Abstract
The sarcolemma of fast-twitch muscle is organized into “costameres,” structures that are oriented transversely, over the Z and M lines of nearby myofibrils, and longitudinally, to form a rectilinear lattice. Here we examine the role of desmin, the major intermediate filament protein of muscle in organizing costameres. In control mouse muscle, desmin is enriched at the sarcolemmal domains that lie over nearby Z lines and that also contain β-spectrin. In tibialis anterior muscle from mice lacking desmin due to homologous recombination, most costameres are lost. In myofibers from desmin −/− quadriceps, by contrast, most costameric structures are stable. Alternatively, Z line domains may be lost, whereas domains oriented longitudinally or lying over M lines are retained. Experiments with pan-specific antibodies to intermediate filament proteins and to cytokeratins suggest that control and desmin −/− muscles express similar levels of cytokeratins. Cytokeratins concentrate at the sarcolemma at all three domains of costameres when the latter are retained in desmin −/− muscle and redistribute with β-spectrin at the sarcolemma when costameres are lost. Our results suggest that desmin associates with and selectively stabilizes the Z line domains of costameres, but that cytokeratins associate with all three domains of costameres, even in the absence of desmin.
INTRODUCTION
Duchenne Muscular Dystrophy and related muscular dystrophies are caused by the mutation or loss of dystrophin and dystrophin-associated proteins (Campbell, 1995; Bonnemann et al., 1996; Straub and Campbell, 1997; Ozawa et al., 1998), respectively, but the functions of these proteins in healthy skeletal muscle are still poorly understood. Dystrophin, which is a member of the spectrin superfamily of membrane skeletal proteins (Davison and Critchley, 1988; Koenig et al., 1988; Dhermy, 1991; Ahn and Kunkel, 1993), accumulates in healthy muscle on the cytoplasmic face of the sarcolemma in linear structures that are oriented both longitudinally and transversely (Masuda et al., 1992; Minetti et al., 1992; Porter et al., 1992; Straub et al., 1992; Williams and Bloch, 1999b). The transverse structures, which lie at the sarcolemma over the Z and M lines of nearby myofibrils, are organized in a rib-like pattern and so are referred to as “costameres” (Pardo et al., 1983a). We also use this term to include the longitudinal elements, which, with the transverse domains, form a lattice-like network that underlies most of the skeletal muscle sarcolemma. All three costameric domains are enriched in dystrophin (Porter et al., 1992). We have found that, in the absence of dystrophin, the longitudinal and M line domains of costameres are more susceptible to disruption in Duchenne muscle and in muscle from the mdx mouse (Porter et al., 1992; Williams and Bloch, 1999b; see also Ehmer et al., 1997), suggesting that dystrophin functions more to stabilize these sarcolemmal domains than the domains that overlie Z lines. These studies also suggest that other structures associated with the sarcolemma help stabilize the Z line domains. Here we test the role of desmin in this stabilization.
Desmin is the major intermediate filament (IF) protein of skeletal muscle and is believed to be the predominant component of IFs at the level of the Z line (Lazarides, 1978; Granger and Lazarides, 1979; Schmid et al., 1979; O'Shea et al., 1981; Richardson et al., 1981). The other IF proteins that are expressed in adult skeletal muscle, synemin, paranemin, syncoilin, and desmuslin, distribute together with desmin at Z lines (Breckler and Lazarides, 1982; Granger et al., 1982; Price and Lazarides, 1983; Hemken et al., 1997; Mizuno et al., 2001; Poon et al., 2002). Additional members of the IF superfamily identified in skeletal muscle, other than the lamins at the myonuclear membrane, are vimentin, nestin, and cytokeratins, all of which are reported to be expressed in embryonic muscle but suppressed as muscle matures (Lazarides, 1978; Bennett et al., 1979; Kosmehl et al., 1990; Sejersen and Lendahl, 1993; Kachinsky et al., 1994; but see Carlsson et al., 1999). Thus, desmin and lower amounts of paranemin, synemin, syncoilin, and desmuslin, are likely to form the IFs that surround the Z disks and may help to organize the myofibrils in the sarcoplasm (Ferrans and Roberts, 1973; Lazarides and Hubbard, 1976; Behrendt, 1977; Granger and Lazarides, 1978; Wang and Ramirez-Mitchell, 1983; Tokuyasu et al., 1983a, 1983b; Milner et al., 1996; Li et al., 1997; Mizuno et al., 2001; Poon et al., 2002; reviewed in Lazarides, 1980; Capetanaki and Milner, 1998).
IFs containing desmin are also likely to link the Z disks of the superficial myofibrils to the sarcolemma (Lazarides and Hubbard, 1976; Campbell and Chamley-Campbell, 1979; Saetersdal et al., 1989). Ultrastructural studies have confirmed that costameres are linked to the contractile apparatus of nearby myofibrils by 10-nm filaments (Garamvölgyi, 1965; Pierobon-Bormioli, 1981; Chiesi et al., 1981; Street, 1983; Shear and Bloch, 1985), the diameter typical of IFs (Ishikawa et al., 1968; reviewed in Lazarides, 1982; Fuchs and Weber, 1994). The creation of mice that are missing desmin due to homologous recombination (Milner et al., 1996; Li et al., 1997) has made it possible to evaluate the possible role in organizing costameres of desmin and other IF proteins.
Compared with normals, the sarcoplasm of desmin −/− mice is poorly organized, and perhaps as a result, the skeletal muscle is myopathic (Milner et al., 1996; Li et al., 1997; reviewed in Capetanaki et al., 1997). In normal myofibers, the sarcomeres in neighboring myofibrils are closely aligned, and Z and M lines run from one side of the myofiber to the other almost without interruption. In desmin −/− muscle, however, this alignment is reduced, and occasional muscle fibers show no regular organization at all. Different muscles in the desmin −/− mouse are disrupted to different extents (Milner et al., 1996; Li et al., 1997), however, suggesting that other factors may mitigate the effects of the desmin null phenotype.
Here we describe experiments in which we have used fast-twitch skeletal muscles from the desmin −/− mouse to investigate the role of desmin in organizing costameres at the sarcolemma. We show that desmin is preferentially associated with the sarcolemmal domains that overlie Z lines and that these domains are selectively lost in muscle lacking desmin. Surprisingly, however, we find that some muscle fibers in desmin −/− mice retain completely organized costameres. Through the use of pan-specific antibodies, we show that such costameres are associated with cytokeratins, which are expressed in both control and desmin −/− muscle.
MATERIALS AND METHODS
Animals
We used 2- to 3-month-old 129 SV mice (desmin +/+) and mice of the same strain that were either heterozygous (desmin +/−) or homozygous (desmin −/−) for a null mutation in the desmin gene, introduced by homologous recombination. These mice were generated as previously described (Milner et al., 1996) and were bred and raised in the Animal Facilities of the Baylor University College of Medicine.
Tissue for Immunofluorescence
Animals were anesthetized and perfusion fixed, and tissue was dissected and processed, exactly as described (Williams and Bloch, 1999b). Frozen, longitudinal sections of the tibialis anterior (TA), extensor digitorum longus (EDL), gastrocnemius and quadriceps muscles were prepared (Williams and Bloch, 1999b), collected on glass slides coated with 0.5% gelatin and 0.05% chromium potassium sulfate, and stored desiccated at −70°C.
Antibodies
Monoclonal mouse antibodies to desmin were purchased as an ascites fluid from ICN Biomedicals, Inc. (Costa Mesa, CA) and were used at a dilution of 1:100. Monoclonal antibodies from Novocastra Laboratories (Newcastle-upon-Tyne, UK) to the C terminus (amino acids 3669–3685) of human dystrophin (Dys2), to the C terminus of human β-dystroglycan (Bewick et al., 1993), and to a fusion protein containing the N-terminal region of utrophin (NCL-DRP-2), were used at dilutions of 1:5, 1:10, and 1:5, respectively. Monoclonal mouse antibody 1351, against all known syntrophin isoforms, was provided by Dr. S. Froehner (University of Washington, Seattle, WA) and was used at 16 μg/ml. Rabbit antiserum to ankyrin 3 was from Dr. J. Morrow (Yale University, New Haven, CT) and was used at a dilution of 1:200. Monoclonal mouse antibodies, obtained from Affinity Bioreagents (Golden, CO), against the α subunit of the dihydropyridine receptor (DHPR, clone 1A) and the sarcoplasmic/endoplasmic reticulum Ca -ATPase from rabbit skeletal muscle (SERCA 1) were used at dilutions of 1:200 and 1:100, respectively. Monoclonal mouse antibodies against α-actinin from rabbit skeletal muscle (EA-53), vinculin from chicken gizzard (VIN-11–5), and fast-twitch myosin (MY32) were from Sigma Immuno Chemicals (St. Louis, MO) and were used at dilutions of 1:200, 1:50, and 1:400, respectively. Pan-specific mouse antibodies to IF proteins (Pruss, 1985) were from Chemicon (Temecula, CA). Pan-specific mouse and rabbit antibodies to cytokeratins were from Biogenex (San Ramon, CA) and ICN (Aurora, OH), respectively.
