Abstract
The global malaria program has faced setbacks due to disruptions in health services caused by COVID-19 pandemic. Despite these challenges, Asia that primarily comprised of low and middle-income countries (LMICs), continues to make strides towards malaria elimination. This scoping review explored the impact of the COVID-19 pandemic on malaria control programs in Asian countries with varying levels of malaria endemicity. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was applied to search for articles published between January 2020 and May 2024 that examined the impact of COVID-19 on malaria control programs in Asia on six databases (PubMed, Scopus, Google Scholar, WHO COVID-19 Research Database, Garuda and Sinta). The findings of these articles were organized into five themes: epidemiology and surveillance, case management (including diagnosis and coinfection), vector control, prevention, and program management. Overall, 54 articles from countries with various endemicity levels were included. These studies focused on malaria epidemiology, surveillance, and case management, with few studies on vector control. The COVID-19 pandemic has affected malaria control differently in different regions. In malaria-free, low-, and high-endemic countries, malaria cases were reduced mainly due to strict public health measures such as travel restrictions, quarantines, and COVID-19-related stigma, which reduced clinic attendance. Conversely, increased malaria cases owing to increased imports, relapses of malaria cases triggered by COVID-19, social conflicts, and underreporting have contributed to this surge. The priority shift to COVID-19 has affected malaria centers, resulting in personnel shortages, budget limits, and an increased number of malaria cases and outbreaks. The pandemic has also spurred innovative malaria prevention methods, such as using social media to raise awareness in China. The COVID-19 pandemic has had a mixed impact on the number of malarial cases reported across Asia. The three main factors were travel restrictions, COVID-19-related stigma, and shifting priorities to COVID-19. Integrating malaria control and COVID-19 strategies, strengthening the healthcare system, developing flexible malaria control strategies during crises, and developing innovative solutions could mitigate these impacts.
Keywords: Malaria elimination, Asia, COVID-19, impacts, scoping review
Introduction
Malaria is a deadly disease caused by parasites and it mainly affects people in tropical and subtropical areas [1]. Despite being preventable and curable, it significantly harms health and livelihoods, particularly those residing in Africa and Southeast Asia [1]. As part of their Sustainable Development Goals, many countries focus on malaria prevention and aim to eliminate the disease by 2030 [2]. The Asia-Pacific region has seen notable success in fighting malaria since 2000, with intense political support and financial investment, and has focused on high-risk groups such as migrant workers and military personnel [3,4]. Unfortunately, not all areas progress equally owing to drug resistance (such as in the Greater Mekong Subregion), financial constraints, and health system challenges [4].
Globally, malaria cases per 1,000 at-risk individuals fell from 81 (2000) to 56 (2019) before rising again to 59 in 2020 [5]. Following coronavirus disease 2019 (COVID-19), a 3 per 1,000 population at risk increase in the malaria incidence rate was observed in 2020 (from 56 to 59 per 1,000 population at risk in 2019 and 2020, respectively), and the spike was attributed to service disruption caused by the COVID-19 [5]. This situation requires significant changes in global malaria control to get back on track. The incidence rate has remained stable between 2020–2022, with the last incidence was 58 per 1000 at-risk population in 2022 [6]. The WHO African Region estimated approximately 233 million malaria cases in 2022, representing nearly 94% of global cases, while the WHO South-East Asia Region contributed approximately 2% of malaria cases worldwide [6].
The COVID-19 pandemic had various impacts on WHO regions, with the American, European, and Southeast Asian regions bearing the heaviest burdens [7]. In contrast, the African and Western Pacific regions continue to report comparatively low numbers of cases and deaths due to COVID-19 [7]. The health system was disrupted during the pandemic, affecting the implementation of malaria control programs, and malaria remains a priority problem in many low- and middle-income countries (LMIC) [8,9].
In highly endemic areas such as Sub-Saharan Africa, the COVID-19 pandemic led to an increase number of malaria cases [7]. Multiple factors have contributed to this increase, including insufficient financial investments, high native malaria burden, ineffective surveillance systems, limited medical resources, and low socioeconomic development [1,7] In the early stages of the COVID-19 pandemic, the shift in medical resources (e.g., health workers and personal protective equipment, diagnostic reagent manufacturing, and drugs) from malaria control to emergency COVID-19 response caused further disruptions, reductions, and delays in malaria control measures [1]. Likewise, lockdown measures significantly hindered the mobilization of community health workers, resources, and access to malaria control services [2].
However, the effect of COVID-19 on malaria control programs in Asia has remained unexamined. Asia is mainly composed of LMICs, many of which is malaria-free and low-endemic countries. Additionally, 14 out of 37 countries have been certified malaria-free by the WHO, including Qatar, Jordan, Lebanon, Bahrain, Kuwait, the United Arab Emirates, the Maldives, Sri Lanka, Japan, China, Mongolia, Taiwan, Singapore, and Brunei Darussalam [10]. The Asian region also includes countries with a high burden of malaria cases, such as India, which accounts for 79% of transmissions, and areas with significant concerns, such as drug resistance, in the Greater Mekong Subregion [6]. With the COVID-19 pandemic imposing an additional burden on the already overstretched health system, a poor understanding of the impact of the COVID-19 pandemic on malaria cases in Asia may further delay the elimination of global malaria by 2030. The aim of this scoping review was to understand the impact of the COVID-19 pandemic on malaria control in Asian countries with various levels of endemicity. This knowledge is critical for renewing strategies to get back on track in eliminating malaria by 2030.
Methods
Data source and search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement was used to guide this scoping review. The search was conducted on May 17, 2024, using several electronic databases, including PubMed, Scopus, WHO COVID-19 Research Database, and national electronic databases from the Ministry of Education and Culture, Indonesia (e.g., Garuda and Sinta) to identify relevant studies. Citations were obtained from Google Scholar using Anne-Wil Harzing's “Publish or Perish” software, which allows up to 1,000 articles to be downloaded [11]. The combinations of search terms included “COVID-19” AND “malaria.” A search filter was used to limit the search results to those published before 2024. Details of the search strategy are provided in Appendix 1.
Inclusion and exclusion criteria
In the first round of screening, the results were pooled using Rayyan.ai (Rayyan, Cambridge, United States), and duplicates were removed. The second round of screening retrieved the search results, and articles eligible for inclusion were selected in two stages. The first stage involved two independent reviewers (RRA and GNDS) who selected articles from the search results based on the titles, abstracts, and specific inclusion and exclusion criteria. Publications in English or Indonesian relevant to COVID-19 and malaria were included. Original articles published between January 2020 – May 2024 (including pre-prints from WHO COVID-19 database) were included. In the second stage, all articles were subjected to full-text review by two reviewers (RRA and GNDS), focusing on the impact of COVID-19 on malaria in Asia.
Data extraction
Two reviewers (RRA and GNDS) performed data extraction using pre-tested data extraction forms and stored them in Google spreadsheets. The results of the extraction data were then presented in a table and chart using a Microsoft Excel 2019 spreadsheet (Microsoft Corporation, Inc., Redmond, United States). Disagreements were first resolved through discussion between the two reviewers. If there was no consensus, a third author was then involved to make the decision (RAA). The following publication characteristics were extracted from each study: author, year of publication, title, publication type, population, country, study design, data collection period, and outcomes. The category of the level of endemicity still referred to the World Malaria Report 2010 [12], while the annual incidence rate per 1,000 people in the countries was taken from the reported malaria cases published in the World Malaria Report 2021 [5]. The level of endemicity was categorized as certified malaria-free, low-endemic (annual parasite incidence (API) < 1 per 1,000 population at risk), and high-endemic (API ≥1 per 1000 population at risk). A map was then created to describe the malaria endemicity for countries in the Asian region. To describe the impact of COVID-19 on malaria, articles were characterized by the year of publication and period of data collection. Five themes were identified and grouped the articles into: epidemiology and surveillance, case management (diagnosis and coinfection), vector control, prevention, and program management.
