, as well as those with lung, gastric, breast, and pancreatic cancers (NCT02349724). Only two allogeneic CAR-T cell therapies specifically targeting CRC have entered clinical trials (NCT04107142 and NCT03692429), starting in 2019 and 2020, respectively [81]. Although a phase I trial in CRC showed stable disease in 70 % of CEA+ patients treated with CAR-T cells, challenges persist due to the short-lived presence of cells in the bloodstream and the occurrence of toxic reactions such as cytokine release syndrome (CRS) [82]. CRS typically occurs 1–14 days post-infusion, with variability based on CAR-T product, trial design, and patient population. Resolution of CRS usually occurs 2–3 weeks after CAR-T infusion [83]. Previous meta-analyses reported CRS incidence rates of approximately 55.3 % in patients with hematologic malignancies treated with CAR-T cell therapy, with severe CRS occurring in approximately 18.5 % [84,85]. Overcoming these challenges is crucial to fully harness the potential of CAR-T cell therapy for CRC treatment.
ACT, akin to many other therapeutic approaches, also has several limitations. The method presents significant technical and economic challenges for both the industry and patients, primarily due to the requirement of generating tumor-specific lymphocytes tailored for each individual. Furthermore, patients who receive allogeneic transplants are frequently at risk of developing graft-versus-host disease (GVHD). Additionally, toxicity is a major concern when targeting antigenic sites, such as TAAs, which are not only expressed in normal tissues but are also often overexpressed in tumors.
Cell therapy has emerged as a promising therapeutic strategy for the treatment of CRC, offering advantages such as high specificity, prolonged effects, and the ability to overcome drug resistance. Nevertheless, safety concerns, constraints imposed by the tumor microenvironment, and production-related challenges limit its widespread application. To address these limitations, improvement strategies include optimizing cell selection, enhancing treatment safety, and improving production efficiency while reducing costs. Cell therapy involves the preparation of cell “medicines” primarily in local production facilities. Obstacles affecting time-to-market and manufacturing costs include extended manufacturing durations, complex delivery systems, and decentralized, patient-specific production [86]. Although the wholesale acquisition costs for CAR-T cell therapy in treating B-cell lymphomas amount to $373,000, recent real-world studies by Prime Therapeutics indicate average total costs exceeding $700,000, potentially surpassing $1 million in certain cases [86]. The CRISPR/Cas system represents an efficient and straightforward method for precise gene editing, offering new possibilities for optimizing CAR-T cells by enhancing functionality and reducing manufacturing costs [87]. Efforts are actively evaluating numerous approaches to make ACTs more affordable, suggesting that this goal may soon become more than just an idealistic aspiration. Continued research and innovation in the field of cell therapy hold significant promise for achieving substantial breakthroughs in the treatment of CRC, thereby offering more effective and personalized therapeutic options.
5. Cancer vaccines
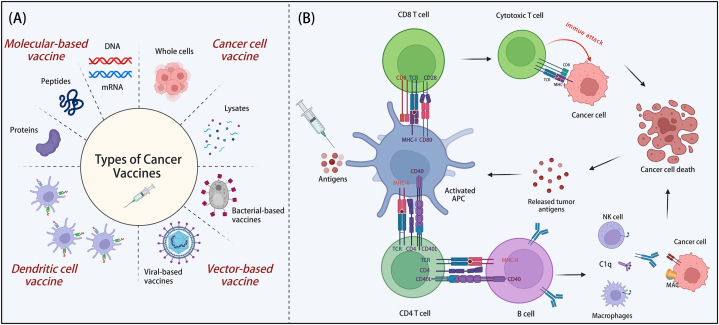
Cancer vaccines are formulated to elicit a robust immune response against tumor-specific antigens (TSAs), thereby targeting and eliminating cancer cells (Fig. 4). Since the discovery of tumor antigens in 1991, substantial advancements have been achieved in the domain of cancer vaccines. Over the intervening decades, there has been a sustained escalation in the number of clinical trials focused on CRC, concurrent with the emergence of innovative vaccine strategies. To date, the FDA has not granted approval for any CRC vaccines, with the majority remaining in the clinical trial phase.
Fig. 4.
Classification and mechanistic insights of cancer vaccines. (A) Types of cancer vaccines. This section outlines the various categories of cancer vaccines available. (B) Mechanisms of cancer vaccine action. DC vaccines: These vaccines employ DCs that are either loaded with tumor antigens ex vivo or transfected to express tumor antigens. Molecular-based and cancer cell vaccines: These types of vaccines stimulate the autologous APCs, leading to the activation of effector immune cells and the enhancement of an antitumor immune response. Abbreviations: MHC, major histocompatibility complex; TCR, T cell receptor; MAC, membrane attack complex; DC, dendritic cell; APC, antigen-presenting cell. Created with BioRender.com.
5.1. Molecular-based vaccine
Molecular-based vaccines encompass peptide/protein, DNA, and mRNA vaccines (Fig. 4A). Protein-based vaccines possess multiple immunogenic sites that are amenable for processing and presentation by MHC I/II epitopes. However, despite phase II trials demonstrating the safety of vaccines when combined with oxaliplatin-based chemotherapy, no significant differences in treatment efficacy were observed [88]. Phase I trials of DNA vaccines have indicated both vaccine safety and the capacity to elicit an immune response by introducing gene sequences that encode tumor antigens [89]. In contrast, mRNA vaccines present significant advantages, such as high potency, ease of modification, shorter production time, and have shown clinical efficiency and safety in phase I trials. Promising mRNA vaccines, such as mRNA-4157, demonstrate potential [90].
5.2. Cancer cell vaccine
Cancer cell vaccines are formulated to stimulate the immune system by introducing either whole cancer cells or their lysates (Fig. 4A). These vaccines can be categorized as autologous or allogeneic based on the source of the cancer cells utilized. Autologous vaccines provide a high degree of specificity, while allogeneic vaccines offer the benefits of rapid production and broader applicability. However, the presence of antigens in normal tissues may elevate the risk of autoimmune responses. Encouraging results have been observed with vaccines such as OncoVax and GVAX, which have effectively enhanced antitumor immune responses. Further research is warranted to explore alternative vaccine strategies [91,92].