The rabbit polyclonal antibody, 9050, was prepared against purified human erythrocyte β-spectrin. It was affinity purified over a column of erythrocyte β-spectrin and cross-adsorbed against β-fodrin and α-fodrin, purified from bovine brain, as previously described (Porter et al., 1997; Zhou et al., 1998). Antibodies to erythrocyte β-spectrin were also generated in chickens. IgY was purified from egg yolk using the EggStract kit (Promega, Madison, WI) and anti–β-spectrin antibodies were affinity purified, as reported (Porter et al., 1997). Specificity for β-spectrin was demonstrated by immunoblotting (Ursitti et al., 2001). Both affinity-purified antibodies to β-spectrin were used at 3 μg/ml. Affinity-purified, subunit-specific antibodies to α-fodrin (Porter et al., 1997) were used at 2 μg/ml.
Nonimmune mouse monoclonal antibodies, MOPC21, were obtained from Sigma Chemical Co. (St. Louis, MO). An affinity-purified rabbit antibody to the C-terminal sequence of the erythroid form of β-spectrin, which is not detected in skeletal muscle (Porter et al., 1997; Zhou et al., 1998), was also used as a control. Nonimmune chicken IgY was purified from eggs collected from chickens before they were immunized with β-spectrin. Normal rabbit serum was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Secondary antibodies included goat anti-rabbit and goat anti-mouse IgGs, and donkey anti-chicken IgY. All secondary antibodies, purchased from Jackson ImmunoResearch as fluorescein or tetramethylrhodamine conjugates, were species-specific with minimal cross-reactivity and were used at a dilution of 1:100.
The specificities of all these antibodies have been established by immunoblotting or immunoprecipitation, as reported by the suppliers or in the relevant publications.
Fluorescent Immunolabeling
Sections were incubated in PBS/BSA (PBS containing 1 mg/ml BSA and 10 mM NaN3) for 15 min to reduce nonspecific binding and then placed in primary antibody in PBS/BSA for 2 h at room temperature or overnight at 4°C. Samples were washed with PBS/BSA and incubated for 1 h with fluorescein- or tetramethylrhodamine-conjugated secondary antibodies diluted in PBS/BSA. After additional washing, samples were mounted in a solution containing nine parts glycerol, one part 1 M Tris-HCl, pH 8.0, supplemented with 1 mg/ml p-phenylenediamine to reduce photobleaching (Johnson et al., 1982). Slides were observed with a Zeiss 410 confocal laser scanning microscope (Carl Zeiss, Inc., Tarrytown, NY) equipped with a 63×, NA 1.4 plan-apochromatic objective. The pinholes for both fluorescein and tetramethylrhodamine fluorescence were set to 18. Images were collected and stored with software provided by Zeiss.
To generate figures, images were arranged into montages, labeled, and given scale bars with Corel Draw (Corel Corporation Limited, Ottawa, Ontario, Canada). Inset pictures were prepared with Metamorph (Universal Imaging, West Chester, PA) and magnified twofold with Corel Draw. No other processing was used for any of the images.
For quantitation of fluorescence, we measured the intensity within a small square (0.04 μm2) placed immediately over nearby M line, Z line, or longitudinal domains. The resulting values as well as ratios (M line domain/Z line domain, longitudinal domain/Z line domain) were compared for significant differences between desmin −/− and control samples.
For the quantitations shown in Figure 2, images were prepared by one of the authors and scored blind by another. Images were taken of all sarcolemmal regions from controls, desmin −/−, and heterozygotes that did not show obvious tears, holes, freeze damage, or other processing artifacts. Sampling was otherwise random. Images of control and sarcolemma were coded and mixed randomly. Each image was then evaluated for the pattern of β-spectrin distribution that was most common over the region of the sarcolemma that was in clear focus. Images were sorted into one of four categories (Williams and Bloch, 1999b): A, clear costameric distribution, including regular labeling over Z and M lines, and in longitudinal domains; B, label present at two costameric domains but absent from the third; C, label present at one costameric domain but absent from the other two; and D, label absent from all three costameric domains but present uniformly or in irregular structures at the sarcolemma. We obtained consistent results when other, naive observers scored the same or a similar set of images.
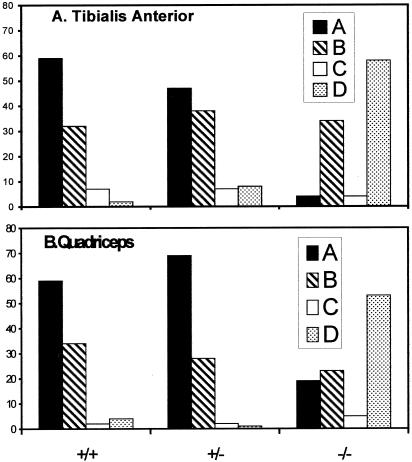
Figure 2.
Quantitation of β-spectrin patterns in wild-type, desmin +/−, and desmin −/− fibers. Images of samples processed as in Figure 1 were evaluated for the dominant patterns of distribution of β-spectrin in the TA (A) and quadriceps (B) muscles. Images were sorted into four categories, as described in detail in MATERIALS AND METHODS: (A, black bars), clear labeling of all three costameric domains; (B, cross-hatched bars), labeling of only two costameric domains; (C, open bars), labeling of only one domain predominates; (D, stippled bars), uniform or irregular labeling. Images of 97 desmin −/−, 87 desmin +/−, and 102 control fibers were examined in the TA muscle; 100 desmin −/−, 107 desmin +/−, and 91 control fibers were examined in the quadriceps.
SDS-PAGE and Immunoblotting
Hindlimb muscles from adult mice were removed, cleaned of fascia and tendons, and frozen. Sections from the bellies of the frozen muscles were used for subsequent steps. Homogenates were prepared in buffer (0.5 ml/25 mg of tissue) containing 1% NP40 and protease inhibitors, as described (Porter et al., 1997). After incubation for 1 h at 0°C, samples were boiled in SDS-PAGE sample buffer (Laemmli, 1970). Aliquots containing 30 μg protein/lane were subjected to electrophoresis (Laemmli, 1970) and then transferred electrophoretically to nitrocellulose paper (Burnette, 1981). Nitrocellulose blots were incubated with a 1:100 dilution of an ascites preparation of mouse anticytokeratin (Chemicon), followed by species-specific goat anti-rabbit IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch). Bound antibody was detected by chemiluminescence (Western Light Detection, Tropix Laboratories, Bedford, MA).
Materials
Unless otherwise stated, all materials were purchased from Sigma Chemical Co. and were the highest grade available.
RESULTS
Loss of Costameres in Desmin −/− Muscle
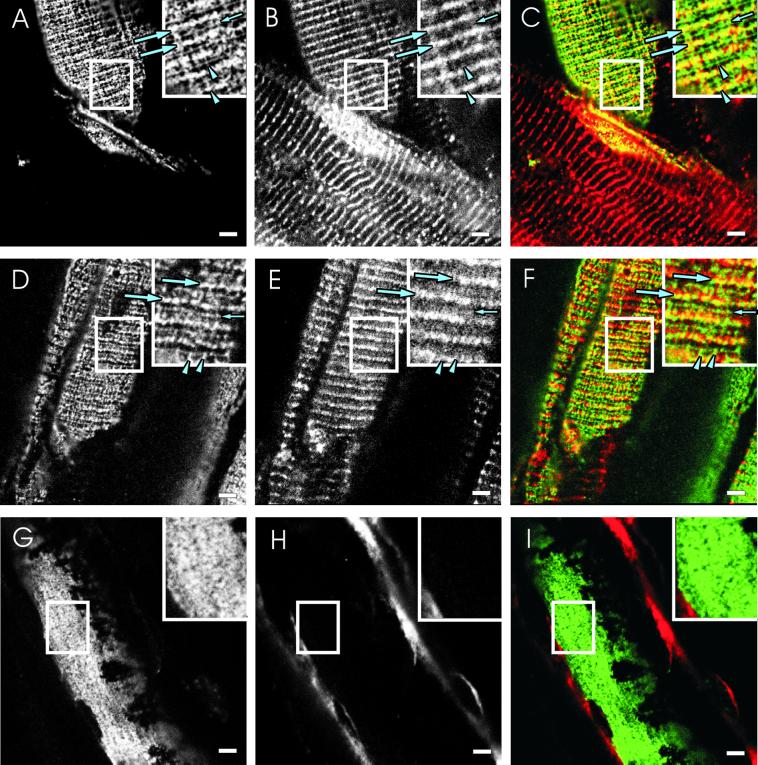
We used double immunofluorescence labeling of frozen, longitudinal sections of mouse muscle to examine the relationship between β-spectrin at the sarcolemma and desmin, the major IF protein of the Z line. In control TA muscles, antibodies to β-spectrin labeled the rectilinear, costameric elements lying in longitudinal strands and over the Z lines and M lines of nearby myofibrils (Figure 1A), in agreement with previous reports (Porter et al., 1992, 1997; Williams and Bloch, 1999a, 1999b). We refer to these structures at the sarcolemma as longitudinal domains, Z line domains, and M line domains. Desmin, visualized in the same optical plane as β-spectrin through the use of confocal optics, was concentrated at Z line domains and was occasionally also found in longitudinal domains (Figure 1B). Desmin was only rarely present in M line domains. Desmin was of course also concentrated at the level of the Z line within each myofiber, but this labeling could easily be distinguished from desmin close to the sarcolemma by the absence of β-spectrin or other sarcolemmal markers (e.g., fiber in the lower portion of Figure 1, A–C). Color composite images confirmed that only the Z line domains of costameres, shown in yellow in Figure 1C, are labeled by both antidesmin and anti–β-spectrin, whereas the longitudinal and M line domains, shown in green, are labeled primarily by anti–β-spectrin.