Results
Characteristics of the included studies
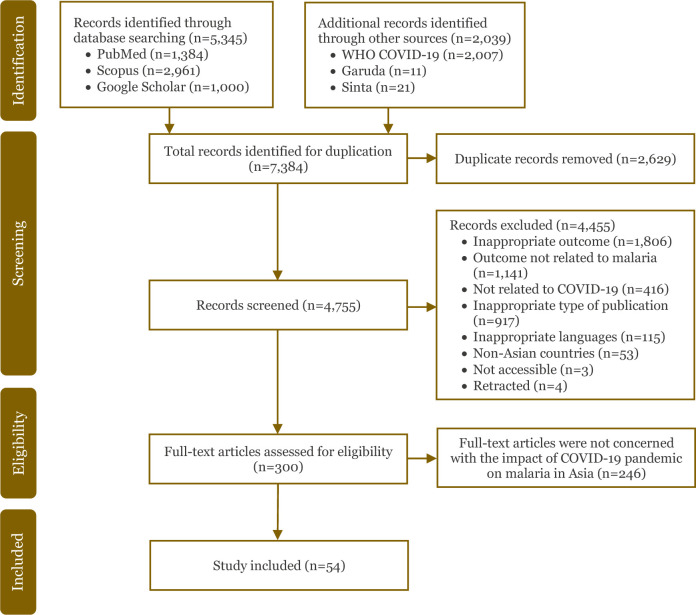
Overall, 7,384 articles were identified from six electronic databases from January 2020 to May 2024: 1,384 articles from PubMed; 2,961 articles from Scopus; 1,000 articles from Google Scholar; 2,007 articles from WHO COVID-19; 11 articles from Garuda; and 21 articles from Sinta. After the duplicate articles were removed (n = 2,629), the titles and abstracts of the remaining 4,755 articles were screened, and additional 4,455 articles were excluded. The reasons for exclusion were inappropriate outcomes (n = 1,806), the outcome was not related to malaria (n = 1,141) or COVID-19 (n = 416), inappropriate type of publication (n = 917), inappropriate language (neither in English nor Indonesian) (n = 115), non-Asian countries (n = 53), not accessible (n = 3), and retracted articles (n = 4). A total of 300 full-text articles were assessed for eligibility and those that were not concerned with the impact of the COVID-19 pandemic on malaria in Asia were excluded (n = 246). Finally, 54 articles were included in this scoping review that met the eligibility criteria [13-66]. Detailed flow chart of the study selection is presented in Figure 1.
Figure 1.
PRISMA flow chart of the study selection.
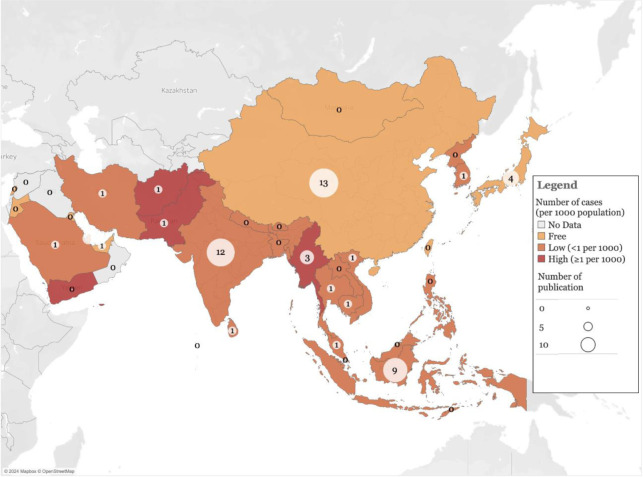
One-third of the articles were from malaria-free countries, with China making the most significant contribution (n = 13, 24.1%) [13-25] followed by Japan (n = 4, 7.4%) [28,29,47,48]. India (n = 12, 22.2%) [36,37,41,42,46,49,50,52,56,59,65,66] and Indonesia (n = 9, 16.6%) [26,27,35,39,45,51,53,57,58] contributed the most publications from low-endemic countries and five publications from high-endemic countries [34,54,60,61,64] (Figure 2).
Figure 2.
Map of malaria endemicity in Asian countries by annual incidence rate per 1,000 populations and the number of publications reviewed in respective countries.
Study settings
Most of the studies were conducted in hospitals (n = 20, 37%) [13,20,22,26,29-31,36-38,41- 44,46,50,56-58,65], followed by primary care facilities [27,53] and immigration centers [28,47] (3.7%, two articles for each setting), clinics [66], COVID-19 quarantine sites [18], district hospitals [35], commune health stations [33], and social media platforms (1.8%, one article for each setting) [23]. The scope of the studies varied from country (n = 7, 12.9%) [25,48,49,52,54,63,64], province (14.8%, eight articles) [15-17,19,21,24,34,40], district (n = 4, 7.4%) [14,45,51,61], and community (n = 6, 11.1%) [32,39,55,59,60,62].
Study designs
Most publications used quantitative methods (n = 47, 88.8%) [13-16,18-31,33-44,46-50,52- 62,65,66], with one mixed-methods study [63] and five qualitative studies [17,32,45,51,64]. Among the quantitative studies, nearly half (n = 23, 47.9%) used descriptive studies, including 14 case reports [13,20,22,26,29,31,35,38,42-44,50,57,58], three case series [36,40,65], five descriptive studies [18,39,47,48,55], and one time series [25]. Analytical observational studies were used in 25 articles (52.1%), consisting of 12 retrospective studies [14- 16,19,21,30,34,37,41,52,56,60], five cross-sectional studies [23,54,61,62,66], one comparative study [53], and one case-control study [46]. Only three articles used an intervention study design [27,33,59], while three others used modeling studies [24,28,49] (Appendix 2).
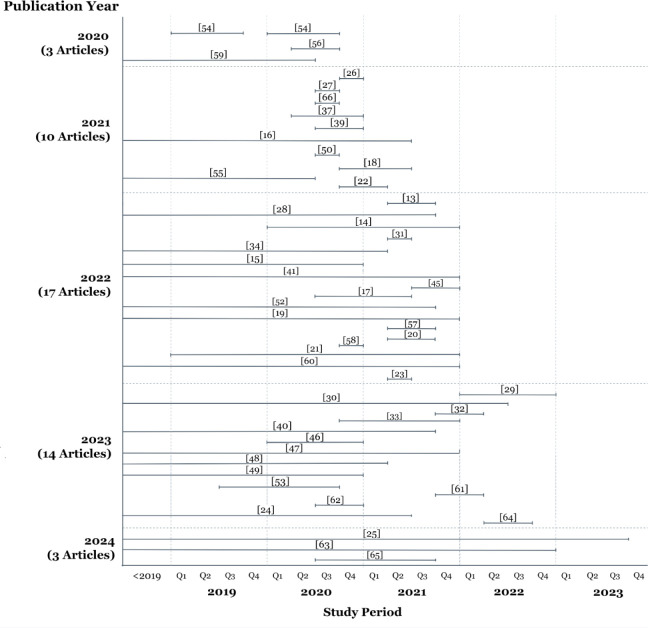
Most articles were published in 2022 (n = 17, 31.4%) [13–15,17,19- 21,23,28,31,34,41,45,52,57,58,60]. Only 47 of 54 publications mentioned the study period. Most articles published in 2021 had shorter study periods than those published in the other years (Figure 3). Publications from 2022 to 2024 consistently covered a longer study period, spanning from before 2019 to the fourth quarter of 2021. The most extended study period was found in a modelling study published in 2022, which used data from 1999 to 2021 [28], while the shortest study period was 12 days, published as a case report in 2021 [22].