5.3. Dendritic cell vaccine
DC vaccines, which are induced to mature through cytokine culture, have exhibited promising outcomes in clinical trials for melanoma and prostate cancer [93]. MAGE-A3 is a tumor-associated antigen (TAA) that exhibits overexpression in CRC. The vaccine MelCancerVac has demonstrated promising potential for the treatment of CRC due to its targeting of the highly expressed MAGE-A3 [94]. Further investigation of the varying DC subpopulations is warranted to enhance vaccine development strategies [95].
5.4. Vector-based vaccine
Biological vectors, such as viruses, live attenuated bacteria, and yeast, can be genetically engineered to express cancer-specific antigens, thereby initiating immune responses. Vector vaccines primarily encompass viral vector vaccines and bacterial vector vaccines (Fig. 4A). Viral vector vaccines are recombinant and utilize viruses as carriers to deliver specific tumor antigens along with co-stimulatory molecules. These viral vectors possess inherent immunogenicity, functioning as adjuvants to enhance the induction of antitumor immune responses. Commonly used viral vectors include adenoviruses, fowlpox viruses, and vaccinia viruses. Adenovirus serotype 5 (Ad5)-based immunotherapy has been repeatedly employed in humans to induce robust T cell-mediated immune responses while maintaining a high degree of safety [96]. Ad5 vaccines have demonstrated excellent safety and efficacy in treating mCRC [97]. PANVAC is a cancer vaccine therapy delivered via two viral vectors, recombinant vaccinia and recombinant fowlpox, and early clinical trials are assessing PANVAC both as a monotherapy and in combination with conventional chemotherapy and/or radiotherapy. Current research aims to establish PANVAC as a means to stimulate the immune system against malignant tumors and provide clinical benefits [98].
Oncolytic viruses (OVs) constitute an emerging approach in tumor immunotherapy, utilizing naturally occurring or genetically modified viruses that specifically infect and lyse tumor cells without harming normal cells. In addition to their direct oncolytic effects, OVs can induce immunogenic cell death and enhance antitumor immunity. Moreover, their toxicity profiles rarely overlap with those of other cancer therapies, which contributes to an acceptable safety profile [99]. Clinical trials are actively exploring the use of OVs in various solid tumors, including melanoma, glioma, and bladder cancer [[100], [101], [102]]. OVs offer new promise to the field of tumor treatment. Given their tolerable safety and unique antitumor mechanisms, combination strategies that integrate OVs with other therapies are demonstrating promising early clinical data [103].
Overcoming tolerance in the tumor microenvironment presents a significant challenge; therefore, considerable efforts have been dedicated to stimulating the immune system to generate robust responses against TAAs and TSAs [104]. Key characteristics of effective cancer vaccines include proficient antigen delivery, minimal impact on normal healthy tissues, and the capacity to elicit a strong antitumor immune response (Fig. 4B). In this context, Listeria monocytogenes, a facultative intracellular Gram-positive bacterium, serves as an attractive platform for cancer vaccine development due to its ability to activate tumor antigens and selectively deliver them to APCs, thereby generating potent antitumor cell-mediated immune responses [105,106].
Listeria-based vaccines have undergone extensive testing in numerous preclinical and clinical trials, targeting a spectrum of tumors including cervical cancer, melanoma, pancreatic cancer, breast cancer, prostate cancer, and malignant pleural mesothelioma [[107], [108], [109], [110], [111], [112], [113]]. Recently, a study on CRC indicated that a Listeria-based vaccination strategy, targeting the pericyte antigen RGS5, could elicit anti-angiogenic effects and trigger protective immune responses against colon cancer [114]. Similarly, Salmonella has been employed as a vector in CRC treatment. Recent research has shown that attenuated Salmonella strains delivering PD-1 small interfering (siRNA) can enhance the antitumor efficacy of EZH2 inhibitors in CRC [115]. It is essential to distinguish between bacterial immunogenicity and virulence during the production of recombinant vaccine vectors. Although substantial progress has been achieved in the development of cancer vaccines, their principal role seems to be as adjuvant therapies for patients with minimal residual disease or those in advanced stages of cancer. Further clinical research is warranted to evaluate their actual impact.
Cancer vaccination therapy offers several advantages, including high specificity, broad applicability, and a variety of vaccine types. However, challenges persist, particularly regarding immune tolerance and variability in treatment response. To augment the efficacy and practicality of CRC vaccinations, it is necessary to implement improvement strategies. These strategies include optimizing the selection of tumor-specific antigens, integrating multiple treatment modalities, personalizing vaccine formulations, and intensifying clinical research efforts.
In recent years, the combination therapy involving CAR-T cells and cancer vaccines has been extensively investigated across a range of cancer types. Reinhardt et al. discovered that a therapeutic approach targeting the tight junction protein claudin 6 (CLDN6), when combined with an RNA-lipoplex (RNA-LPX) vaccine encoding CLDN6, can significantly enhance the proliferation of CLDN6-specific CAR-T cells. This strategy has effectively eliminated human ovarian cancer and murine lung cancer cells in mouse models, as validated in both murine and human cancer cells [116]. Wang et al. developed CD19-CAR variants utilizing cytomegalovirus (CMV)-specific T cells. CAR-T cells engineered from CMV-specific T cells may exhibit sensitivity to CMV vaccination, potentially leading to the persistent proliferation of CAR-T cells in vivo. Furthermore, this strategy has been demonstrated to augment the measured antitumor capabilities in lymphoma-bearing mouse models [117].
Due to their high antigenic heterogeneity, cancers present significant challenges in the design of therapeutic cancer vaccines. RNA-based cancer vaccines have the capacity to encode newly mutated antigens for delivery, which contributes to their status as highly personalized medical products. However, this personalization is associated with high costs. These costs are driven by the expensive process of tumor sequencing and often necessitate complex logistics and the use of centralized sequencing facilities. One potential approach to reducing costs could involve the adoption of new decentralized third-generation sequencing technologies, which may offer improved cost efficiency.