Figure 1.
Desmin and β-spectrin at the sarcolemma of normal, heterozygous, and homozygous desmin −/− mice. Perfusion-fixed TA muscles from wild-type mice (A–C), desmin −/− mice that lack desmin due to homologous recombination (G–I), and desmin +/− heterozygous mice (D–F), were snap frozen and cryosectioned (see MATERIALS AND METHODS). The sections were double-labeled with affinity-purified chicken anti–β-spectrin (A, D, and G) and a mouse mAb to desmin (B, E, and H), followed by appropriate secondary antibodies. Typical costameric structures overlying Z lines (large arrows), M lines (small arrows), and in longitudinally oriented lines (arrowheads), are indicated in the insets to A–F. Color composite images were created to show β-spectrin in green, desmin in red, and areas containing both proteins in yellow (C, F, and I). (A–C) The costameric lattice, visualized with anti–β-spectrin (A) is intact. Desmin, visualized in the same optical plane (B), is detected primarily at or near Z line domains, where it overlaps with β-spectrin (large arrows; transverse yellow lines in C). Desmin is largely absent from M line and longitudinal domains of costameres (small arrows, arrowheads), which appear green in the color composite image (C). Desmin is also present in the interior of muscle fibers (e.g., fiber in bottom half of image in B and C). (D–F) As in the wild-type, muscle that is heterozygous for desmin expression has normal costameres, visualized with anti–β-spectrin (D), displaying areas of overlap with desmin that are limited primarily to Z line domains (E and F). (G–I) In desmin −/− muscle, desmin is missing from Z lines near the sarcolemma and in the interior of the fiber (H), and there is no costameric organization of β-spectrin at the sarcolemma (G). The labeling seen in H and in red in I is nonspecific and is due to the endogenous mouse antibodies in the connective tissue that are visualized with goat anti-mouse IgG. β-Spectrin in the desmin −/− sample is almost uniformly distributed at the sarcolemma (G and I). Bars, 5 μm.
We performed similar experiments with the TA muscles of mice that are missing desmin due to homologous recombination (desmin −/−) or heterozygotes that have only half the normal complement of desmin (desmin +/−). Although small changes cannot be ruled out, the organization of the sarcolemma in the heterozygotes resembled that of the wild-type, with a regular, rectilinear latticework of costameres, associated with desmin primarily at Z line domains (Figure 1, D–F). Quantitative comparisons of sarcolemmal organization in control and heterozygotic TA muscles (see MATERIALS AND METHODS) revealed no significant differences (Figure 2A; p > 0.14 by χ2 analysis). By contrast, costameric organization in the TA muscle of the desmin −/− mouse tended to be severely disturbed (Figure 1, G–I). The most prevalent morphology, present in >50% of the fibers (Figure 2A), showed β-spectrin in a nearly uniform pattern at the sarcolemma (Figure 1G). Linear elements could be detected at the sarcolemma of the remaining ∼45% of TA myofibers (Figure 2A), but they were rarely as clear or as well organized as in controls (see below). Quantitative comparisons of the organization of β-spectrin at the desmin −/− sarcolemma to that of wild-type and heterozygotic mice showed differences that were highly significant (Figure 2A; p < 0.0001 by χ2 analysis). These results suggest that the presence of desmin at or near Z line domains helps to organize β-spectrin into costameres at the sarcolemma.
Other Sarcolemmal Proteins
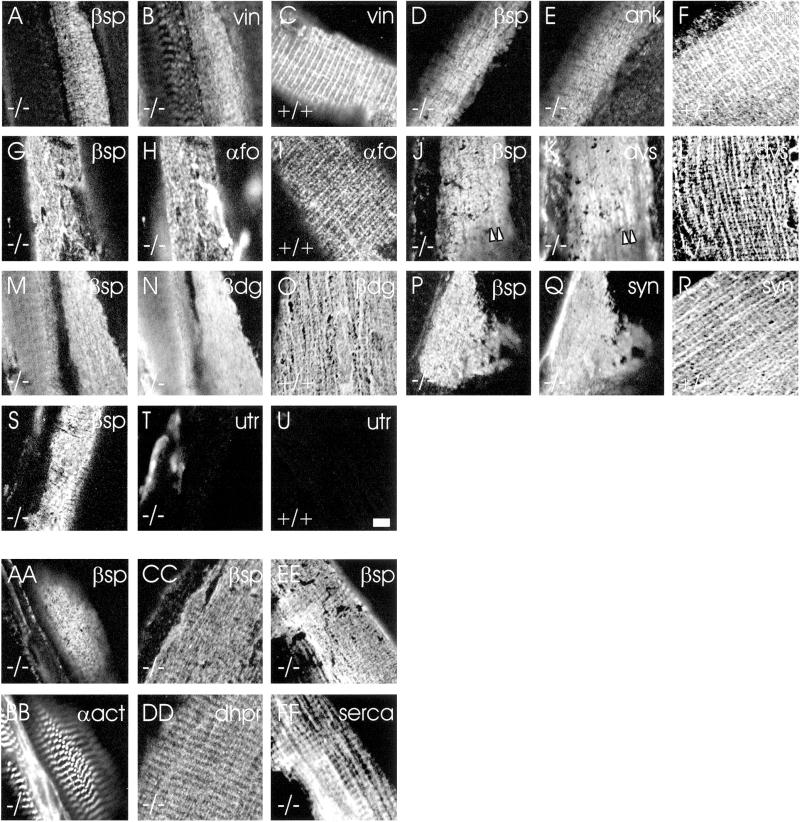
We studied the distribution of other proteins present in the membrane skeleton of costameres to learn if they, like β-spectrin, were organized differently at the sarcolemma of the desmin −/− mouse. In control TA muscles, β-spectrin is concentrated in costameres together with several other membrane skeletal proteins (Porter et al., 1992; Williams and Bloch, 1999b; Williams et al., 2001). Like β-spectrin, vinculin (Figure 3C), ankyrin 3 (Figure 3F), and α-fodrin (Figure 3I) are present in a costameric distribution in wild-type TA muscles, but they lose this distribution in the desmin −/− mouse (vinculin: Figure 3B; ankyrin 3: Figure 3E; α-fodrin, Figure 3H). Likewise, dystrophin (Figure 3L), β-dystroglycan (Figure 3O), and syntrophin (Figure 3R) are enriched in costameres in wild-type but not in desmin −/− TA muscle (dystrophin: Figure 3K; β-dystroglycan, Figure 3N; syntrophin: Figure 3Q), although limited regions of the desmin −/− sarcolemma occasionally retain some remnants of costameres (e.g., longitudinal domains enriched in dystrophin, Figure 3K, arrowheads). Utrophin, which in the mdx mouse is upregulated and codistributes with β-spectrin (Williams and Bloch, 1999b), is not present in significant amounts at the sarcolemma of the TA muscle of the desmin −/− mouse (compare Figure 3T with 3U). These results suggest that the costameric organization of many of the proteins normally in the membrane skeleton of TA muscles requires desmin.
Figure 3.