Figure 3.
Number of publications by year and their study period: three studies in 2020 [54,56,59], ten studies in 2021 [16,18,22,26,27,37,39,50,55,66], 17 studies in 2022 [13-15,17,19- 21,23,28,31,34,41,45,52,57,58,60], 14 studies in 2023 [24,29,30,32,33,40,46-49,53,61,62,64], and three studies in 2024 [25,63,65].
Thematic analysis
The effect of COVID-19 on malaria based on the level of endemicity was summarized as positive and negative effects for each theme (Table 1), followed by detailed explanation of the effect in Table 2. The analysis revealed that case management (diagnosis and coinfection), epidemiology, and surveillance were the most frequently mentioned themes, whereas vector control was the least frequently reported (Table 2).
Table 1. Summary of positive and negative effects of COVID-19 on malaria based on level of endemicities.
| Aspect | Free | Low-endemic | High-endemic |
|---|---|---|---|
| Epidemiology and surveillance | Positive and negative effects | Negative effects | Negative effects |
| Case management | Positive and negative effects | Positive and negative effects | N/A |
| Vector control | N/A | Negative effects | N/A |
| Prevention | Positive effects | Positive and negative effects | N/A |
| Program management | Positive and negative effects | Positive and negative effects | Positive and negative effects |
N/A: not available
Table 2.
Thematic impact of COVID-19 on malaria control by level of endemicities
| Aspect | Themes | Malaria endemicity | ||
|---|---|---|---|---|
| Free malaria | Low endemicity | High endemicity | ||
| Epidemiology and surveillance | Decreased | China Significant reduction in imported malaria cases during the COVID-19 [19,21,24]. Decrease in malaria cases during COVID-19 [15,16,25]. Japan Stagnation of international traffic during COVID-19 caused a 50% decrease in malaria cases in 2021 [28]. Decrease imported malaria cases during the COVID-19 [47]. Sri Lanka COVID-19 restrictions lead to decreased parasitological surveillance [63]. |
India Decline in the number of reported malaria cases in 2020 [52]. Decreased malaria cases during the COVID-19 pandemic might result from lower clinic attendance [66]. Decrease in monsoon-related illnesses due to efforts to curb the COVID-19 pandemic [41,49]. Indonesia Malaria-endemic areas saw fewer cases during the pandemic than before COVID-19 [53]. No malaria cases were found in endemic areas because of the decreased number of patients who visited health centers [27]. Iran COVID-19 lockdowns caused a drop in malaria cases[40]. |
Afghanistan Provision of malaria services, neglected tropical diseases, and community outreach programs decreased [61]. Myanmar Decline in malaria cases during the COVID-19 [54]. Pakistan Decline in malaria cases during the first and second COVID-19 waves in some regions of Pakistan [34]. |
| Increased | China Decrease and sudden increase of imported malaria cases during the COVID-19 pandemic in Shanghai [14]. Effective migration surveillance of malaria at entry points during COVID-19 [18]. Japan Almost threefold increase in proportion of malaria cases in 2020 (from 6.5% to 18.8%) [48]. |
India Over 4% of healthcare workers with COVID-19 also tested positive for P. vivax malaria [56]. Coinfection of COVID-19 and P. vivax in healthcare workers was over 5% [37]. Indonesia Social conflict disrupts the malaria surveillance and detection of imported cases [45]. Malaysia COVID-19 has restricted check-ups in healthcare facilities and led to a decrease in surveillance migration [62]. Saudi Arabia Malaria cases increased by 93% during COVID-19 compared to before the pandemic[30]. |
Myanmar Increase in P. falciparum and P. vivax cases due to restrictions of the military coup and COVID-19 [60]. |
|
| Case management | Diagnosis and treatment | China Patients with fever and travel history from endemic areas were screened for malaria and COVID-19 coinfection during the pandemic [13,20,22]. Delayed care seeking and diagnosis of imported malaria cases [19]. Malaria case investigations were delayed due to waiting period for COVID-19 test confirmation before collecting blood smears [17]. Japan COVID-19 may lead to delayed diagnosis, coinfection with malaria P. falciparum, or misdiagnosis of malaria as COVID-19 [29]. Qatar Patients with fever and travel history from endemic areas were screened for malaria and COVID-19 coinfection during the pandemic [43,44]. United Arab Emirates (UAE) Screening of malaria and COVID-19 coinfection conducted in patients with fever and a history of travel from endemic areas during the pandemic [38]. |
India Malaria screening in COVID-19 patients was delayed due to persistent fever and travel history from endemic areas[42]. Diagnosis was delayed because of overlapping symptoms in malaria and COVID-19 coinfection [50]. Similar symptoms between malaria and COVID-19 can lead to misdiagnosis of P. vivax in pregnant women as late-onset COVID-19, causing severe conditions [36]. Malaria clinic attendance dropped by one-third during COVID-19, as most patients refused COVID-19 tests [66]. Indonesia Screening of malaria and COVID-19 coinfection was conducted in patients with fever and a history of travel from endemic areas during the pandemic [26,35]. Early case management of malaria (non-falciparum) in coinfection with COVID-19 led to early recovery [35]. Malaria was diagnosed as a secondary infection in COVID-19 patients with a travel history to endemic areas and prior malaria infection [57]. Screening of malaria and COVID-19 conducted in malaria- endemic areas and history of P. vivax infection [58]. During the COVID-19 pandemic, the results of the malaria rapid diagnosis test (RDT) and blood smear showed the same result [27]. Vietnam Expanding G6PD tests into case management practice was feasible for malaria control during COVID-19 [33]. |
N/A |
| Coinfection | China Coinfection of COVID-19 and P. falciparum for imported cases[20]. P. falciparum coinfection of patient with recurrent COVID-19 might lead to high inflammation results [13]. COVID-19 might induce relapses in P. ovale and cause coinfection [22]. Qatar Coinfection of P. vivax and COVID-19 [43]. Persistent fever in COVID-19 after treatment due to coinfection with P. vivax [44]. UAE Coinfection of COVID-19 and P. falciparum could occur in persistent fever and generalized fatigue [38]. |
India People from malaria-endemic areas are at risk of malaria and COVID-19 coinfection [65]. Coinfection of P. vivax and COVID-19 caused persistent fever with chills in patients [42]. Coinfection of P. vivax with COVID-19 [56]. P. vivax and COVID-19 coinfection are linked to milder disease and quicker recovery with early virus clearance [37,46]. P. falciparum and COVID-19 coinfection leads to severe symptoms in a short time [50]. Indonesia Coinfection of COVID-19 and relapsed P. vivax causes prolonged fever and hyper coagulopathy [57]. Coinfection of COVID-19 and P. vivax led to pericarditis [58]. Coinfection of COVID-19 and P. falciparum has the potential to cause hyperinflammation and hypercoagulation [26]. Coinfections of malaria non-falciparum with COVID-19, especially in endemic areas [35]. South Korea Afebrile P. falciparum might be caused by cross-immunity from previous COVID-19 infection [31]. |
N/A | |
| Vector control | N/A | N/A | Indonesia COVID-19 disrupted integrated vector management (IVM) for malaria, limiting prevention and control efforts [51]. |
N/A |