6. Gene therapy
Genetic and epigenetic alterations are hallmarks of cancer progression. While chemotherapy is often effective, it frequently results in severe adverse effects. Targeted gene therapy presents a safer and more efficient treatment approach, aiming to improve patient survival and minimize disease recurrence. This therapy entails the introduction of new genes into cancer cells or adjacent tissues, inducing cell death or inhibiting the spread of cancer. It can be integrated with conventional therapies and utilizes targeted genetic approaches to enhance immune responses against tumors, facilitating the direct delivery of cytotoxic agents or genes to tumor cells. Over the past three decades, there have been significant advancements in cancer gene therapy, leading to the approval of several drugs and the initiation of ongoing clinical trials [118].
6.1. Targeting p53 and KRAS
CRC arises from the accumulation of genetic and epigenetic alterations that disrupt signaling pathways regulating cancer. Six key driver genes in CRC have been identified: APC, KRAS, B-Raf proto-oncogene, serine/threonine kinase (BRAF), Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), SMAD family member 4 (SMAD4), and TP53 [119]. Gene therapy targeting the p53 gene is being explored for various solid tumors, with the aim of inhibiting mutant p53 function and halting CRC progression. Gendicine, the first gene therapy product for head and neck squamous cell carcinoma (HNSCC), has demonstrated tumor suppressor activity by restoring wild-type p53 expression, leading to cell cycle arrest, DNA repair, or programmed cell death in response to cellular stress. Other targeted gene therapies, such as Rexin-G for pancreatic cancer and VB-111 for glioblastoma, have shown promise [120,121]. In contrast, KRAS-targeted gene therapies are still underdeveloped, despite indications of potential efficacy. The KRAS gene is one of the most commonly mutated oncogenes, accounting for approximately 40 % of all CRC cases when mutated [122]. RAS proteins are small membrane-associated GTP-binding proteins that participate in multiple signaling pathways, ultimately regulating cell growth, motility, angiogenesis, and survival in various cancer types [123]. These mutations can lead to the permanent activation of KRAS, resulting in uncontrolled cell growth and proliferation. The downstream signaling pathways activated by mutated KRAS involve a complex network of proteins that promote cell proliferation and survival, which are associated with tumor progression and resistance to targeted therapies [124].
Recommended treatment regimens for KRAS-mutated CRC include fluoropyrimidine-based therapies in combination with oxaliplatin and/or irinotecan, as well as the incorporation of anti-VEGF therapies in both first- and second-line treatments [125]. Clinical efficacy has been observed with the use of KRAS G12C inhibitors, such as adagrasib and sotorasib, in previously treated patients with various tumor types, including CRC [126,127]. Currently, the KRYSTAL-10 trial is a randomized Phase III study comparing the combination of adagrasib with cetuximab against chemotherapy. This trial has completed patient recruitment and is awaiting results in the coming months.
Additionally, novel KRAS inhibitors, such as divarasib and garsorasib, have demonstrated significant clinical activity, achieving disease control rates of 84 % and 95 %, respectively, in monotherapy. They have also shown considerable clinical activity when used in combination with anti-EGFR therapies. Specifically, when divarasib is used in conjunction with cetuximab, the disease control rate can increase to 95.8 % [128,129]. However, despite the promising data from these trials, careful interpretation is essential due to the small size and the non-randomized nature of the Phase I and II clinical trials. Therefore, standardized treatment regimens involving p53 and KRAS gene therapy require further development and rigorous evaluation.
Gene therapy for CRC offers several advantages, including high specificity, safety, and a diversity of approaches. However, it faces significant challenges due to technological limitations and variability in treatment effectiveness. To date, the FDA has approved a total of 19 gene therapies, many of which now include the first CRISPR genome editing therapy for sickle cell disease, CASGEVY. This therapy is currently the most expensive drug on the market, with a price tag exceeding $4 million per patient. Jennifer A. Doudna has proposed a pricing structure that, once implemented, could potentially reduce costs per patient by an order of magnitude and has suggested a business model that allocates responsibility while leveraging diverse funding sources [130]. By enhancing gene delivery efficiency, integrating multimodal therapies, implementing personalized treatment strategies, and advancing clinical research, the efficacy and feasibility of gene therapy for CRC can be significantly improved.
7. Future directions
Given the escalating global prevalence of CRC, there is an urgent need to develop superior treatment options for advanced and metastatic disease. Immunotherapy has demonstrated promising responses in a significant number of CRC patients, improving patient survival, minimizing adverse effects, and in some cases, facilitating complete recovery. However, a fundamental challenge is overcoming primary resistance to immunotherapy, which is prevalent among the majority of CRC patients. Potential mechanisms of resistance in CRC encompass downregulation of tumor antigen presentation, loss of T cell functionality in the host, and the emergence of mutant variants enabling cancer cells to evade immune surveillance. Combination therapy, targeting multiple pathways, is regarded as the most potent antitumor strategy. Various pathways are being targeted using a combination of multiple drugs, increasing the likelihood of arresting disease progression. This therapeutic approach can effectively tackle tumor heterogeneity, enhance treatment efficacy, and counteract drug resistance, thereby aiding in the eradication of cancer stem cells (Table 2).
Table 2.