Membrane skeletal and intracellular proteins in desmin −/− and control muscle. Samples of wild-type (C, F, I, L, O, R, and U) and desmin −/− (A, B, D, E, G, H, J, K, M, N, P, Q, S, T, and AA–FF) TA muscle were double-immunolabeled with affinity-purified chicken antibodies to β-spectrin (A, D, G, J, M, P, S, AA, CC, and EE) and mouse monoclonal antibodies to vinculin (B and C), ankyrin 3 (E and F), α-fodrin (H and I), dystrophin (K and L), β-dystroglycan (N and O), syntrophin (Q and R), utrophin (T and U), α-actinin (BB), dihydropyridine receptor (DD), or SERCA 1 (FF), followed by appropriate secondary antibodies. The results show that vinculin, ankyrin 3, α-fodrin, dystrophin, β-dystroglycan, and syntrophin are costameric in control muscles (C, F, I, L, O, and R) but lose their normal costameric organization in desmin −/− muscle (B, E, H, K, N, and Q). In some samples, longitudinally oriented structures were retained in desmin −/− samples (e.g., K, arrowheads). Utrophin is not present at detectable levels in desmin −/− (T) or wild-type muscle (U). Intracellular organization in desmin −/− muscle is not altered (BB, DD, and FF). Scale bar in U = 5 μm and applies to all panels.
Intracellular Organization near the Sarcolemma
Previous studies of desmin −/− muscle have shown that most myofibers retain their normal sarcomeres but that the side-to-side alignment of neighboring myofibrils is disrupted. A small percentage of myofibers show much more extensive reorganization of their myoplasm, however. We examined the distributions of three proteins associated with sarcomeres, α-actinin, DHPR, and SERCA, to determine if the altered organization of the sarcolemma was associated with extensive changes in the nearby myoplasm.
We observed no consistent relationship between myoplasmic organization and the loss of costameres at the sarcolemma in desmin −/− mice. All fibers that lacked regular sarcomeric structures also lacked organized costameres (not shown). However, many fibers that retained their regular myofibrillar organization near the sarcolemma (α-actinin: Figure 3BB; DHPR: Figure 3DD; SERCA: Figure 3FF) also lacked organized costameres, as visualized with antibodies to β-spectrin (Figure 3, AA, CC, and EE). The loss of costameres in desmin −/− muscle is therefore not associated with extensive changes in the organization of the nearby myoplasm.
Costameric Domains Retained in Less Affected Myofibers
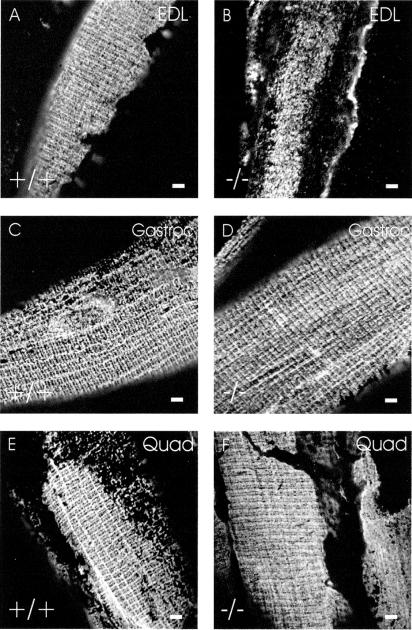
The preceding studies were all performed with the TA muscle of desmin −/− mice, in which the typical organization of the sarcolemma is severely altered by the desmin −/− phenotype (e.g., Figures 1, 2A, and 3). Different skeletal muscles of desmin −/− mice are reported to suffer different degrees of myopathy, however (Milner et al., 1996; Capetanaki et al., 1997; Li et al., 1997). We therefore labeled the sarcolemmae of several different fast-twitch muscles in the desmin −/− mouse with antibodies to β-spectrin to learn if the organization of costameres was also affected to different extents.
We found that the EDL, which in wild-type has a typical costameric organization at the sarcolemma (Figure 4A), showed extensive reorganization in the −/− mouse (Figure 4B), similar to the TA. By contrast, two other muscles with a high percentage of fast twitch myofibers, the gastrocnemius and the quadriceps, both of which resemble the EDL in the wild type (Figure 4, C and E), showed a milder phenotype in the desmin −/− mouse (Figure 4, D and F). Although the sarcolemma of quadriceps myofibers in the desmin −/− mouse were significantly different from controls (Figure 2B; p < 0.0001 by χ2 analysis), a significant number showed what appeared to be a normal costameric pattern, with distinct elements in longitudinal, Z line and M line domains (Figures 2B and 4F). Quantitative measurements showed that, in desmin −/− fibers retaining these three domains, the relative intensities of labeling by anti–β-spectrin antibodies were not significantly affected, compared with controls (p > 0.11, t-test). Additional myofibers in the quadriceps selectively retained one or two of these domains (see below). Some TA myofibers also retained intact costameres in the absence of desmin, but the number of these fibers was significantly less than the number seen in the quadriceps (p < 0.001 by χ2 analysis). This suggests that the costameres do not require desmin to the same extent in all muscles and that structures other than desmin-based IFs that emerge from the contractile apparatus may also contribute to the costameric organization of the sarcolemma.
Figure 4.
Sarcolemmal organization in other fast-twitch skeletal muscles. Cryosections of perfusion-fixed EDL (A and B), gastrocnemius (C and D), and quadriceps (E and F) muscles from control (A, C, and E) and desmin −/− (B, D, and F) mice were labeled for immunofluorescence with chicken anti–β-spectrin. The results show that costameric organization of β-spectrin at the sarcolemma is lost in desmin −/− EDL muscle, but is largely retained in some desmin −/− gastrocnemius and quadriceps myofibers. Bars, 5 μm.
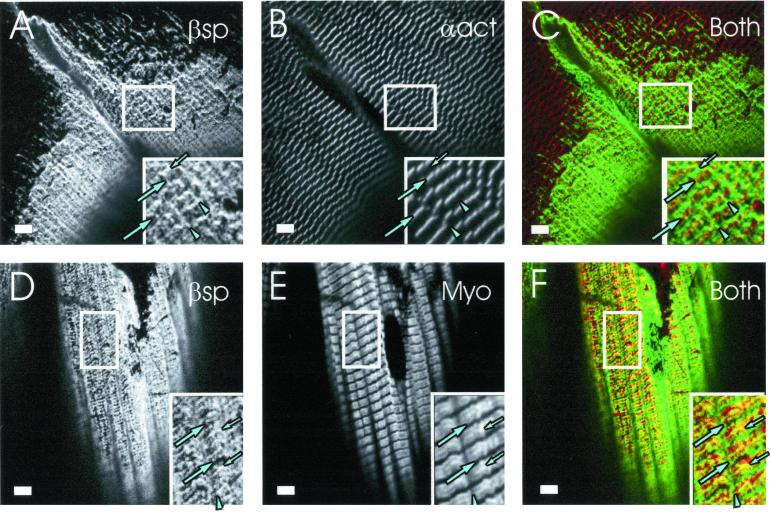
We examined the fibers of the quadriceps to learn if the absence of desmin was associated with the preferential loss of some but not other costameric domains. Because desmin is primarily associated with Z lines, we predicted that the Z line domains would be selectively lost, even when some costameric organization is retained. Muscle fibers were immunolabeled with anti–β-spectrin (Figure 5, A and D) to mark the costameres, and with either anti–α-actinin (Figure 5B) or antimyosin (Figure 5E) to mark the Z lines and A bands, respectively, of nearby myofibrils. In both samples, we found limited regions of the sarcolemma that showed labeling of M lines (Figure 5, C and F, small arrows) and longitudinal domains (Figure 5, C and F, arrowheads) without labeling of nearby Z line domains (Figure 5, C and F, large arrows). These results suggest that M line and longitudinal domains can be selectively retained when Z line domains are lost in desmin −/− quadriceps muscle.
Figure 5.
M line and longitudinal domains are selectively retained in desmin −/− quadriceps. Quadriceps muscle from desmin −/− mice were fixed, collected, frozen, sectioned, and immunolabeled as described in Figures 1 and 3. Double labeling utilized chicken anti–β-spectrin (A and D) and mouse monoclonal antibodies to α-actinin (B) or myosin (E). In color composite images (C and F), β-spectrin is shown in green, myosin or α-actinin in red, and regions that contain each protein pair in yellow. In insets, Z line domains are indicated by large arrows, M line domains by small arrows, and longitudinal domains by arrowheads. (A–C) Z lines near the sarcolemma, labeled with anti–α-actinin (B and C), show only intermittent colabeling with anti–β-spectrin (A). Nearby longitudinal and M lines tend to be intact. This is especially clear in C (inset), where there is little overlap indicated and strong green lines appear at longitudinal and M line domains. (D–F) A bands labeled with antimyosin appear red and consistently show a central yellow line (F, small arrows), consistent with the presence of β-spectrin at M line domains at the nearby sarcolemma. Longitudinal domains are also intact (arrowhead), but several Z line domains are absent, as indicated by their failure to label with anti–β-spectrin (large arrows). Bars, 5 μm.