| Prevention | N/A | China Individuals with high number of followers on social media provided new channels for people to find and share information on malaria prevention during the COVID-19 pandemic [23]. |
Indonesia Villagers’ knowledge, attitude, and action towards malaria during the COVID-19 pandemic were less attention [39]. Behavior changes, like sleeping outside to guard land during social conflicts, caused malaria outbreaks, not COVID-19 [45] Malaysia Fishing and hunting during the night and self-treatment for mild symptoms contributed to the P. malariae outbreak during COVID-19 [62]. Thailand Reactive drug administration (RDA) for malaria during COVID-19 was well accepted by household members and health staff [32]. |
N/A |
| Program management | N/A | China Integrating rapid malaria screening at COVID-19 quarantine sites helped detect imported malaria in a timely manner, preventing its further spread [18]. Malaria surveillance and case management were neglected during COVID-19, with issues like missing standard procedures, limited public health provider mobility, and delays in the “1– 3–7” surveillance strategy [17]. Two imported malaria cases took over three days to investigate due to staff being focused on the COVID-19 response [21]. Sri Lanka COVID-19 restrictions affected advocacy and training activities in the anti-malaria campaign (AMC) [63]. |
Cambodia Malaria service coverage and utilization rates remained steady in 2020, with program activities adapted to COVID-19 [55]. India Healthcare systems in low-resource areas were overwhelmed by COVID-19, and the situation worsened with concurrent malaria or dengue infections [36]. Village malaria workers (VMWs) and malaria field coordinators (MFCs) of the Malaria Elimination Demonstration Project (MEDP) used door-to-door surveillance and migration databases to integrate COVID-19 and malaria screening [59]. Malaysia COVID-19 strained healthcare facilities, prioritizing severe cases and causing malaria outbreaks [62]. Thailand COVID-19 worsened the staff shortage for the RDA malaria program and cut budgets for training and awareness. Travel to remote areas and RDA activities were hindered by competing health priorities like COVID-19 [32]. |
Afghanistan Half of health facilities saw no change in malaria prevention campaigns during COVID-19, but 47.1% reported increased home visits [61]. Myanmar Tailored malaria intervention amid COVID-19 to continue maintaining essential services [54]. Due to COVID-19 and the military coup, the majority of case investigations and monitoring evaluations were conducted remotely through cell phones [60]. Military coups increased malaria risk for internally displaced persons (IDPs) living in the jungle, while COVID-19 restricted access to health services [64]. |
N/A: not available
Epidemiology and surveillance
The COVID-19 pandemic has profoundly affected the epidemiology and surveillance of malaria at all levels of endemicity (Table 2). Most studies have reported reduced malaria cases during the pandemic in malaria-free countries (China, Japan, and Sri Lanka) [15,16,19,21,24,25,28,47,63], low-endemic countries (India, Indonesia, and Iran) [27,40,41,49,52,53,66], and high-endemic countries (Afghanistan, Pakistan, and Myanmar) [34,54,61]. Studies in China and Japan have shown that mobility restrictions at the border positively affect cases of imported malaria [24,28,47]. This reduction can be attributed to stringent international travel restrictions, leading to decreased international travel and thus reducing the number of traveler entries [24,28,47]. Another study in China reported possible underreporting of malaria cases due to delayed care-seeking and diagnosis [19]. Additionally, the fear of COVID-19 exposure and financial constraints have deterred individuals from seeking malaria care [15]. In Sri Lanka, COVID-19 restrictions have led to decreased parasitological surveillance [63].
In low-endemic countries, such as India, Indonesia, and Iran, malaria cases have decreased due to travel restrictions [27,40], fear of getting infected from COVID-19 [27,40,66] and fear of being tested and diagnosed with COVID-19 [66]. Travel restrictions reduced attendance at malaria clinics [66]. Additionally, COVID-19-related stigma reduced clinic attendance due to fear of being tested and diagnosed with COVID-19 [27]. During the pandemic, the number of malaria cases reported in India was relatively low during the monsoon season while historically malaria cases increased during this season [41,49].
The reduction in reported malaria cases in high-endemic countries such as Afghanistan, Myanmar, and Pakistan are caused by different factors. In Afghanistan and Pakistan, the reduction was caused by weaker health system performance and seasonality during winter, when infections were less common [34,61]. Myanmar experienced a continued decline in malaria cases, severe cases, and deaths because the National Malaria Control Program (NMCP) effectively used pre-pandemic supplies and additional funds to maintain malaria services, mitigating the impact of COVID-19 on malaria control [54].
Nine studies reported an increase in malaria-free (China and Japan) [14,18,48], low- endemic (India, Saudi Arabia, Indonesia, and Malaysia) [30,37,45,56,62], and high-endemic (Myanmar) countries [14,18,30,37,45,48,56,60,62]. Between 2020 and 2021, the number of imported malaria cases in China increased from 18 to 94. The surge was linked to the high volume of overseas flights to Shanghai and the mandatory 14-day quarantine, which contributed to the detection of more cases among returning labor workers [14]. These findings underscore the need for enhanced surveillance and screening, which have proven to be effective in the early detection of malaria [18]. In Japan, the proportion of imported malaria cases increased almost threefold in 2020 (from 6.5% to 18.8%) [48].
A study in the Eastern region of Saudi Arabia showed an increase in imported malaria cases, from 32 cases pre-COVID to 60 cases during COVID-19 [30]. During COVID-19, there was a higher rate of infection among stateless tribal patients (21.7% vs 3.1%), with a similar pattern observed for mixed malarial infections involving Plasmodium falciparum (P. falciparum) and P. vivax. Symptoms, such as fever and headaches, which are common to both malaria and COVID-19 triggered an increase in malaria diagnoses as more individuals sought medical care because of fear of contracting COVID-19 [30].
In low-endemic countries such as India, Indonesia, and Malaysia, there were unique challenges that increased malaria cases during the COVID-19 pandemic. The prevalence of malaria and COVID-19 coinfections among healthcare workers increased, particularly during the monsoon season, correlating with the pandemic's peak in urban areas such as Mumbai, India [37,56]. Social conflicts in Indonesia have disrupted malaria surveillance and increased infection risk, especially outdoor sleeping behavior during the evening to guard the land [45]. An outbreak of P. malariae linked to nighttime outdoor activities such as fishing and hunting was observed in Malaysia [62]. Furthermore, the strain on the healthcare system due to the COVID-19 pandemic has led to the underreporting and undertreatment of mild malaria symptoms, as severe cases are prioritized for management [62]. This strain has also contributed to the increasing positivity rates of P. falciparum and P. vivax in Myanmar, a high-endemic country, owing to the restrictions of military coups and COVID-19 [60].