Summary of clinical studies on immunotherapy for CRC from January 1, 2023, to August 11, 2024, as indexed on PubMed.
| No. | Drug name | Target | Immunotherapy type | ClinicalTrials.gov identifier | CRC type | References |
|---|---|---|---|---|---|---|
| 1 | ELI-002 2P | G12D and G12R mutant KRAS | Cancer vaccine | NCT04853017 | CRC | Pant et al. [131] |
| 2 | Nivolumab plus Ipilimumab | PD-1 | Monoclonal antibody | NCT03026140 | dMMR CRC | Chalabi et al. [132] |
| 3 | Bevacizumab | anti-VEGF-A | Monoclonal antibody | NCT03950154 | mCRC | Pan et al. [133] |
| 4 | Divarasib plus Cetuximab | KRAS G12C | Monoclonal antibody | NCT04449874 | KRAS G12C | Desai et al. [129] |
| 5 | Oleclumab | CD73 | Monoclonal antibody | NCT02503774 | Advanced CRC | Bendell et al. [134] |
| 6 | Nivolumab and Metformin | PD-1 | Monoclonal antibody | NCT03800602 | MSS CRC | Akce et al. [135] |
| 7 | PexaVec | / | Oncolytic viruses | NCT03206073 | pMMR CRC | Monge et al. [136] |
| 8 | Camrelizumab | PD-1 | Monoclonal antibody | / | pMMR CRC | Li et al. [137] |
| 9 | Personalized neoantigen vaccine | / | Cancer vaccine | / | MSS CRC | Yu et al. [138] |
| 10 | T-VEC | / | Oncolytic viruses | / | CRC with liver metastases | Hecht et al. [139] |
| 11 | Nivolumab | PD-1 | Monoclonal antibody | NCT03414983 | mCRC | Lenz et al. [140] |
| 12 | Pixatimod | TLR9 | NCT05061017 | MSS CRC | Lemech et al. [141] | |
| 13 | NIS793 | TGF-β | Monoclonal antibody | NCT02947165 | MSS CRC | Bauer et al. [142] |
| 14 | MUC1Peptide Vaccine | MUC1 | / | Colorectal Adenoma | Schoen et al. [143] | |
| 15 | Durvalumab and Tremelimumab | PD-L1 CTLA-4 | Monoclonal antibody | / | mCRC | Loree et al. [144] |
| 16 | Durvalumab | PD-L1 | Monoclonal antibody | NCT04083365 | Advanced CRC | Grassi et al. [145] |
| 17 | Monalizumab plus Durvalumab | NKG2A/CD94 and PD-L1 | Monoclonal antibody | NCT02671435 | MSS CRC | Patel et al. [146] |
| 18 | PD-1 blockade plus COX inhibitors | COX and PD-1 | Monoclonal antibody | NCT03638297 | dMMR mCRC | Wu et al. [147] |
| 19 | Enadenotucirev | / | Adenoviral vector | NCT02636036 | MSI-low/MSS CRC | Fakih et al. [148] |
| 20 | Urelumab | CD137 agonist | Monoclonal antibody | NCT02110082; NCT02253992 | mCRC | Khushalani et al. [149] |
| 21 | VB-111 | Tumor microenvironment | Adenoviral vector | NCT04166383 | MSS CRC with liver metastases | Coffman-D'Annibale et al. [150] |
| 22 | hTERT | / | Vaccination | / | CRC | Zareian et al. [151] |
| 23 | Cibisatamab | CD3 and CEA | Bispecific antibody |
NCT02324257 NCT02650713 |
MSS CRC | Segal et al. [152] |
| 24 | TERTiNTs | 4-1BB | Cell therapy | / | CRC | Choi et al. [153] |
| 25 | NEO-201 | CEACAM-5/6 | Monoclonal antibody | NCT03476681 | CRC | Cole et al. [154] |
| 26 | Sapanisertib | mTORC1/2 | Monoclonal antibody | / | CRC | Coleman et al. [155] |
Emerging tools, such as nanotherapies that leverage specific characteristics of the TME, offer novel avenues for immunotherapy. When nanotherapies are used in combination with other tumor-targeted therapeutics, they have demonstrated superior outcomes compared to single-targeted agents. Numerous studies have confirmed that nanoparticle-mediated, TME-targeted antitumor therapies exhibit potent immunosuppressive effects and hold potential for treating tumors when used in conjunction with other therapeutic approaches [156]. Recent advancements in computational analysis and artificial intelligence (AI) have deepened our understanding of the TME. By integrating and analyzing complex molecular data from immune cells, stromal cells, and other components of the TME, AI and computational biology can elucidate the molecular mechanisms underlying the TME and its pathological outcomes. While questions regarding their reliability and accuracy persist, the widespread application of these techniques and their potential as effective adjuncts to tumor immunotherapy are anticipated to expand as the technology matures.
CRediT authorship contribution statement
Yuan Li: Writing – original draft, Visualization, Investigation. Zewei Cheng: Writing – original draft, Visualization, Data curation. Shengli Li: Writing – review & editing, Supervision, Investigation, Funding acquisition, Conceptualization. Jiwei Zhang: Writing – review & editing, Investigation, Funding acquisition, Conceptualization.
Declaration
We confirm that this manuscript is not under consideration elsewhere and that all authors have consented to its submission.
Data availability statement
No data were utilized in the research presented within this article.
Ethics approval and consent to participate
Not applicable. This article contains no studies performed by authors with human participants or animals. It is a comprehensive review, synthesizing insights from previously published articles.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Sponsored by Shanghai Pujiang Program (20PJ1413000) and the National Natural Science Foundation of China (82173106, 82130115, 82004004, 8129010803, 82074011).
Contributor Information
Shengli Li, Email: shengli.li@sjtu.edu.cn.
Jiwei Zhang, Email: joezhang@shutcm.edu.cn.