We quantitated these observations in two ways. First, we determined the frequency with which sarcolemmal regions containing M line domains were retained in desmin −/− quadriceps muscle, compared with regions containing Z line domains. Of the myofibers in quadriceps muscle that showed partial costameric organization (Figure 2B, striped bar), <20% had Z line domains, whereas most (>70%) retained M line domains. This suggests that the contribution of fibers containing M line but not Z line domains to this phenotype was considerable. Because we do not observe a similar number of desmin −/− muscle fibers that retain only one costameric domain (Figure 2, open bars), these results further suggest that M line and longitudinal domains may be lost simultaneously in many desmin −/− myofibers. Second, we measured the fluorescence intensities of anti–β-spectrin labeling in 10 neighboring Z line, M line, and longitudinal domains in the images of Figure 5 and compared them with controls. The values we obtained indicated that the mean intensity of Z line domains in these desmin −/− samples was reduced by 34%. This difference was significant (p < 0.001, t test), despite the considerable range of intensities we sampled (e.g., Figure 5, C and F). By contrast, the mean intensities of M line and longitudinal domains were not consistently altered (5% reduction at M line domains, p > 0.67; 12% reduction at longitudinal domains, p > 0.095). These results confirm that Z line domains are selectively lost in desmin −/− muscle and indicate that M line and longitudinal domains need not be affected by this loss.
Cytokeratins Identified at Costameres in Desmin −/− Muscle
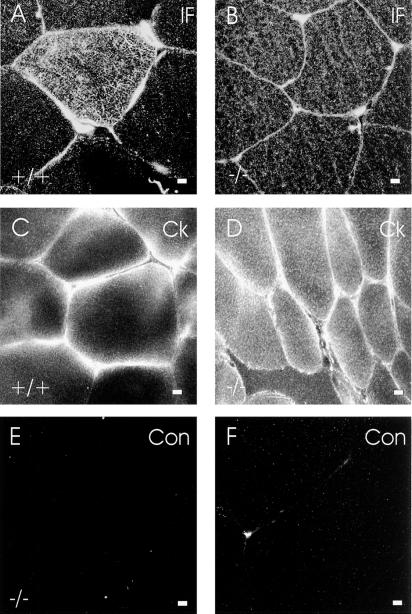
Our results with gastrocnemius and quadriceps muscles suggest that costameres can be stabilized by structures that remain intact when desmin is absent. Ultrastructural studies have suggested that IFs may link the sarcolemma to nearby M lines (Pierobon-Bormioli, 1981; Li et al., 1997), and immunocytochemical studies have revealed that both developing muscle and muscle tumors express cytokeratins (Langbein et al., 1989; Miettinen and Rapola, 1989; Kosmehl et al., 1990). We therefore determined if cytokeratins were present in muscle near the sarcolemma. We first used pan-specific antibodies to IF proteins (Pruss, 1985) to address this question by immunofluorescence. Immunofluorescence images of cross sections of quadriceps muscle labeled with pan-specific anti-IF antibodies showed bright labeling of the sarcolemma and less intense labeling of the cytoplasm both in desmin −/− (Figure 6B) and wild-type (Figure 6A) muscle. This labeling was specific, because desmin −/− (Figure 6E) and wild-type muscle incubated with a nonimmune mouse antibody, MOPC-21, showed no sarcolemmal or cytoplasmic labeling under identical conditions. These results suggest that IF proteins other than desmin are present in skeletal muscle, and that they are concentrated at or near the sarcolemma.
Figure 6.
IF proteins at the sarcolemma in wild-type and desmin −/− skeletal muscle. Frozen cross sections of wild-type (A and C) and desmin −/− (B and D–F) quadriceps muscles were immunolabeled with pan-specific mouse monoclonal antibodies to IF proteins (A and B), with affinity-purified, pan-specific rabbit antibodies to cytokeratins (C and D), or with nonimmune controls (E and F). The results show that IF proteins (B) and cytokeratins (D) are present at the sarcolemma in desmin −/− skeletal muscle. Bars, 5 μm.
We next used pan-specific rabbit antibodies to the cytokeratins to determine if they labeled the sarcolemmal region in similar samples. As we found with the pan-specific mouse antibodies to IF proteins, rabbit anticytokeratin labeled primarily at and near the sarcolemma in both wild-type (Figure 6C) and desmin −/− (Figure 6D) quadriceps muscle. In this case, too, labeling was specific, because it was not apparent in either desmin −/− (Figure 6F) or wild-type (not shown) muscle incubated with a control, affinity-purified rabbit antibody. These results indicate that cytokeratins are present at or near the sarcolemma in normal skeletal muscle and that they persist there when desmin is absent.
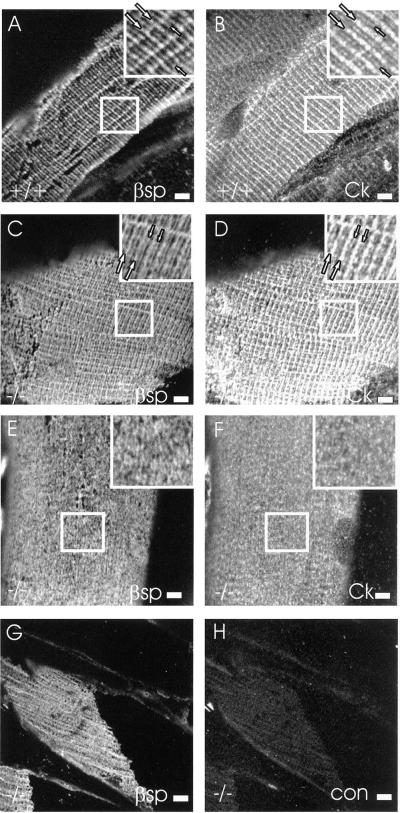
We tested the presence of cytokeratins at costameres by examining longitudinal sections. The pan-specific rabbit anticytokeratin labeled the costameres in wild-type muscle (Figure 7B) as well as those that were retained in desmin −/− quadriceps muscle (Figure 7D). By contrast, labeling by anticytokeratin antibodies of the sarcolemma of EDL myofibers, which undergo more extensive reorganization in the desmin −/− mouse (e.g., Figure 4), showed a nearly uniform pattern (Figure 7F). Similar results were obtained with the myofibers from the desmin −/− TA muscle. Labeling was not seen with an irrelevant rabbit IgG (Figure 7H), indicating that the labeling by the anticytokeratin antibodies was specific. Comparisons to β-spectrin revealed very similar patterns (compare Figure 7, A,B; C,D; and E,F). Thus, the cytokeratins are present at all three costameric domains of the sarcolemma, and their presence there does not require desmin.
Figure 7.
Cytokeratins at costameres in wild-type and desmin −/− skeletal muscle. Longitudinal cryosections of wild-type (A, B) and desmin −/− (C–H) quadriceps (A–D, G, and H) and EDL (E and F) muscle were immunolabeled with chicken anti–β-spectrin (A, C, E, and G) and either affinity-purified, pan-specific rabbit antibodies to cytokeratins (B, D, and F) or with a nonimmune rabbit antibody (H). Cytokeratins are specifically labeled at costameres present in wild-type (B), and retained in desmin −/− (D) quadriceps muscle. Cytokeratins are more uniformly distributed at the sarcolemma of EDL muscle of desmin −/− mice (F). In the insets in A–D, Z line domains and M line domains are indicated by large and small arrows, respectively. Bars, 5 μm.
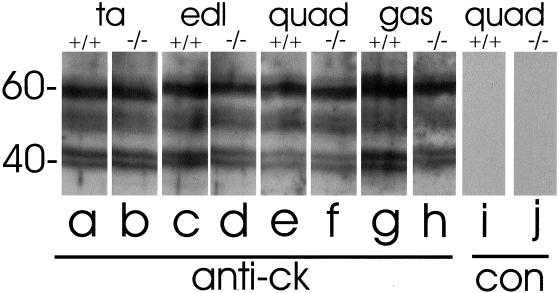
We tested the pan-specific anticytokeratin antibodies to determine if they reacted with proteins of the appropriate molecular weights in blots prepared from control and desmin −/− muscle (Figure 8, lanes a–h). In extracts of both wild-type and mutant muscle, anticytokeratin labels three major bands at ∼40,000, 54,000 and 60,000. These are consistent with the sizes of several cytokeratin subunits, in particular cytokeratins 19, 8, and 5, respectively (see DISCUSSION). Labeling of each of these bands was specific, because a control antibody failed to label any of them (Figure 8, lanes i and j). The intensity of labeling of the immunoblots was similar in the quadriceps, gastrocnemius, EDL, and TA muscles of both wild-type and desmin −/− samples. This suggests that the absence of desmin has no significant effect on the amounts of these proteins expressed in skeletal muscle and that the preservation of costameres in the desmin −/− quadriceps and gastrocnemius muscles cannot be ascribed solely to an increased level of expression of the cytokeratins. Nevertheless, our results are consistent with the conclusion that adult skeletal muscle expresses cytokeratins and concentrates them in costameres at the sarcolemma of both normal and desmin −/− myofibers.