Case management
Amidst the COVID-19 pandemic, healthcare professionals in malaria-free countries such as China, Japan, Qatar, and the UAE have screened patients presenting with fever and travel history from malaria-endemic regions for possible coinfections of malaria and COVID-19 [13,20,22,38,43,44]. Overlapping symptoms common to both diseases, such as fever, muscle pain, and headaches, were carefully monitored [13,20,22,43,44]. Additionally, delays in malaria diagnosis have been noted in China, which is potentially exacerbated by COVID-19, as blood smears were often obtained once the patient tested negative for COVID-19 [17,19]. In Japan, COVID-19 with comorbidities can lead to a delayed diagnosis of coinfection with malaria falciparum [29]. Furthermore, immune responses to these coinfections can influence the progression and recurrence of symptoms [20]. Cytokine responses triggered by systemic illnesses such as COVID-19 could lead to malaria relapses, particularly in P. vivax, although the exact mechanisms remain under investigation [44].
Coinfection of COVID-19 with malarial parasites, such as P. falciparum and P. vivax, presents complex diagnostic and clinical challenges. In China, coinfection may alter symptom presentation, potentially due to the immune response from previous malarial infections, which could inhibit the multiplication of SARS-CoV-2, clear the virus, or lead to high inflammation [13]. Additionally, cytokine responses induced by COVID-19 can trigger relapses in diseases such as those caused by P. ovale [22]. In Japan, comorbidities such as diabetes mellitus, hypertension, spinal cord injury, and indwelling urinary catheter-associated pyelonephritis with coinfections can lead to severe health conditions [29]. Qatar reported that sudden or persistent fever in COVID-19 patients with a history of P. vivax infection raised suspicions of malaria relapse, possibly due to the reactivation of previous infections or local transmission [43,44]. In the UAE, the presentation of COVID-19 in patients can vary widely, and coinfections with P. falciparumare particularly suspected in cases of persistent fever and generalized fatigue [38].
A similar situation also occurs in low-endemic countries such as India, Indonesia, and Vietnam [26,27,33,35,36,50,57,58,66]. The COVID-19 pandemic has highlighted challenges in diagnosing malaria and COVID-19 coinfections due to overlapping symptoms such as fever, fatigue, and headaches, which often lead to delayed diagnoses and treatment. India reported that similar symptoms led to misdiagnoses in pregnant women and a drop in malaria clinic attendance due to fear of contracting COVID-19 [36,66]. Indonesia has also faced similar diagnostic challenges with stigmatization and reduced healthcare visits, further complicating the situation [27,57]. Early case management of non-falciparum malaria in patients co-infected with COVID-19 led to early recovery [35]. Vietnam successfully expanded the use of G6PD tests within its malaria control programs during the pandemic, integrating them into case management practices to enhance diagnostic accuracy and treatment outcomes [33].
In low-endemic countries, such as India, Indonesia, and South Korea, the dynamics of coinfections involving COVID-19 and malaria parasites, such as P. vivax and P. falciparum, have presented various clinical outcomes [26,31,35,37,42,46,50,56-58,65]. In India, coinfection with P. vivax in COVID-19 patients, especially frontline healthcare workers, has been associated with continued fever symptoms, potentially less severe COVID-19, and quicker recovery, possibly due to cross-reactive antibodies [37,42,46,65]. However, coinfection with P. falciparum may increase disease severity by affecting multiple body systems and altering clinical presentations [50]. In Indonesia, COVID-19 is known to trigger P. vivax relapse and increase cytokine levels, potentially leading to complications such as acute pericarditis [57,58]. COVID-19 and P. falciparum infections can enhance hyperinflammation and coagulation, raising concerns regarding blood clotting markers [26,35]. In South Korea, afebrile P. falciparum infections have been speculated to result from cross-immunity caused by previous COVID-19 infections [31].
Vector control
Only one study from a low-endemic country (Indonesia) has reported the impact of COVID-19 on malaria vector control [51]. The study was conducted in a South Sumatra Province subdistrict, highlighted the reduction in malaria integrated vector management (IVM) only for the socialization component. The other element of IVM was presumably halted during the pandemic [51].
Prevention
Five studies from a malaria-free country (China) and low-endemic countries (Indonesia, Malaysia, and Thailand) reported malaria prevention activities during the pandemic [23,32,39,45,62]. Social conflict causes a lack of trust in healthcare workers and malaria- prevention efforts. For example, sleeping outside to guard the land during the evening in a receptive area contributed to the malaria outbreak in Indonesia [45].
In 2021, the intensity of access to information and prevention of malaria was limited owing to the shifting priority to the COVID-19 program. Therefore, there was less awareness of malaria during the COVID-19 pandemic [39]. Practices that increase the risk of malarial infection have also been reported [62]. A malaria outbreak during COVID-19 in Malaysia identified unprotected night fishing and hunting, as well as self-treatment for minor symptoms, as risk factors [62].
Efforts to prevent malaria transmission, particularly among mobile migrant populations, should continue. Despite Thailand's changing health priorities (COVID-19), the 2023 Reactive Drug Administration report was widely supported and well accepted. Improving community and healthcare provider education (especially for rural health workers) and knowledge of the purpose of the Reactive Drug Administration program in addressing concerns about the demand for malaria medication when sick [32].
Despite these circumstances, innovative approaches to malaria prevention have been discovered in malaria-free countries such as China [23]. During the COVID-19 pandemic, the use of social media with large followers has provided new channels for finding and sharing information on malaria prevention, especially on the day of malaria [23]. This could be a new method to promote malaria, especially with limited face-to-face interactions.
Program management
Within the study period, malaria management programs were mostly affected from 2020 to 2022, especially in low-endemic countries such as Cambodia, India, Malaysia, and Thailand (Table 2) [32,36,55,59,62]. The COVID-19 pandemic has affected malaria control programs in several ways, but they face common challenges in maintaining healthcare services. In Cambodia, despite the pandemic, malaria service coverage and utilization did not decline because of proactive planning, data monitoring, and strong community engagement with mobile malaria workers who continued to provide uninterrupted services [55]. In India, the health care system was heavily strained, particularly in low-resource settings, leading to disruptions in malaria and dengue care when they occur alongside COVID-19 [36]. The country adjusted its malaria operational plans to integrate COVID-19 support tasks, such as door-to-door surveillance, and equipped staff with the necessary training and personal protective equipment [59].
Malaysia faced strained healthcare resources due to COVID-19, which led to the prioritization of severe cases and contributed to malaria outbreaks [62]. Similarly, in Thailand, the pandemic has exacerbated personnel shortages and budget constraints for the Reactive Drug Administration program, challenging the continuation of malaria control activities. These constraints included difficulties reaching remote areas and carrying out necessary health interventions because of competing health priorities, leading to a need for increased resources and support for healthy volunteers [32].
In high-endemic countries such as Afghanistan and Myanmar, the COVID-19 pandemic has significantly impacted malaria control and healthcare services [54,60,61,64]. In Afghanistan, service disruptions have occurred due to limited medicines, supplies, and medical staff, with some healthcare facilities maintaining services despite funding cuts from international donors and government collapse [61]. This situation has led to reduced malaria prevention campaigns and the reassignment of healthcare staff to manage changing patient volumes [61]. Despite the challenges posed by COVID-19 and the military coup in Myanmar, essential malaria services have been sustained [64]. The procurement of mosquito nets, diagnostic tools, and antimalarial drugs completed before the pandemic, along with the strategic use of secured savings and additional funds by the National Malaria Control Program, helped mitigate the impact on malaria control [54]. Moreover, the need for a flexible surveillance system has been highlighted by the increased late reporting of malaria cases and the use of cell phone communication to monitor activities in malaria posts [60].