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M., et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–349. doi: 10.1053/j.gastro.2020.02.068. e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidler M.M., Soerjomataram I., Bray F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer. 2016;139:2436–2446. doi: 10.1002/ijc.30382. [DOI] [PubMed] [Google Scholar]
- 6.Keum N., Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 7.Kuipers E.J., et al. 2015. Colorectal Cancer. Nat Rev Dis Primers 1, 15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz S.D., Bertagnolli M.M. Molecular origins of cancer: molecular basis of colorectal cancer. N. Engl. J. Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 11.Hardiman K.M. Update on sporadic colorectal cancer genetics. Clin. Colon Rectal Surg. 2018;31:147–152. doi: 10.1055/s-0037-1602234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Perochon J., Carroll L.R., Cordero J.B. Wnt signalling in intestinal stem cells: lessons from mice and flies. Genes. 2018;9 doi: 10.3390/genes9030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seton-Rogers, Oncogenes S. Driving immune evasion. Nat. Rev. Cancer. 2018;18:67. doi: 10.1038/nrc.2018.5. [DOI] [PubMed] [Google Scholar]
- 15.Mascaux C., et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019;571:570–575. doi: 10.1038/s41586-019-1330-0. [DOI] [PubMed] [Google Scholar]
- 16.Brown K.G.M., Solomon M.J., Mahon K., O'Shannassy S. Management of colorectal cancer. BMJ. 2019;366:l4561. doi: 10.1136/bmj.l4561. [DOI] [PubMed] [Google Scholar]
- 17.Andrew A.S., et al. Risk factors for diagnosis of colorectal cancer at a late stage: a population-based study. J. Gen. Intern. Med. 2018;33:2100–2105. doi: 10.1007/s11606-018-4648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colucci G., et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J. Clin. Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy J., et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J. Clin. Oncol. 2004;22:2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg R.M., et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Bach S.P., et al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. Lancet Gastroenterol Hepatol. 2021;6:92–105. doi: 10.1016/S2468-1253(20)30333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Gundin J., Fernandez-Carballido A.M., Martinez-Valdivieso L., Barreda-Hernandez D., Torres-Suarez A.I. New trends in the therapeutic approach to metastatic colorectal cancer. Int. J. Med. Sci. 2018;15:659–665. doi: 10.7150/ijms.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Stok E.P., Spaander M.C.W., Grunhagen D.J., Verhoef C., Kuipers E.J. Surveillance after curative treatment for colorectal cancer. Nat. Rev. Clin. Oncol. 2017;14:297–315. doi: 10.1038/nrclinonc.2016.199. [DOI] [PubMed] [Google Scholar]
- 24.Baretti M., Le D.T. DNA mismatch repair in cancer. Pharmacol. Ther. 2018;189:45–62. doi: 10.1016/j.pharmthera.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Olave M.C., Graham R.P. Mismatch repair deficiency: the what, how and why it is important. Genes Chromosomes Cancer. 2022;61:314–321. doi: 10.1002/gcc.23015. [DOI] [PubMed] [Google Scholar]
- 26.Boland C.R., Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. e2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner H., Kloor M., Pox C.P. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venderbosch S., et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le D.T., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilbur H.C., Le D.T., Agarwal P. Immunotherapy of MSI cancer: facts and hopes. Clin. Cancer Res. 2024;30:1438–1447. doi: 10.1158/1078-0432.CCR-21-1935. [DOI] [PubMed] [Google Scholar]
- 32.Bhamidipati D., Subbiah V. Tumor-agnostic drug development in dMMR/MSI-H solid tumors. Trends Cancer. 2023;9:828–839. doi: 10.1016/j.trecan.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhao W., et al. Colorectal cancer immunotherapy-Recent progress and future directions. Cancer Lett. 2022;545 doi: 10.1016/j.canlet.2022.215816. [DOI] [PubMed] [Google Scholar]
- 34.Ott P.A., et al. A phase ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183:347–362. doi: 10.1016/j.cell.2020.08.053. e324. [DOI] [PubMed] [Google Scholar]
- 35.Ganesh K. Optimizing immunotherapy for colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2022;19:93–94. doi: 10.1038/s41575-021-00569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loree J.M., et al. Expanded low allele frequency RAS and BRAF V600E testing in metastatic colorectal cancer as predictive biomarkers for cetuximab in the randomized CO.17 trial. Clin. Cancer Res. 2021;27:52–59. doi: 10.1158/1078-0432.CCR-20-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe J., et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329:1271–1282. doi: 10.1001/jama.2023.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arteaga C.L., Engelman J.A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickler J.H., et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. 2023;24:496–508. doi: 10.1016/S1470-2045(23)00150-X. [DOI] [PubMed] [Google Scholar]
- 40.Yang L., et al. Depleting receptor tyrosine kinases EGFR and HER2 overcomes resistance to EGFR inhibitors in colorectal cancer. J. Exp. Clin. Cancer Res. 2022;41:184. doi: 10.1186/s13046-022-02389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez A., Harada K., Vasilakopoulou M., Shanbhag N., Ajani J.A. Targeting angiogenesis in colorectal carcinoma. Drugs. 2019;79:63–74. doi: 10.1007/s40265-018-1037-9. [DOI] [PubMed] [Google Scholar]
- 42.Vimalraj S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022;221:1428–1438. doi: 10.1016/j.ijbiomac.2022.09.129. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q., Yang S., Wang K., Sun S.Y. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J. Hematol. Oncol. 2019;12:63. doi: 10.1186/s13045-019-0759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Argiles G., et al. Regorafenib plus modified FOLFOX6 as first-line treatment of metastatic colorectal cancer: a phase II trial. Eur. J. Cancer. 2015;51:942–949. doi: 10.1016/j.ejca.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Van Cutsem E., et al. Nintedanib for the treatment of patients with refractory metastatic colorectal cancer (LUME-Colon 1): a phase III, international, randomized, placebo-controlled study. Ann. Oncol. 2018;29:1955–1963. doi: 10.1093/annonc/mdy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price T.J., et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569–579. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 47.Terazawa T., et al. Phase II study of panitumumab monotherapy in chemotherapy-naive frail or elderly patients with unresectable RAS wild-type colorectal cancer: ogsg 1602. Oncol. 2021;26:17. doi: 10.1002/ONCO.13523. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham D., et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 49.Passardi A., et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first-line treatment from the ITACa randomized clinical trial. Ann. Oncol. 2015;26:1201–1207. doi: 10.1093/annonc/mdv130. [DOI] [PubMed] [Google Scholar]