Figure 8.
Immunoblotting of cytokeratins in wild-type and desmin −/− skeletal muscle. Homogenates of TA (a and b), EDL (c and d), quadriceps (e, f, i, and j) and gastrocnemius (g and h) muscles from wild-type (a, c, e, g, and i) and desmin −/− (b, d, f, h, and j) mice were subjected to SDS-PAGE at 30 μg protein/lane and immunoblotted with mouse monoclonal anticytokeratin antibody (a–h) or nonimmune mouse IgG, MOPC-21 (i and j). The blots show several bands between 40 and 60 kDa, including a broad band at ∼52–56 kDa, that label specifically with the pan-specific antibody to cytokeratins. The intensities of these bands do not change significantly with the desmin null phenotype or with the muscle examined.
DISCUSSION
The observation that the sarcolemma of skeletal muscle is organized into structures that align with the Z lines of nearby myofibrils was made nearly two decades ago, when costameres were first discovered and named (Craig and Pardo, 1983; Nelson and Lazarides, 1983; Pardo et al., 1983a, 1983b; Nelson and Lazarides, 1984), but the nature of the structure(s) that mediate this alignment have never been elucidated. Ultrastructural studies suggested that this parallel organization is mediated by IFs. Because desmin, the major IF protein of skeletal muscle, is concentrated at Z lines and is present near the sarcolemma (see INTRODUCTION for references), we predicted that costameres would be disrupted in desmin −/− mice. Our studies of fast-twitch muscles confirm this prediction, but they also indicate that desmin's stabilizing role is limited. In some desmin −/− myofibers, the longitudinal and M line domains of costameres remain intact, whereas in others, the entire costameric lattice appears unaffected by the desmin null phenotype. In studying the latter, we discovered the presence in adult skeletal muscle of cytokeratins and showed that they can concentrate at all three domains of costameres, even when desmin is absent. Cytokeratins are the first IF proteins of striated muscle that are known to have this distribution.
Although ours is the first report of cytokeratins in healthy, adult skeletal muscle, cytokeratins have been reported in developing muscle (Kosmehl et al., 1990) as well as in rhabdomyosarcomas (Langbein et al., 1989; Miettinen and Rapola, 1989). Of particular interest to us is our observation that the cytokeratins appear to concentrate at the sarcolemma in costameres (e.g., Figures 6 and 7). This suggests that, unlike desmin, which not only interacts with the sarcolemma at Z line domains but also surrounds the Z disks throughout the myoplasm, cytokeratins may interact preferentially with the sarcolemma. Because other members of the IF superfamily can bind to β-spectrins (Langley and Cohen, 1987; Frappier et al., 1992a, 1992b; Macioce et al., 1999), cytokeratin-sarcolemmal interactions may even be mediated by members of the spectrin superfamily, as suggested by the model shown in Figure 9. Understanding these potential interactions will require more definitive characterization of the cytokeratins in muscle.
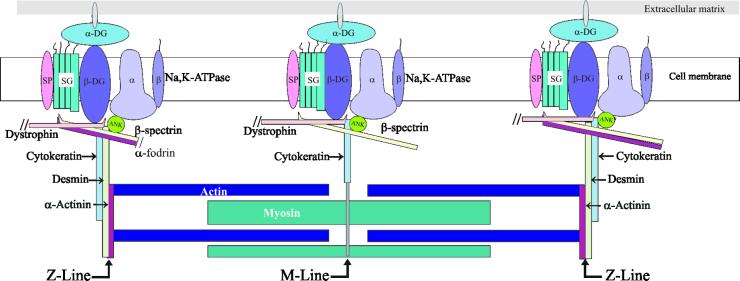
Figure 9.
Model of the sarcolemmal membrane skeleton and its relationship to desmin and cytokeratin. This figure depicts a model of the organization of the muscle cell surface, from the extracellular space to the contractile apparatus. The membrane skeletal and intermediate filament proteins that we have studied at costameres are emphasized, whereas many proteins known to be at or near the sarcolemma or in the contractile structures have been omitted for clarity. Longitudinal domains, which are similar in composition to M line domains, and intercostameric regions are not illustrated. The only extracellular protein depicted is α-dystroglycan (α-DG). Integral proteins of the sarcolemma shown are the α and β chains of the Na,K-ATPase, β-dystroglycan (β-DG), sarcoglycans (SG), and sarcospan (SP). The membrane skeletal proteins illustrated are ankyrin 3 (Ank), dystrophin, αII-spectrin (α-fodrin), and βIΣ2-spectrin (β-spectrin). Sarcomeric proteins shown are actin, myosin and α-actinin. Our results suggest that two sets of intermediate filaments connect the contractile apparatus to the costameres at the sarcolemma: desmin, which links the Z disks to the Z line domains of costameres, and cytokeratin, which links the contractile apparatus to all three costameric domains. Cytokeratin filaments were referred to as “connectors” in an earlier version of this cartoon (Williams et al., 2001). Not drawn to scale.
In preliminary experiments, we have used RT-PCR to confirm the presence of at least two cytokeratins in adult striated muscle, cyokeratins 8 and 19 (our unpublished results). This is consistent with the results of our immunoblots (Figure 8), which identify two bands with molecular masses of ∼40 and ∼54 kDa, expected for these two proteins. Thus, cytokeratins 8 and 19 can account for two of the three major bands we detect in immunoblots of skeletal muscle probed with pan-specific antibodies to the cytokeratins (e.g., Figure 8). Other bands detected in immunoblots may be due to additional cytokeratins expressed either in myofibers or in the other cell types present in skeletal muscle (e.g., blood vessels, connective tissue). Experiments at the molecular level are now in progress to characterize more thoroughly each of the cytokeratins of adult striated muscle and the IFs that they form, to localize them within muscle cells, and to learn how they interact with the sarcolemma.
Our results indicate that, like desmin in the interior of muscle fibers, desmin at the sarcolemma is selectively enriched at the level of Z lines. It was therefore to be expected that Z line domains would be lost in the desmin −/− mouse. Because different muscles are affected by the desmin null mutation to varying extents (Milner et al., 1996; Li et al., 1997), it was not surprising to find that the sarcolemma in the TA and EDL was more severely disrupted than in the quadriceps and gastrocnemius (e.g., Figures 2, 4, and 7). This cannot be explained by variations in the levels of the cytokeratins in these muscles (Figure 8) or by a total loss of organization in the myoplasm (Figure 3), reported to occur in a small number of desmin −/− fibers (Milner et al., 1996; Li et al., 1997). We currently favor the idea that these differences are due to the distinct duty cycles of these muscles during normal locomotory activity and the extents to which the connections between the sarcolemma and the contractile apparatus in these muscles rely on desmin-based IFs. Alternatively, proteins that help to stabilize the connections of IFs to the sarcolemma may be expressed in varying amounts in different desmin −/− muscles (but see Carlsson et al., 2000). In any case, our results clearly indicate that, although the cytokeratins are present at costameres and may well be necessary for sarcolemmal organization, their simple presence at the sarcolemma may not be sufficient to stabilize costameres when desmin is absent.
Although they are lost in most TA and EDL muscles, the costameric structures most likely to persist in desmin −/− quadriceps muscle are the longitudinal and M line domains. This is in striking contrast to results with muscle that lacks dystrophin, in which the longitudinal and M line domains are selectively lost, whereas the Z line domains are selectively retained (Porter et al., 1992; Williams and Bloch, 1999b). Our observations on muscle from the mdx mouse suggest that the costameric structures that remain at the sarcolemma of dystrophic muscle may be stabilized by association with desmin IFs, even when obvious Z line domains are lost and replaced with irregular, polygonal structures (our unpublished results). Thus, desmin appears to help to maintain Z line domains and related structures, but not M line or longitudinal domains. The cytokeratins at those domains (e.g., Figure 9) may play a role in stabilizing them when desmin is absent, perhaps by interacting with the dystrophin-based membrane skeleton. Dystrophin at costameres may associate indirectly with still other IF proteins, including syncoilin and desmuslin (Mizuno et al., 2001; Newey et al., 2001, Poon et al., 2002), two newly identified members of the IF superfamily that bind the dystrophin-associated protein, α-dystrobrevin. Neither desmuslin nor syncoilin has been unambiguously localized to costameres, however.