A similar challenge was found in malaria-free countries, such as China and Sri Lanka [17,18,21,63]. In China, malaria screening tests were implemented at COVID-19 quarantine sites, designated hospitals, and surrounding areas, as well as surveillance of Anopheles vectors and mosquito density control [18]. This funding cut interrupted the supply of malaria-related diagnostic tools and drugs, and a shift in priority towards COVID-19 led to neglected malaria surveillance and case management [17]. Additionally, the investigation of imported malaria cases was delayed as staff were redirected to focus on COVID-19, and the outbreak hindered the distribution of bed nets [17,21]. In Sri Lanka, COVID-19 restrictions have reduced advocacy and training activities in anti-malaria campaigns [63].
Discussion
The present study found both positive and negative effects of the COVID-19 pandemic on malaria programs across various endemic levels. Most studies have reported a reduction in malaria cases during the COVID-19 pandemic in malaria-free countries (China, Japan, and Sri Lanka) [15,16,19,21,24,25,28,47,63], low-endemic countries (India, Indonesia, and Iran) [27,40,41,49,52,53,66], and high-endemic countries (Afghanistan, Pakistan, and Myanmar) [34,54,61]. This reduction was primarily attributed to stringent international travel restrictions, which reduced imported malaria by limiting traveler entries [28,40,41,47,48,52]. However, in China, factors like underreporting due to delayed care-seeking and diagnosis, along with decreased parasitological surveillance in Sri Lanka, have also been noted. In low-endemic countries, such as India, Indonesia, and Iran, travel restrictions and COVID-19-related stigma further reduced clinic attendance. Meanwhile, reductions in high-endemic countries such as Afghanistan and Pakistan have been linked to weaker health system performance and seasonality.
Myanmar maintained a decline in cases by effectively using pre-pandemic supplies and additional funding. In contrast, nine studies reported an increase in malaria cases in China, Japan, Saudi Arabia, India, Indonesia, Malaysia, and Myanmar. This increase was due to factors such as enhanced migration surveillance, COVID-19-induced relapses, especially P. vivax and P. ovale, malaria outbreaks due to social conflicts disrupting active case-finding, and the healthcare system burden prioritizing COVID-19 over malaria.
Mobility restriction policy implemented in response to COVID-19 has had several effects, including reduced passenger arrivals and fewer imported malaria cases [28,40,47,48,52]. However, the policy also led to an increase of overseas workers returning home [14]. Mandatory quarantine and integrated screening for COVID-19 and malaria among these travelers contributed to the detection of more cases among returning workers. Quick screening for malaria at COVID-19 quarantine sites effectively prevents the retransmission of imported malaria cases [18]. Sri Lanka successfully maintained its malaria elimination status by screening imported cases through an integrated case surveillance system that monitored people returning from malaria- endemic countries during quarantine as part of a coordinated multisectoral response [63].
Lockdowns and travel restrictions have reduced the frequency of visits to health services and malaria clinics, hampered anti-malarial campaigns and training operations, and decreased vigilance against malaria transmission [19,21,44,61,63]. This also results in delays and hampers the procurement of commodities for vector control [2,67]. The pandemic has significantly affected the traditional methods of controlling mosquitoes, leading to a global disruption of malaria control supplies from 2020 to 2021. In Sub-Saharan Africa, activities such as distributing mosquito nets and educating the community are particularly difficult. However, the challenges posed by the pandemic have also spurred innovation in vector control technologies, suggesting a shift towards developing and adopting new strategies to combat mosquito-borne diseases [67]. Other approaches included promoting malaria prevention through social media, deploying village malaria workers to carry out services, and monitoring malaria post-activities in remote areas by telephone [23,55,60].
This review also highlighted COVID-19-related stigma. Fear of COVID-19 exposure, testing, and diagnosis led to decreased patient attendance [15,27]. In India, similar symptoms resulted in misdiagnoses in pregnant women and reduced clinic visits due to fear of contracting COVID-19 [36,66]. Indonesia has experienced diagnostic challenges with stigma and fewer healthcare visits, which further complicate matters [27,57]. In China, malaria diagnosis delays were noted and were potentially worsened by COVID-19, as blood smears were often obtained only after a negative COVID-19 test [17,19]. The fear of COVID-19 and its stigma discourages people from receiving malaria treatment, worsening the burden of malaria. This fear has constrained access to healthcare and delayed the diagnosis and treatment [68]. Furthermore, stigmatizing healthcare staff and COVID-19 patients has had an indirect negative impact on malaria care by increasing stress and limiting provider resources [69].
This study discovered that the pandemic has prompted priority shifts to COVID-19, affecting malaria control. Some malaria centers became COVID-19 facilities, healthcare prioritized severe patients, personnel shortages, budget limits, inadequate supplies, fewer vector control efforts, and disruptions in surveillance and detection of imported cases, contributing to increased malaria cases and outbreaks [30,45,62]. The overwhelming health system and difficulty in diagnosis due to overlapping malaria and COVID-19 symptoms, such as fever and headache, have resulted in delays, misdiagnoses, and complications, such as false positives in COVID-19 tests due to malaria [13,29,31,34,36,44]. The epidemic was also detected in most of the eliminated countries, including Bhutan, because of a delay in the introduction of routine malaria preventative treatments, which contributed to an increase in the incidence of indigenous malaria. Consequently, Bhutan is unlikely to fulfill its national malaria elimination goals by 2020 [70]. Despite this, malaria elimination performance in Myanmar was claimed to have improved during the pandemic owing to increased case investigations. Providing resources to implement early mitigation plans and accommodating vital malaria services during the pandemic reduced further disruptions by providing continuous malaria services [54]. Thus, ensuring funds for uninterrupted malaria services is critical for resilient malaria programs.
This scoping review has limitations. First, the impact of the COVID-19 pandemic on malaria control, as described in this review, may not fully represent the actual situation because only 17 out of 37 countries in Asia have publications. Second, journal databases such as EBSCO, Ovid, and ProQuest were not included. However, it is expected that potential articles in EBSCO, Ovid, and ProQuest would have already been identified in the Google Scholar database. Despite these limitations, this study depicts diverse Asian situations with diverse healthcare systems.
For future research, findings from this scoping review and the Global Malaria Program Operational Strategy 2024–2030 encourage programmatic interventions for malaria control program to get back-to-elimination track [71]. Provision of malaria services relies on a strong public health service system that can respond to emerging needs and technical leadership of the global malaria response.
Conclusion
The COVID-19 pandemic has had a mixed effect on malaria control, with three main factors: travel restrictions, COVID-19-related stigma, and shifting priority to COVID-19. Generally, there has been a decline in malaria cases in countries with varying levels of endemicity, including China, Japan, India, Indonesia, Iran, Afghanistan, Myanmar, and Pakistan. This is primarily because of stringent public health measures alongside behavioral changes that decreased clinic visits due to COVID-19 concerns. Other regions have experienced an increase in malaria cases, often due to heightened screening, complications in diagnosing coinfections with similar symptoms, and an overwhelmed healthcare system with limited resources. The pandemic also highlighted the need to cope with malaria and COVID-19 simultaneously, emphasizing the need for quick detection, treatment, and constant monitoring. This situation demonstrated the need for flexibility and resiliency within malaria control strategies during health emergencies, allowing innovative solutions to fight malaria alongside the COVID-19 challenges.
Acknowledgments
We thank the Department of Biostatistics, Epidemiology, and Population Health, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia, for covering the PhD tuition fee.