- 50.Shah M.A., et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3:620–627. doi: 10.1001/jamaoncol.2016.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spigel D.R., et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J. Clin. Oncol. 2017;35:412–420. doi: 10.1200/JCO.2016.69.2160. [DOI] [PubMed] [Google Scholar]
- 52.Bendell J.C., et al. A phase II randomized trial (GO27827) of first-line FOLFOX plus bevacizumab with or without the MET inhibitor onartuzumab in patients with metastatic colorectal cancer. Oncol. 2017;22:264–271. doi: 10.1634/theoncologist.2016-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbst R.S., et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin. Cancer Res. 2010;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 54.LoRusso P., et al. Eftozanermin alfa (ABBV-621) monotherapy in patients with previously treated solid tumors: findings of a phase 1, first-in-human study. Invest. N. Drugs. 2022;40:762–772. doi: 10.1007/s10637-022-01247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overman M.J., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 56.Ganesh K., et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris V.K., et al. Treatment of metastatic colorectal cancer: ASCO guideline. J. Clin. Oncol. 2023;41:678–700. doi: 10.1200/JCO.22.01690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoos A., et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin. Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu T., et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest. N. Drugs. 2016;34:347–354. doi: 10.1007/s10637-016-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mettu N.B., et al. Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: a randomized clinical trial. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.49040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson B., et al. Phase II study of durvalumab (anti-PD-L1) and trametinib (MEKi) in microsatellite stable (MSS) metastatic colorectal cancer (mCRC) J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-005332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thibaudin M., et al. First-line durvalumab and tremelimumab with chemotherapy in RAS-mutated metastatic colorectal cancer: a phase 1b/2 trial. Nat. Med. 2023;29:2087–2098. doi: 10.1038/s41591-023-02497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linke R., Klein A., Seimetz D. Catumaxomab: clinical development and future directions. mAbs. 2010;2:129–136. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hidalgo M., et al. First-in-Human phase I study of single-agent vanucizumab, A first-in-class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin. Cancer Res. 2018;24:1536–1545. doi: 10.1158/1078-0432.CCR-17-1588. [DOI] [PubMed] [Google Scholar]
- 65.Lind H., et al. Dual targeting of TGF-beta and PD-L1 via a bifunctional anti-PD-L1/TGF-betaRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strauss J., et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFbeta, in advanced solid tumors. Clin. Cancer Res. 2018;24:1287–1295. doi: 10.1158/1078-0432.CCR-17-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitamura N., et al. Current trends and future prospects of molecular targeted therapy in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2020;22 doi: 10.3390/ijms22010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao X., et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front. Immunol. 2017;8:774. doi: 10.3389/fimmu.2017.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., et al. Growth of human colorectal cancer SW1116 cells is inhibited by cytokine-induced killer cells. Clin. Dev. Immunol. 2011;2011 doi: 10.1155/2011/621414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan Q.Z., et al. Retrospective analysis of the efficacy of cytokine-induced killer cell immunotherapy combined with first-line chemotherapy in patients with metastatic colorectal cancer. Clin Transl Immunology. 2020;9 doi: 10.1002/cti2.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li C.M.Y., et al. Use of cytokine-induced killer cell therapy in patients with colorectal cancer: a systematic review and meta-analysis. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2023-006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ying Li C.M., et al. Clinical application of cytokine-induced killer (CIK) cell therapy in colorectal cancer: current strategies and future challenges. Cancer Treat Rev. 2024;122 doi: 10.1016/j.ctrv.2023.102665. [DOI] [PubMed] [Google Scholar]
- 73.Wang S., et al. DC-CIK as a widely applicable cancer immunotherapy. Expet Opin. Biol. Ther. 2020;20:601–607. doi: 10.1080/14712598.2020.1728250. [DOI] [PubMed] [Google Scholar]
- 74.Xu H., et al. Analysis of the clinical efficacy of dendritic cell -cytokine induced killer cell-based adoptive immunotherapy for colorectal cancer. Immunol. Invest. 2021;50:622–633. doi: 10.1080/08820139.2020.1781881. [DOI] [PubMed] [Google Scholar]
- 75.Li C.M.Y., et al. Generation and assessment of cytokine-induced killer cells for the treatment of colorectal cancer liver metastases. Cancer Immunol. Immunother. 2024;73:6. doi: 10.1007/s00262-023-03591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C., et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol. Ther. 2017;25:1248–1258. doi: 10.1016/j.ymthe.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chao Z., Liu W., Liu Y. Advances of CAR-T cell therapy in treating colorectal cancer] Sheng Wu Gong Cheng Xue Bao. 2024;40:1365–1379. doi: 10.13345/j.cjb.230741. [DOI] [PubMed] [Google Scholar]
- 78.Huang R., et al. Recent advances in CAR-T cell engineering. J. Hematol. Oncol. 2020;13:86. doi: 10.1186/s13045-020-00910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y., et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 2021;5:1336–1347. doi: 10.1038/s41551-021-00779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chuang L., Qifeng J., Shaolei Y. The tumor immune microenvironment and T-cell-related immunotherapies in colorectal cancer. Discov Oncol. 2024;15:244. doi: 10.1007/s12672-024-01117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aparicio C., et al. Cell therapy for colorectal cancer: the promise of chimeric antigen receptor (CAR)-T cells. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morris E.C., Neelapu S.S., Giavridis T., Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022;22:85–96. doi: 10.1038/s41577-021-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frey N., Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transplant. 2019;25:e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 84.Shimabukuro-Vornhagen A., et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grigor E.J.M., et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus. Med. Rev. 2019;33:98–110. doi: 10.1016/j.tmrv.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Schaft N., et al. The future of affordable cancer immunotherapy. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1248867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tao R., et al. Revolutionizing cancer treatment: enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1354825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murahashi M., et al. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin. Immunol. 2016;166–167:48–58. doi: 10.1016/j.clim.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Shahnazari M., Samadi P., Pourjafar M., Jalali A. Therapeutic vaccines for colorectal cancer: the progress and future prospect. Int. Immunopharm. 2020;88 doi: 10.1016/j.intimp.2020.106944. [DOI] [PubMed] [Google Scholar]