The distinct effects of null mutations in dystrophin and desmin suggest that different structural elements are involved in stabilizing the three domains that make up the costameric lattice of fast-twitch skeletal muscle. As mentioned above, Z line domains are likely to be stabilized at least in part through their association with desmin. In addition to desmin, the only other structural protein that is currently known to concentrate selectively at Z line domains is α-fodrin (αII-spectrin). Although it is tempting to speculate that desmin IFs anchor to α-fodrin at the sarcolemma at Z line domains, binding of desmin to α subunits of the spectrin superfamily has yet to be reported. Desmin and other IF proteins bind to spectrin and to ankyrin (Georgatos and Blobel, 1987; Langley and Cohen, 1987; Frappier et al., 1991, 1992; Macioce et al., 1999), however, so anchoring of desmin IFs to the sarcolemma may be mediated by the αβ-spectrin heteromers and ankyrin that are present at Z line domains (Porter et al., 1997; Williams and Bloch, 1999b; Williams et al., 2001; see Figure 9).
The structures that anchor to and stabilize the longitudinal and M line domains remain to be identified, but our present results suggest that they may be cytokeratin-based IFs. If so, then the loss of cytokeratin-based IFs from the longitudinal and M line domains of the dystrophic sarcolemma may contribute to the myopathy seen in Duchenne dystrophy, and mutations in the cytokeratins themselves may be linked to other muscular dystrophies. In addition, the destabilization of the Z line domains of costameres that occurs in the absence of desmin is likely to contribute to the myopathy and the associated loss of contractile strength observed in desmin −/− mice and in human desminopathies (Milner et al., 1996; Li et al., 1997; Sam et al., 2000; Li and Dalakas, 2001).
ACKNOWLEDGMENTS
We are grateful to Drs. S. Froehner (University of Washington, Seattle, WA) and J. Morrow (Yale University, New Haven, CT) for their gifts of antibodies, and to Dr. E. Fuchs (University of Chicago, Chicago, IL) and our colleagues at the University of Maryland School of Medicine for useful discussions. Our research has been supported by grants from the National Institutes of Health (AR39617 to Y.C.; NS 17282 and HL 64304, to R.J.B.) and from the Muscular Dystrophy Association.
Abbreviations used:
- IF
intermediate filament
- EDL
extensor digitorum longus
- TA
tibialis anterior
- DHPR
dihydropyridine receptor
- SERCA
Ca-ATPase of the sarcoplasmic reticulum
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0576. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0576.
REFERENCES
- Ahn AH, Kunkel LM. The structural and functional diversity of dystrophin. Nature Genetics. 1993;3:283–291. doi: 10.1038/ng0493-283. [DOI] [PubMed] [Google Scholar]
- Behrendt H. Effect of anabolic steroids on rat heart muscle cells. I. Intermediate filaments. Cell Tiss Res. 1977;180:303–315. doi: 10.1007/BF00227598. [DOI] [PubMed] [Google Scholar]
- Bennett GS, Fellini SA, Toyama Y, Holtzer H. Redistribution of intermediate filament subunits during skeletal myogenesis and maturation in vitro. J Cell Biol. 1979;82:577–584. doi: 10.1083/jcb.82.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Nicholson LVB, Young C, Slater CR. Relationship of a dystrophin-associated glycoprotein to junctional acetylcholine receptor clusters in rat skeletal muscle. Neuromusc Disord. 1993;3:503–506. doi: 10.1016/0960-8966(93)90105-s. [DOI] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bonnemann CG, McNally EM, Kunkel LM. Beyond dystrophin: current progress in the muscular dystrophies. Curr Opin Pediatr. 1996;8:569–82. [PubMed] [Google Scholar]
- Breckler J, Lazarides E. Isolation of a new high molecular weight protein associated with desmin and vimentin filaments from avian embryonic skeletal muscle. J Cell Biol. 1982;92:795–806. doi: 10.1083/jcb.92.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Chamley-Campbell J. Antibody staining of 10-nm (100-Å) filaments in cultured smooth, cardiac and skeletal muscle cells. J Cell Sci. 1979;37:303–322. doi: 10.1242/jcs.37.1.303. [DOI] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y, Milner DJ. Desmin cytoskeleton in muscle integrity and function. Subcell Biochem. 1998;31:463–495. [PubMed] [Google Scholar]
- Capetanaki Y, Milner DJ, Weitzer G. Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct Funct. 1997;22:103–116. doi: 10.1247/csf.22.103. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Li ZL, Paulin D, Price MG, Breckler J, Robson RM, Wiche G, Thornell L-E. Differences in the distribution of synemin, paranemin, and plectin in skeletal muscles of wild-type and desmin knock-out mice. Histochem Cell Biol. 2000;114:39–47. doi: 10.1007/s004180000158. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Li Z, Paulin D, Thornell L-E. Nestin is expressed during development and in myotendinous and neuromuscular junctions in wild type and desmin knock-out mice. Exp Cell Res. 1999;251:213–223. doi: 10.1006/excr.1999.4569. [DOI] [PubMed] [Google Scholar]
- Chiesi M, Ho MM, Inesi G, Somlyo AV, Somlyo AP. Primary role of sarcoplasmic reticulum in phasic contractile activation of cardiac myocytes with shunted myolemma. J Cell Biol. 1981;91:728–742. doi: 10.1083/jcb.91.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SW, Pardo JV. Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motil. 1983;3:449–462. doi: 10.1002/cm.970030513. [DOI] [PubMed] [Google Scholar]
- Davison MD, Critchley DR. Alpha-actinins and the DMD protein contain spectrin-like repeats. Cell. 1988;52:159–160. doi: 10.1016/0092-8674(88)90503-x. [DOI] [PubMed] [Google Scholar]
- Dhermy D. The spectrin super-family. Biol Cell. 1991;71:249–254. [PubMed] [Google Scholar]
- Ehmer S, Herrmann R, Bittner R, Voit T. Spatial distribution of β-spectrin in normal and dystrophic human skeletal muscle. Acta Neuropathol. 1997;94:240–246. doi: 10.1007/s004010050699. [DOI] [PubMed] [Google Scholar]
- Ferrans VJ, Roberts WC. Intermyofibrillar and nuclear-myofibrillar connections in human and canine myocardium. An ultrastructural study. J Mol Cell Cardiol. 1973;5:247–257. doi: 10.1016/0022-2828(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Frappier T, Derancourt J, Pradel L-A. Actin and neurofilament binding domain of brain spectrin beta subunit. Eur Biochem. 1992;205:85–91. doi: 10.1111/j.1432-1033.1992.tb16754.x. [DOI] [PubMed] [Google Scholar]
- Frappier T, Stetzkowski-Marden F, Pradel L-A. Interaction domains of neurofilament light chain and brain spectrin. Biochem J. 1991;275:521–527. doi: 10.1042/bj2750521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Ann Rev Biochem. 1994;63:345–82. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Garamvölgyi N. Inter-Z bridges in the flight muscle of the bee. J Ultrastruct Res. 1965;13:435–443. doi: 10.1016/s0022-5320(65)90006-7. [DOI] [PubMed] [Google Scholar]
- Georgatos SD, Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avain erythroyctes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987;105:105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger BL, Lazarides E. The existence of an insoluble Z disc scaffold in chicken skeletal muscle. Cell. 1978;15:1253–1268. doi: 10.1016/0092-8674(78)90051-x. [DOI] [PubMed] [Google Scholar]
- Granger BL, Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979;18:1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Granger BL, Repasky EA, Lazarides E. Synemin and vimentin are components of intermediate filaments in avian erythrocytes. J Cell Biol. 1982;92:299–312. doi: 10.1083/jcb.92.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemken PM, Bellin RM, Sernett SW, Becker B, Huiatt TW, Robson RM. Molecular characteristics of the novel intermediate filament protein paranemin. Sequence reveals EAP-300 and IFAPa-400 are highly homologous to paranemin. J Biol Chem. 1997;272:32489–32499. doi: 10.1074/jbc.272.51.32489. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Bischoff R, Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968;38:538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GD, Davidson RS, McNamee KC, Russell G, Goodwin D, Holborow EJ. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Meth. 1982;55:231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994;166:216–28. doi: 10.1006/dbio.1994.1248. [DOI] [PubMed] [Google Scholar]
- Koenig M, Monaco AP, Kunkel LM. A complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–223. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kosmehl H, Langbein L, Katenkamp D. Transient cytokeratin expression in skeletal muscle during murine embryogenesis. Anat Anz. 1990;171:39–44. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langbein L, Kosmehl H, Kiss F, Katenkamp D, Neupert G. Cytokeratin expression in experimental murine rhabdomyosarcomas. Intermediate filament pattern in original tumors, allo-transplants, cell culture and re-established tumors from cell culture. Exp Pathol. 1989;36:23–36. doi: 10.1016/s0232-1513(89)80107-0. [DOI] [PubMed] [Google Scholar]