Ethics approval
Not required.
Competing interests
All the authors declare that there are no conflicts of interest.
Funding
The Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia, partially supported research funding (grant number: 2342/UN1/FKKMK.1.3/PPKE/PT/2023).
Underlying data
Derived data supporting the findings of this study are available in the Appendix 1 (http://dx.doi.org/10.6084/m9.figshare.27329985) and Appendix 2 (http://dx.doi.org/10.6084/m9.figshare.27329982).
How to cite
Arisanti RR, Saputri GND, Ahmad RA, Utarini A. Impacts of COVID-19 on malaria elimination strategies in Asia: A scoping review. Narra J 2024; 4 (3): e1492 - http://doi.org/10.52225/narra.v4i3.1492.
References
- 1.Gao L, Shi Q, Liu Z, et al. Impact of the COVID-19 pandemic on malaria control in Africa: A preliminary analysis. Trop Med Infect Dis 2023;8(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J, Kang S, Seok D, et al. Barriers against and strategies for malaria control during the COVID-19 pandemic in low- and middle-income countries: A systematic review. Malar J 2023;22(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shretta R, Silal SP, Celhay OJ, et al. Malaria elimination transmission and costing in the Asia-Pacific: A multi-species dynamic transmission model. Wellcome Open Res 2019;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen S, Harvard KE, Gueye CS, et al. Targeting populations at higher risk for malaria: A survey of national malaria elimination programmes in the Asia Pacific. Malar J 2016;15(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. World malaria report 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 6.World Health Organization. World malaria report 2023. Geneva: World Health Organization; 2023. [Google Scholar]
- 7.Heuschen AK, Lu G, Razum O, et al. Public health-relevant consequences of the COVID-19 pandemic on malaria in sub-Saharan Africa: A scoping review. Malar J 2021;20(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: A modelling study. Lancet Glob Health 2020;8(9):e1132–e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Beeson JG, Laman M, et al. Identifying and combating the impacts of COVID-19 on malaria. BMC Med 2020;18(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global malaria program. Available from: https://www.who.int/teams/global-malaria-programme/elimination/countries-and-territories-certified-malaria-free-by-who. Accessed: 21 April 2024.
- 11.Harzing A. Publish or Perish. Available from: https://harzing.com/resources/publish-or-perish. Accessed: 10 April 2024
- 12.World Health Organization. World malaria report 2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 13.Research Square. A case of recurrent COVID-19 co-infected with Plasmodium falciparum and the SARS-CoV-2 IgG may be an indicator for the discharge criteria for the recurrent COVID-19. Available from: https://www.researchsquare.com/article/rs-1374861/v1. Accessed: 28 April 2024.
- 14.Zhu M, Zhang C, Zhang Y, et al. An epidemiological analysis of imported malaria in Shanghai during a COVID-19 outbreak. Malar J 2022;21(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Ling F, Zhang S, et al. Comparison of 19 major infectious diseases during COVID-19 epidemic and previous years in Zhejiang, implications for prevention measures. BMC Infect Dis 2022;22(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X, Hu J, Luo L, et al. Impact of interventions on the incidence of natural focal diseases during the outbreak of COVID-19 in Jiangsu Province, China. Parasit Vectors 2021;14(1):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Cao Y, Zhang D, et al. Implementation and challenges to preventing the re-establishment of malaria in China in the COVID-19 era. Global Health 2022;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Deng Z, Lin R, et al. Malaria surveillance of entry people during the COVID-19 epidemic - Guangdong Province, China, October 2020-May 2021. China CDC Wkly 2021;3(38):799-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Wang D, Qian Y, et al. Profile and determinants of delayed care-seeking and diagnosis among patients with imported malaria: A retrospective study in China, 2014–2021. Infect Dis Poverty 2022;11(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Q, Xu WJ, Wang XX, et al. SARS-CoV-2 and Plasmodium falciparum co-infection in a returning traveler. Front Public Health 2022;10:871374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Jiang J, Lyu X, et al. Surveillance and response to imported malaria during the COVID-19 epidemic - Anhui Province, China, 2019–2021. China CDC Wkly 2022;4(28):622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Gao S, Ai L, et al. The first reported case of COVID-19 and Plasmodium ovale malaria coinfection - Guangdong Province, China, January 2021. China CDC Wkly 2021;3(21):454-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Li C, Zhang J, et al. Using social media for health education and promotion: A pilot of WeChat-based prize quizzes on China national malaria day. Malar J 2022;21(1):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamana E, Zhao J. Deep learning hybrid model for analyzing and predicting the impact of imported malaria cases from Africa on the rise of Plasmodium falciparum in China before and during the COVID-19 pandemic. PLoS One 2023;18(12):e0287702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Qing S, Lan X, et al. Evaluating the long-term impact of COVID-19-associated public health interventions on zoonotic and vector-borne diseases in China: An interrupted time series analysis. J Transl Med 2024;22(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salim NA, Hudari H, Permata M, et al. A case report of moderate COVID-19 and malaria falciparum co-infection with thrombocytopenia. JKK 2021;8(3):173–178. [Google Scholar]
- 27.Lidia K, Setianingrum ELS, Folamauk CL, Pakan P. A comparative study of malaria diagnosis using rapid diagnostic tests (RDT) and microscopy in fever patients in East and West Kupang district, Indonesia during the COVID-19 pandemic. J Commun Dis 2021;53(2):57–61. [Google Scholar]
- 28.Noda H. A model to estimate the effect of international traffic on malaria cases: The case of Japan from 1999 to 2021. Int J Environ Res Public Health 2022;19(2):880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mito H, Hase R, Ueda H, et al. A pitfall of cognitive bias during the pandemic: Two cases of Plasmodium falciparum malaria coinfected or misdiagnosed with COVID-19. J Infect Chemother 2023;29(9):916–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alhaddad MJ, Alsaeed A, Alkhalifah RH, et al. A surge in malaria cases in the Eastern Health Region of Saudi Arabia during the COVID-19 pandemic. Cureus 2023;15(4):e37740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Hong SK, Kim JH. An imported case of afebrile Plasmodium falciparum malaria infection from Tanzania in a returning traveler to the Republic of Korea following an earlier COVID-19 infection. Trop Med Infect Dis 2022;7(4):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwannarong K, Cotter C, Ponlap T, et al. Assessing the acceptability and feasibility of reactive drug administration for malaria elimination in a Plasmodium vivax predominant setting: A qualitative study in two provinces in Thailand. BMC Public Health 2023;23(1):1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerth-Guyette E, Nguyen HT, Nowak S, et al. Assessing the operational feasibility of integrating point-of-care G6PD testing into Plasmodium vivax malaria management in Vietnam. Pathogens 2023;12(5):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Missaghi B, Malik MW, Shaukat W, et al. Associations of the COVID-19 pandemic with the reported incidence of important endemic infectious disease agents and syndromes in Pakistan. BMC Infect Dis 2022;22(1):887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pusparani A, Henrina J, Cahyadi A. Co-infection of COVID-19 and recurrent malaria. J Infect Dev Ctries 2021;15(05):625–629. [DOI] [PubMed] [Google Scholar]