- 90.Harris J.E., et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J. Clin. Oncol. 2000;18:148–157. doi: 10.1200/JCO.2000.18.1.148. [DOI] [PubMed] [Google Scholar]
- 91.Fu C., et al. Therapeutic antitumor efficacy of cancer stem cell-derived DRibble vaccine on colorectal carcinoma. Int. J. Med. Sci. 2021;18:3249–3260. doi: 10.7150/ijms.61510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang N., et al. A novel vaccinia virus enhances anti-tumor efficacy and promotes a long-term anti-tumor response in a murine model of colorectal cancer. Mol Ther Oncolytics. 2021;20:71–81. doi: 10.1016/j.omto.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang D.K., Zuo Q., He Q.Y., Li B. Targeted immunotherapies in gastrointestinal cancer: from molecular mechanisms to implications. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.705999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berry J., et al. Cancer vaccines in colon and rectal cancer over the last decade: lessons learned and future directions. Expet Rev. Clin. Immunol. 2017;13:235–245. doi: 10.1080/1744666X.2016.1226132. [DOI] [PubMed] [Google Scholar]
- 95.Maruoka S., et al. Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro. Sci. Rep. 2022;12:3295. doi: 10.1038/s41598-022-07305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tatsis N., Ertl H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morse M.A., et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol. Immunother. 2013;62:1293–1301. doi: 10.1007/s00262-013-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madan R.A., Arlen P.M., Gulley J.L. PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expet Opin. Biol. Ther. 2007;7:543–554. doi: 10.1517/14712598.7.4.543. [DOI] [PubMed] [Google Scholar]
- 99.Ban W., et al. Emerging systemic delivery strategies of oncolytic viruses: a key step toward cancer immunotherapy. Nano Res. 2022;15:4137–4153. doi: 10.1007/s12274-021-4031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Desjardins A., et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman G.K., et al. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N. Engl. J. Med. 2021;384:1613–1622. doi: 10.1056/NEJMoa2024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gallego Perez-Larraya J., et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 2022;386:2471–2481. doi: 10.1056/NEJMoa2202028. [DOI] [PubMed] [Google Scholar]
- 103.Shalhout S.Z., Miller D.M., Emerick K.S., Kaufman H.L. Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol. 2023;20:160–177. doi: 10.1038/s41571-022-00719-w. [DOI] [PubMed] [Google Scholar]
- 104.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gunn G.R., et al. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 106.Ding Y.D., et al. Listeria monocytogenes: a promising vector for tumor immunotherapy. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1278011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Basu P., et al. A randomized phase 2 study of ADXS11-001 Listeria monocytogenes-listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int. J. Gynecol. Cancer. 2018;28:764–772. doi: 10.1097/IGC.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duan F., et al. Enhanced therapeutic efficacy of Listeria-based cancer vaccine with codon-optimized HPV16 E7. Hum. Vaccines Immunother. 2021;17:1568–1577. doi: 10.1080/21645515.2020.1839291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gilley R.P., Dube P.H. Checkpoint blockade inhibitors enhances the effectiveness of a Listeria monocytogenes-based melanoma vaccine. Oncotarget. 2020;11:740–754. doi: 10.18632/oncotarget.27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xin G., et al. Pathogen boosted adoptive cell transfer immunotherapy to treat solid tumors. Proc. Natl. Acad. Sci. U. S. A. 2017;114:740–745. doi: 10.1073/pnas.1614315114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stein M.N., et al. ADXS31142 immunotherapy +/- pembrolizumab treatment for metastatic castration-resistant prostate cancer: open-label phase I/II KEYNOTE-046 study. Oncol. 2022;27:453–461. doi: 10.1093/oncolo/oyac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drake C.G., et al. Safety and preliminary immunogenicity of JNJ-64041809, a live-attenuated, double-deleted Listeria monocytogenes-based immunotherapy, in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2022;25:219–228. doi: 10.1038/s41391-021-00402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsujikawa T., et al. Evaluation of cyclophosphamide/GVAX pancreas followed by listeria-mesothelin (CRS-207) with or without nivolumab in patients with pancreatic cancer. Clin. Cancer Res. 2020;26:3578–3588. doi: 10.1158/1078-0432.CCR-19-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson T.S., et al. Listeria-based vaccination against the pericyte antigen RGS5 elicits anti-vascular effects and colon cancer protection. OncoImmunology. 2023;12 doi: 10.1080/2162402X.2023.2260620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lv M., et al. Attenuated Salmonella-delivered PD-1 siRNA enhances the antitumor effects of EZH2 inhibitors in colorectal cancer. Int. Immunopharm. 2023;124 doi: 10.1016/j.intimp.2023.110918. [DOI] [PubMed] [Google Scholar]
- 116.Reinhard K., et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 117.Wang X., Diamond D.J., Forman S.J., Nakamura R. Development of CMV-CD19 bi-specific CAR T cells with post-infusion in vivo boost using an anti-CMV vaccine. Int. J. Hematol. 2021;114:544–553. doi: 10.1007/s12185-021-03215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belete T.M. The current status of gene therapy for the treatment of cancer. Biologics. 2021;15:67–77. doi: 10.2147/BTT.S302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang D., et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018;37:173–187. doi: 10.1007/s10555-017-9726-5. [DOI] [PubMed] [Google Scholar]
- 120.Gordon E.M., et al. First clinical experience using a 'pathotropic' injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int. J. Oncol. 2004;24:177–185. [PubMed] [Google Scholar]
- 121.Gordon E.M., Hall F.L. Rexin-G, a targeted genetic medicine for cancer. Expet Opin. Biol. Ther. 2010;10:819–832. doi: 10.1517/14712598.2010.481666. [DOI] [PubMed] [Google Scholar]
- 122.Zhu G., Pei L., Xia H., Tang Q., Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer. 2021;20:143. doi: 10.1186/s12943-021-01441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ros J., et al. Targeting KRAS G12C mutation in colorectal cancer, A review: new arrows in the quiver. Int. J. Mol. Sci. 2024;25 doi: 10.3390/ijms25063304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y., et al. Impact of KRAS mutation on the tumor microenvironment in colorectal cancer. Int. J. Biol. Sci. 2024;20:1947–1964. doi: 10.7150/ijbs.88779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cervantes A., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023;34:10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Yaeger R., et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. 2023;388:44–54. doi: 10.1056/NEJMoa2212419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hong D.S., et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacher A., et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N. Engl. J. Med. 2023;389:710–721. doi: 10.1056/NEJMoa2303810. [DOI] [PubMed] [Google Scholar]