- Langley RC, Jr, Cohen CM. Cell type-specific association between two types of spectrin and two types of intermediate filaments. Cell Motil Cytoskelet. 1987;8:165–173. doi: 10.1002/cm.970080208. [DOI] [PubMed] [Google Scholar]
- Lazarides E. The distribution of desmin (100 Å) filaments in primary cultures of embryonic chick cardiac cells. Exp Cell Res. 1978;112:265–273. doi: 10.1016/0014-4827(78)90209-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lazarides E, Hubbard BD. Immunological characterization of the subunit of the 100-Å filaments from muscle cells. Proc Natl Acad Sci USA. 1976;73:4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Dalakas MC. Abnormal desmin protein in myofibrillar myopathies caused by desmin gene mutations. Ann Neurol. 2001;49:532–536. [PubMed] [Google Scholar]
- Li Z, Mericskay M, Agbulut O, Butler-Browne GS, Carlsson L, Thornell L-E, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol. 1997;139:129–44. doi: 10.1083/jcb.139.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macioce P, Gandolfi N, Leung CL, Chin SS, Malchiodi-Albedi F, Ceccarini M, Petrucci TC, Liem RK. Characterization of NF-L and βIIΣ1-spectrin interaction in live cells. Exp Cell Res. 1999;250:142–154. doi: 10.1006/excr.1999.4479. [DOI] [PubMed] [Google Scholar]
- Masuda T, Fujimaki N, Ozawa E, Ishikawa H. Confocal laser microscopy of dystrophin localization in guinea pig skeletal muscle fibers. J Cell Biol. 1992;119:543–548. doi: 10.1083/jcb.119.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M, Rapola J. Immunohistochemical spectrum of rhabdomyosarcoma and rhabdomyosarcoma-like tumors. Expression of cytokeratin and the 68-kD neurofilament protein. Am J Surg Pathol. 1989;13:120–132. doi: 10.1097/00000478-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–70. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, Beltrame F, Marcenaro G, Bonilla E. Dystrophin at the plasma membrane of human muscle fibers shows a costameric localization. Neuromusc Disord. 1992;2:99–109. doi: 10.1016/0960-8966(92)90041-4. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM. Desmuslin, an intermediate filament protein that interacts with α-dystrobrevin and desmin. Proc Natl Acad Sci USA. 2001;98:6156–6161. doi: 10.1073/pnas.111153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Lazarides E. Switching of subunit composition of muscle spectrin during myogenesis in vitro. Nature. 1983;304:364–368. doi: 10.1038/304364a0. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Lazarides E. Goblin (ankyrin) in striated muscle: identification of the potential membrane receptor for erythroid spectrin in muscle cells. Proc Natl Acad Sci USA. 1984;81:3292–3296. doi: 10.1073/pnas.81.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ. Syncoilin, a novel member of the intermediate filament superfamily that interacts with α-dystrobrevin in skeletal muscle. J Biol Chem. 2001;276:6645–6655. doi: 10.1074/jbc.M008305200. [DOI] [PubMed] [Google Scholar]
- O'Shea JM, Robson RM, Hartzer MK, Huiatt TW, Rathbun WE, Stromer MH. Purification of desmin from adult mammalian skeletal muscle. Biochem J. 1981;195:345–356. doi: 10.1042/bj1950345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa E, Noguchi S, Mizuno Y, Hagiwara Y, Yoshida M. From dystrophinopathy to sarcoglycanopathy: evolution of a concept of muscular dystrophy. Muscle Nerve. 1998;21:421–438. doi: 10.1002/(sici)1097-4598(199804)21:4<421::aid-mus1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: Transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA. 1983a;80:1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW. Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983b;97:1081–1088. doi: 10.1083/jcb.97.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierobon-Bormioli S. Transverse sarcomere filamentous systems: ‘Z- and M-cables.’. J Musc Res Cell Motil. 1981;2:401–413. [Google Scholar]
- Poon E, Howman EV, Newey SE, Davies KE. Association of syncoilin and desmin: linking intermediate filament proteins to the dystrophin-associated protein complex. J Biol Chem. 2002;277:3433–3439. doi: 10.1074/jbc.M105273200. [DOI] [PubMed] [Google Scholar]
- Porter GA, Dmytrenko GM, Winkelmann JC, Bloch RJ. Dystrophin colocalizes with β-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J Cell Biol. 1992;117:997–1005. doi: 10.1083/jcb.117.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter GA, Scher MG, Resneck WG, Fowler V, Bloch RJ. Two populations of β-spectrin in mammalian skeletal muscle. Cell Motil Cytoskelet. 1997;37:7–19. doi: 10.1002/(SICI)1097-0169(1997)37:1<7::AID-CM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Price MG, Lazarides E. Expression of intermediate filament-associated proteins paranemin and synemin in chicken development. J Cell Biol. 1983;97:1860–1874. doi: 10.1083/jcb.97.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss RM. Efficient detection of intermediate filament proteins using a panspecific monoclonal antibody: anti-IFA. J Neuroimmunol. 1985;8:293–299. doi: 10.1016/s0165-5728(85)80068-0. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Stromer MH, Huiatt TW, Robson RM. Immunoelectron and immunofluorescence localization of desmin in mature avian muscles. Eur J Cell Biol. 1981;26:91–101. [PubMed] [Google Scholar]
- Saetersdal T, Dalen H, Røli J. Immunofluorescence and immunogold electron microscopy of desmin in isolated adult heart myocytes of the rat. Histochemistry. 1989;92:467–473. doi: 10.1007/BF00524758. [DOI] [PubMed] [Google Scholar]
- Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared to wild-type muscles. Am J Physiol Cell Physiol. 2000;279:C1116–C1122. doi: 10.1152/ajpcell.2000.279.4.C1116. [DOI] [PubMed] [Google Scholar]
- Schmid E, Tapscott SJ, Bennett GS, Croop J, Fellini SA, Holtzer H, Franke WW. Differential location of different types of intermediate-sized filaments in various tissues of the chicken embryo. Differentiation. 1979;15:27–40. doi: 10.1111/j.1432-0436.1979.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106:1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Shear CR, Bloch RJ. Vinculin in subsarcolemmal densities in chicken skeletal muscle: localization and relationship to intracellular and extracellular structures. J Cell Biol. 1985;101:240–256. doi: 10.1083/jcb.101.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Bittner RE, Leger JJ, Voit T. Direct visualization of the dystrophin network on skeletal muscle fiber membrane. J Cell Biol. 1992;119:1183–1191. doi: 10.1083/jcb.119.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr Opin Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K, Dutton AH, Singer SJ. Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken skeletal muscle. J Cell Biol. 1983a;96:1727–1735. doi: 10.1083/jcb.96.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT, Dutton AH, Singer SJ. Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken cardiac muscle. J Cell Biol. 1983b;96:1736–1742. doi: 10.1083/jcb.96.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursitti JA, Martin L, Chaney T, Zielke C, Alger BE, Bloch RJ. Spectrins in the developing rat hippocampal cells. Dev Brain Res. 2001;129:81–93. doi: 10.1016/s0165-3806(01)00160-2. [DOI] [PubMed] [Google Scholar]
- Wang K, Ramirez-Mitchell R. A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vertebrate skeletal muscle. J Cell Biol. 1983;96:562–570. doi: 10.1083/jcb.96.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MW, Bloch RJ. Differential distribution of dystrophin and β-spectrin at the sarcolemma of fast twitch skeletal muscle fibers. J Musc Res Cell Motil. 1999a;20:387–398. doi: 10.1023/a:1005512217552. [DOI] [PubMed] [Google Scholar]
- Williams MW, Bloch RJ. Extensive but coordinated reorganization of the membrane skeleton in myofibers of dystrophic (mdx) mice. J Cell Biol. 1999b;144:1259–1270. doi: 10.1083/jcb.144.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MW, Resneck WG, Kaysser T, Ursitti JA, Birkenmeier CS, Barker JE, Bloch RJ. Na,K-ATPase in skeletal muscle: two populations of β-spectrin control localization in the sarcolemma but not partitioning between the sarcolemma and the transverse tubules. J Cell Sci. 2001;114:751–762. doi: 10.1242/jcs.114.4.751. [DOI] [PubMed] [Google Scholar]
- Zhou D, Ursitti JA, Bloch RJ. Developmental expression of spectrins in rat skeletal muscle. Mol Biol Cell. 1998;9:47–61. doi: 10.1091/mbc.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]