- 36.Mahajan NN, Kesarwani SN, Shinde SS, et al. Co-infection of malaria and dengue in pregnant women with SARS-CoV-2. Int J Gynaecol Obstet 2020;151(3):459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahajan NN, Gajbhiye RK, Bahirat S, et al. Co-infection of malaria and early clearance of SARS-CoV-2 in healthcare workers. J Med Virol 2021;93(4):2431–2438. [DOI] [PubMed] [Google Scholar]
- 38.Eid MM. Co-Infection with COVID-19 and malaria in a young man. Dubai Med J 2021;4(2):164–166. [Google Scholar]
- 39.Rading FB, Pijoh VD, Tuda JSB. Perilaku masyarakat desa terhadap penyakit malaria di masa pandemi COVID-19. MSJ 2021;3(1):20. [Google Scholar]
- 40.Gheibi Z, Boroomand M, Soltani A. Comparing the trends of vector-borne diseases (VBDs) before and after the COVID-19 pandemic and their spatial distribution in Southern Iran. J Trop Med 2023;2023:7697421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wankar RL, Chavhan SS, Adsul B, Dhikale P. Comparison of trends of monsoon-related diseases during COVID-19 pandemic in a tertiary care center: A retrospective study. Med J Dr DY Patil Vidyapeeth 2022;15(8) Suppl 2:S248-S252. [Google Scholar]
- 42.Shaikh SW, Mahendrakar SM, Ladhani SS, Khan AH. COVID-19 and malaria co-infection management in post liver transplant- A case report. J Clin of Diagn Res 2021;15(5):OD03-OD05. [Google Scholar]
- 43.Sardar S, Sharma R, Alyamani TYM, Aboukamar M. COVID-19 and Plasmodium vivax malaria co-infection. IDCases 2020;21:e00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahid Z, Karim N, Shahid F, Yousaf Z. COVID-19 associated imported Plasmodium vivax malaria relapse: First reported case and literature review. Res Rep Trop Med 2021;12:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinta S, Manalu HSP. Konflik sosial dan pengendalian malaria pada masa pandemi Covid-19 di Kabupaten Purworejo, Jawa Tengah tahun 2021. Jurnal Kesehatan Lingkungan Indonesia 2022;21(3):274-284. [Google Scholar]
- 46.Rathi P, Mahajan N, Srivastava V, et al. Early virus clearance of SARS-CoV-2 among co-infection with malaria. J Vector Borne Dis 2023;60(2):211–214. [DOI] [PubMed] [Google Scholar]
- 47.Hibiya K, Shinzato A, Iwata H, et al. Effect of voluntary human mobility restrictions on vector-borne diseases during the COVID-19 pandemic in Japan: A descriptive epidemiological study using a national database (2016 to 2021). PLoS One 2023;18(5):e0285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasamatsu A, Kanou K, Fukusumi M, et al. Epidemiologic trends and distributions of imported infectious diseases among travelers to Japan before and during the COVID-19 pandemic, 2016 to 2021: A descriptive study. J Epidemiol 2024;34(4):187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayilsamy M, Vijaykumar A, Veeramanoharan R, et al. Impact of COVID 19 lockdown during 2020 on the occurrence of vector-borne diseases in India. J Vector Borne Dis 2023;60(2):207–210. [DOI] [PubMed] [Google Scholar]
- 50.Indari O, Baral B, Muduli K, et al. Insights into Plasmodium and SARS-CoV-2 co-infection driven neurological manifestations. Biosaf Health 2021;3(4):230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewinsca MY, Raharjo M, Nurjazuli N. Integrated vector management to control malaria during the Covid-19's pandemic in Lawang Kidul District. J Aisyah J Ilmu Kesehatan 2021;6(3):651–660. [Google Scholar]
- 52.Prabhu SR, Ware AP, Saadi AV, et al. Malaria epidemiology and COVID-19 pandemic: Are they interrelated?. OMICS 2022;26(4):179–188. [DOI] [PubMed] [Google Scholar]
- 53.Triwahyuni T, Haryati S, Nusri M. Malaria situation in Lampung Pesawaran during the COVID-19 pandemic. Malahayati Int J Nurs Health Sci 2023;6(3):170–174. [Google Scholar]
- 54.Research Square. Myanmar continues to curb malaria amid coronavirus disease-2019 crisis. Available from: https://www.researchsquare.com/article/rs-101547/v1. Accessed: 5 April 2024.
- 55.Feldman M, Vernaeve L, Tibenderana J, et al. Navigating the COVID-19 crisis to sustain community-based malaria interventions in Cambodia. Glob Health Sci Pract 2021;9(2):344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahajan NN, Mathe A, Patokar GA, et al. Prevalence and clinical presentation of COVID-19 among healthcare workers at a dedicated hospital in India. J Assoc Physicians India 2020;68(12):16–21. [PubMed] [Google Scholar]
- 57.Asmarawati TP, Martani OS, Bramantono B, Arfijanto MV. Prolonged fever and exaggerated hypercoagulopathy in malaria vivax relapse and COVID-19 co-infection: A case report. Malar J 2022;21(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hocin K, Kusuma DY, Dewi AP. SARS-CoV-2 infection complicated by acute pericarditis concurrent with malaria infection. Jurnal Penyakit Dalam Indonesia 2022;9(2):9. [Google Scholar]
- 59.Rajvanshi H, Bharti PK, Nisar S, et al. Study design and operational framework for a community-based Malaria Elimination Demonstration Project (MEDP) in 1233 villages of district Mandla, Madhya Pradesh. Malar J 2020;19(1):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rae JD, Nosten S, Kajeechiwa L, et al. Surveillance to achieve malaria elimination in eastern Myanmar: A 7-year observational study. Malar J 2022;21(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neyazi N, Lindan C, Perdes S, et al. The provision and utilization of essential health services in Afghanistan during COVID-19 pandemic. Front Public Health 2023;10:1097680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naserrudin NA, Jiee SF, Habil B, et al. The public health response to a Plasmodium malariae outbreak in Penampang District, Sabah during a COVID-19 movement control order. Malar J 2023;22(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perera PN, Amarasinghe SN, Fonseka SH, et al. Factors impacting sustained coverage in the context of donor transitions: Experience from Sri Lanka. Health Policy Plan 2024;39 Suppl 1:i33–i49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khai TS. Vulnerability to health and well-being of internally displaced persons (IDPs) in Myanmar post-military coup and COVID-19. Arch Public Health 2023;81:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nivedita S, Mohan M. Syndemic challenges of malaria and COVID-19 and reported coinfection of both: Experience from a tertiary care center. J South Asian Fed Obstet Gynaecol 2024;16(1):12–16. [Google Scholar]
- 66.Guha S, Biswas M, Gupta B, et al. A report on incidence of COVID-19 among febrile patients attending a malaria clinic. Trop Parasitol 2021;11(1):38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu HZ, Sui Y, Lobo NF, et al. Challenge and opportunity for vector control strategies on key mosquito-borne diseases during the COVID-19 pandemic. Front Public Health 2023;11:1207293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherrard-Smith E, Hogan AB, Hamlet A, et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat Med 2020;26(9):1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mostafa A, Sabry W, Mostafa NS. COVID-19-related stigmatization among a sample of Egyptian healthcare workers. PLoS One 2020;15(12):e0244172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penjor K, TobgyalZangpo T. et al. Has COVID19 derailed Bhutan's national malaria elimination goal? A commentary. Malar J 2021;20(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Health Organization. Global malaria programme operational strategy 2024-2030. Geneva: World Health Organization; 2024. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available in the Appendix 1 (http://dx.doi.org/10.6084/m9.figshare.27329985) and Appendix 2 (http://dx.doi.org/10.6084/m9.figshare.27329982).