- 129.Desai J., et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat. Med. 2024;30:271–278. doi: 10.1038/s41591-023-02696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kliegman M., et al. A roadmap for affordable genetic medicines. Nature. 2024;634:307–314. doi: 10.1038/s41586-024-07800-7. [DOI] [PubMed] [Google Scholar]
- 131.Pant S., et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial. Nat. Med. 2024;30:531–542. doi: 10.1038/s41591-023-02760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chalabi M., et al. Neoadjuvant immunotherapy in locally advanced mismatch repair-deficient colon cancer. N. Engl. J. Med. 2024;390:1949–1958. doi: 10.1056/NEJMoa2400634. [DOI] [PubMed] [Google Scholar]
- 133.Pan Q.Z., et al. XELOX (capecitabine plus oxaliplatin) plus bevacizumab (anti-VEGF-A antibody) with or without adoptive cell immunotherapy in the treatment of patients with previously untreated metastatic colorectal cancer: a multicenter, open-label, randomized, controlled, phase 3 trial. Signal Transduct. Targeted Ther. 2024;9:79. doi: 10.1038/s41392-024-01788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bendell J., et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 2023;72:2443–2458. doi: 10.1007/s00262-023-03430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akce M., et al. Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2023-007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Monge C., et al. Phase I/II study of PexaVec in combination with immune checkpoint inhibition in refractory metastatic colorectal cancer. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Y., et al. Total neoadjuvant therapy with PD-1 blockade for high-risk proficient mismatch repair rectal cancer. JAMA Surg. 2024;159:529–537. doi: 10.1001/jamasurg.2023.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu Y.J., et al. Preliminary clinical study of personalized neoantigen vaccine therapy for microsatellite stability (MSS)-advanced colorectal cancer. Cancer Immunol. Immunother. 2023;72:2045–2056. doi: 10.1007/s00262-023-03386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hecht J.R., et al. Phase Ib study of talimogene laherparepvec in combination with atezolizumab in patients with triple negative breast cancer and colorectal cancer with liver metastases. ESMO Open. 2023;8 doi: 10.1016/j.esmoop.2023.100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lenz H.J., et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: phase 2 results from the CheckMate 9X8 randomized clinical trial. J Immunother Cancer. 2024;12 doi: 10.1136/jitc-2023-008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lemech C., et al. Phase Ib open-label, multicenter study of pixatimod, an activator of TLR9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bauer T.M., et al. Phase I/Ib, open-label, multicenter, dose-escalation study of the anti-TGF-beta monoclonal antibody, NIS793, in combination with spartalizumab in adult patients with advanced tumors. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2023-007353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schoen R.E., et al. Randomized, double-blind, placebo-controlled trial of MUC1 peptide vaccine for prevention of recurrent colorectal adenoma. Clin. Cancer Res. 2023;29:1678–1688. doi: 10.1158/1078-0432.CCR-22-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loree J.M., et al. Plasma versus tissue tumor mutational burden as biomarkers of durvalumab plus tremelimumab response in patients with metastatic colorectal cancer in the CO.26 trial. Clin. Cancer Res. 2024;30:3189–3199. doi: 10.1158/1078-0432.CCR-24-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grassi E., et al. Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial. ESMO Open. 2023;8 doi: 10.1016/j.esmoop.2023.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel S.P., et al. Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors. J Immunother Cancer. 2024;12 doi: 10.1136/jitc-2023-007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Z., et al. PD-1 blockade plus COX inhibitors in dMMR metastatic colorectal cancer: clinical, genomic, and immunologic analyses from the PCOX trial. Méd. 2024;5:998–1015. doi: 10.1016/j.medj.2024.05.002. e1016. [DOI] [PubMed] [Google Scholar]
- 148.Fakih M., et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer: a phase I clinical trial (SPICE) J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khushalani N.I., et al. Final results of urelumab, an anti-CD137 agonist monoclonal antibody, in combination with cetuximab or nivolumab in patients with advanced solid tumors. J Immunother Cancer. 2024;12 doi: 10.1136/jitc-2023-007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coffman-D'Annibale K., et al. VB-111 (ofranergene obadenovec) in combination with nivolumab in patients with microsatellite stable colorectal liver metastases: a single center, single arm, phase II trial. J Immunother Cancer. 2024;12 doi: 10.1136/jitc-2023-008079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zareian N., et al. A phase 1 trial of human telomerase reverse transcriptase (hTERT) vaccination combined with therapeutic strategies to control immune-suppressor mechanisms. Exp. Biol. Med. 2024;249 doi: 10.3389/ebm.2024.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Segal N.H., et al. CEA-CD3 bispecific antibody cibisatamab with or without atezolizumab in patients with CEA-positive solid tumours: results of two multi-institutional Phase 1 trials. Nat. Commun. 2024;15:4091. doi: 10.1038/s41467-024-48479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi W., et al. Phase 1 trial of 4-1BB-based adoptive T-cell therapy targeting human telomerase reverse transcriptase in patients with advanced refractory solid tumors. Cytotherapy. 2023;25:1236–1241. doi: 10.1016/j.jcyt.2023.07.006. [DOI] [PubMed] [Google Scholar]
- 154.Cole C.B., et al. First-in-human phase 1 clinical trial of anti-core 1 O-glycans targeting monoclonal antibody NEO-201 in treatment-refractory solid tumors. J. Exp. Clin. Cancer Res. 2023;42:76. doi: 10.1186/s13046-023-02649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Coleman N., et al. Phase I study of sapanisertib (CB-228/TAK-228/MLN0128) in combination with ziv-aflibercept in patients with advanced solid tumors. Cancer Med. 2024;13 doi: 10.1002/cam4.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roma-Rodrigues C., Pombo I., Fernandes A.R., Baptista P.V. Hyperthermia induced by gold nanoparticles and visible light photothermy combined with chemotherapy to tackle doxorubicin sensitive and resistant colorectal tumor 3D spheroids. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21218017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were utilized in the research presented within this article.